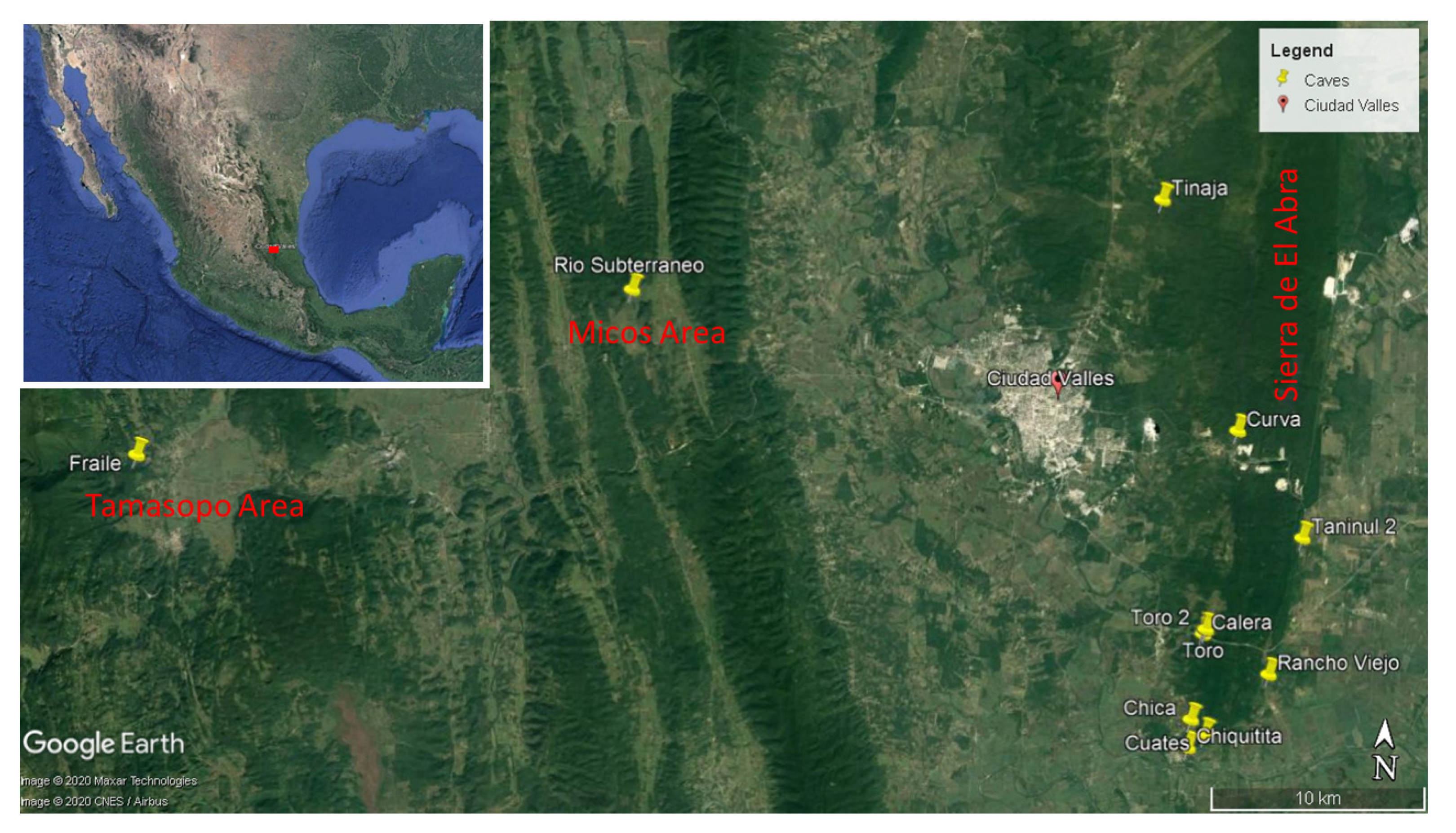
3.1.1. Background on Sótano del Toro, Cueva del Rancho Viejo and Taninul #2
Sótano del Toro is the smallest and shallowest of the known eyeless
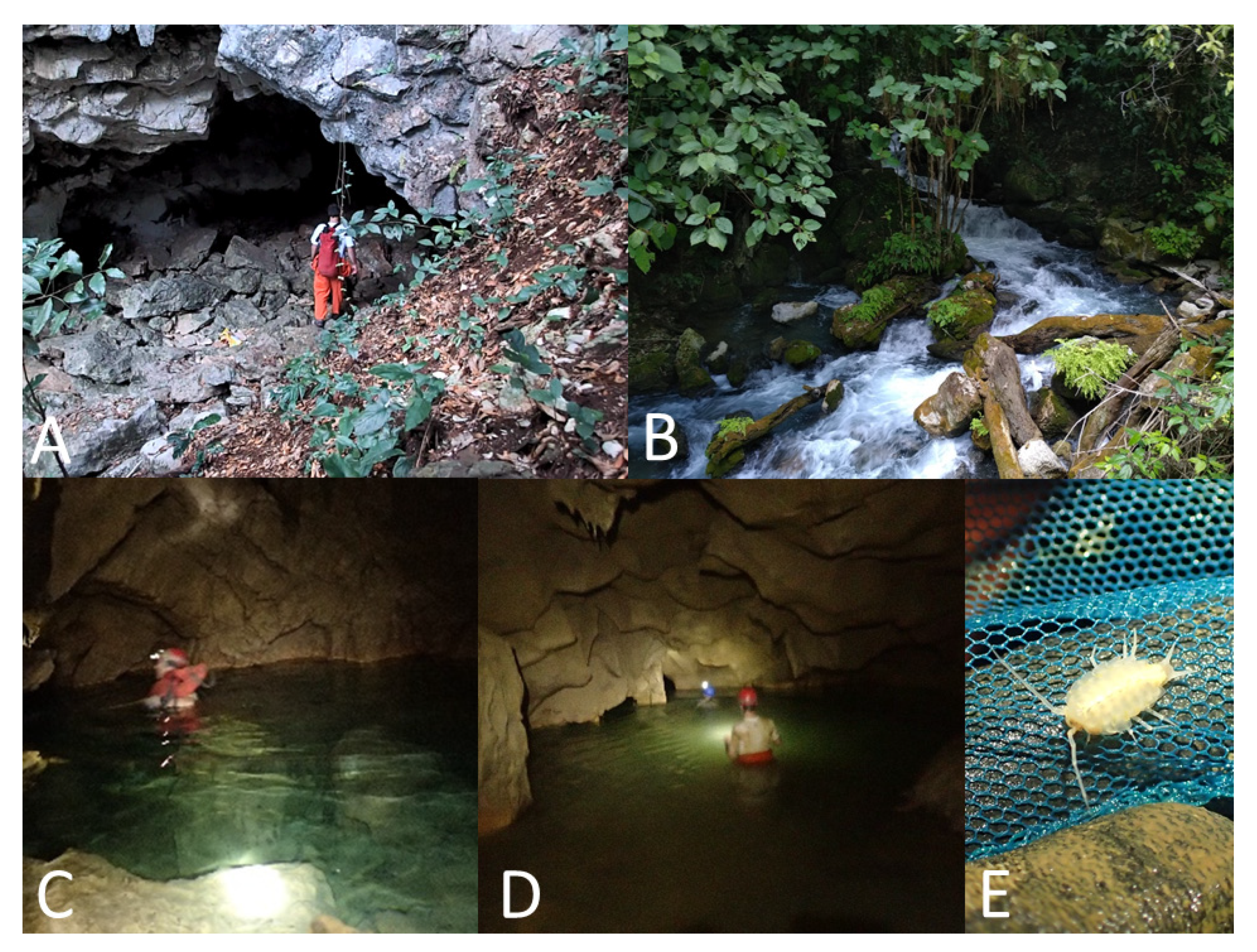
Astyanax caves. It is possible to stand in the entrance sink and see the 0.5 m wide by 2.5 m long pool. A gallery can be seen continuing underwater. Light reaches this small pool, as can be seen in
Figure 2. The cave was visited in 1969, when three days of work resulted in only about eight fish being collected. On March 2008, three cavefish were obtained for genomic work [
9]. Previous reports [
11] conclude that “None of these fishes appeared to have hybridized with surface
Astyanax, even though Cueva Chica (which has large amounts of surface and hybrids) is located only 4 km to the south”. They further report that “Another cave may exist in the vicinity, but it was not found in 1974 by local guides who knew of it”.
The Cueva del Rancho Viejo is near a small resurgence called Nacimiento del Rancho Viejo, which only discharges water after a rain. The cave was explored by Elliott et al. in 1974. A climbdown of 8 m reaches the first pool, 5-10 m deep. A low gallery 100 m long reaches a second pool. Eyed
Astyanax and cichlids were observed and collected at that time [
11]. During the dry season, no water comes out of the resurgence, and fish are isolated in pools that lie in complete darkness. This resurgence is of interest because it is only 3.6 km east of Sótano del Toro, and 4.2 km northeast of Cueva Chica, so they could be hydrologically related. Before this study it was unclear if troglomorphic fish were also present in this cave.
Cueva de Taninul #2 is 6 km northeast of Sótano del Toro. This cave is only 100 m away from the fresh water, non-sulphur resurgence pool at the hotel Taninul. This cave is about 200 m long, and has a 30 m pit. At the bottom of this pit there is a sump. This sump is at about the same level as the resurgence. There were no published records of fish inhabiting this cave before this study.
3.1.2. Results from Taninul #2, Cueva del Rancho Viejo, and Toro Area
Taninul #2 was explored to the final sump, where half a dozen fully eyed and pigmented fish were observed. They were comparable to surface fish found above ground, and were responsive to light. No troglomorphic fish were seen, despite careful observations while snorkelling with an underwater lamp.
We visited Cueva del Rancho Viejo on 6 January 2020. About 10 fully pigmented fish were observed in the pools. A baited trap was left with the aim of attracting troglomorphic fish, since they have enhanced smell [
1,
17]. The trap was then recovered on the 10th of January, 2020. Around 15 individuals were collected, but all had normal eyes and pigment. Fish were released unharmed after careful observation. Despite two days of effort and a baited trap, we found no evidence that troglomorphic or hybrid fish inhabit these caves. This supports the 1974 observation that only epigeomorphic fish inhabit this cave [
11].
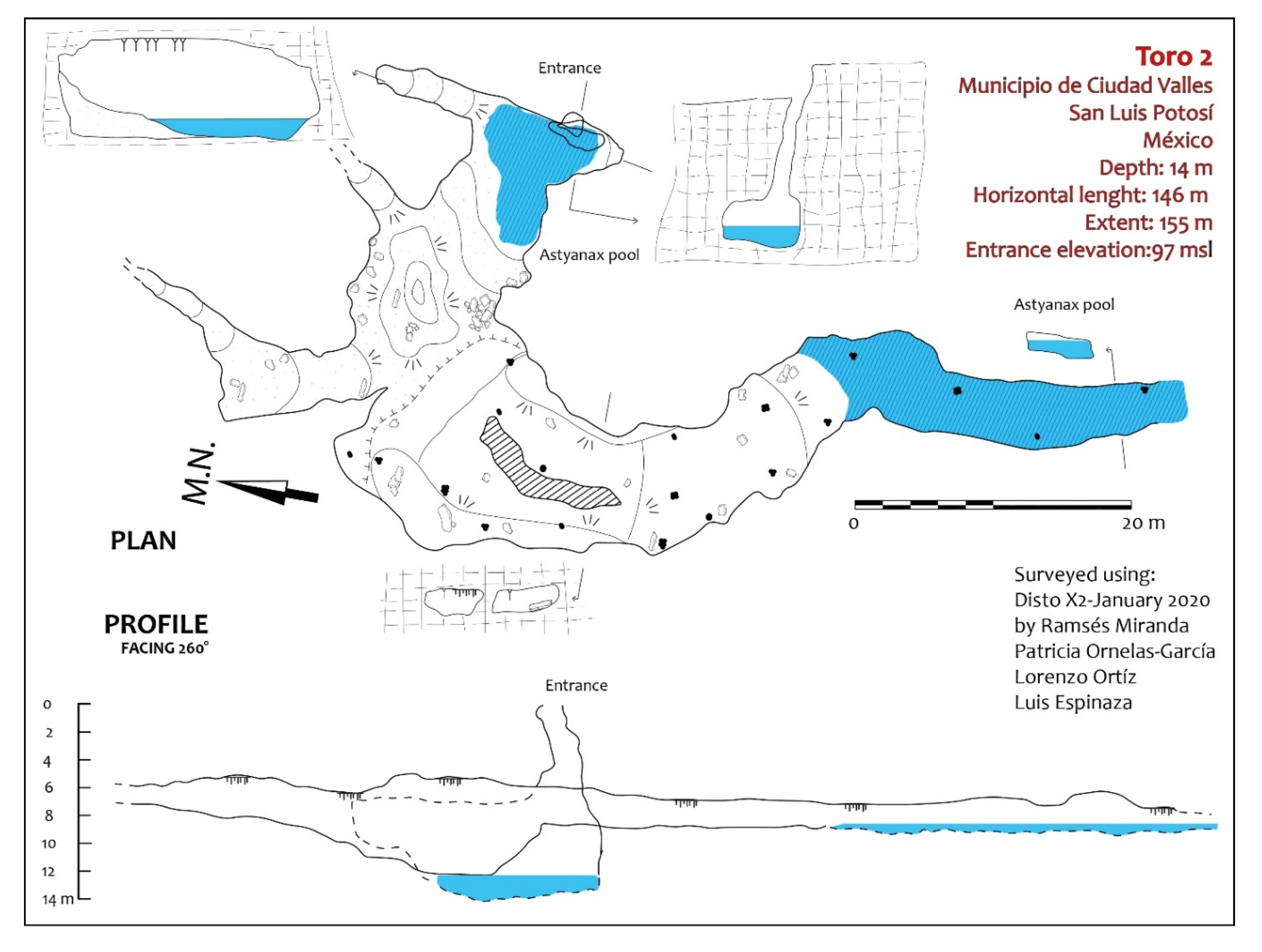
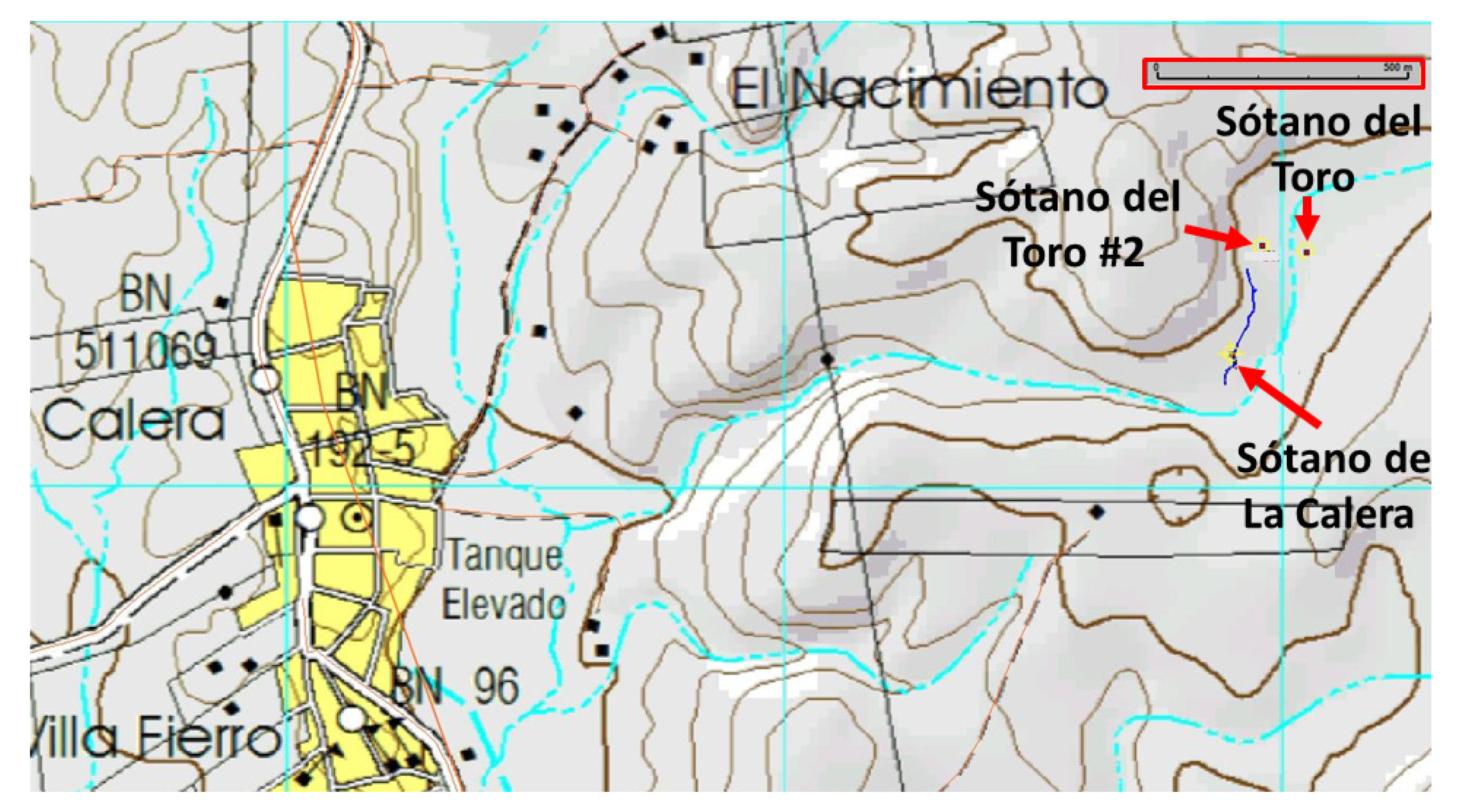
Exploration of the area around Sótano del Toro yielded two new entrances to caves with troglomorphic Astyanax: Sótano del Toro #2 is only 68 m west of Sótano del Toro, and Sótano de La Calera is 239 m south of Sótano del Toro #2.
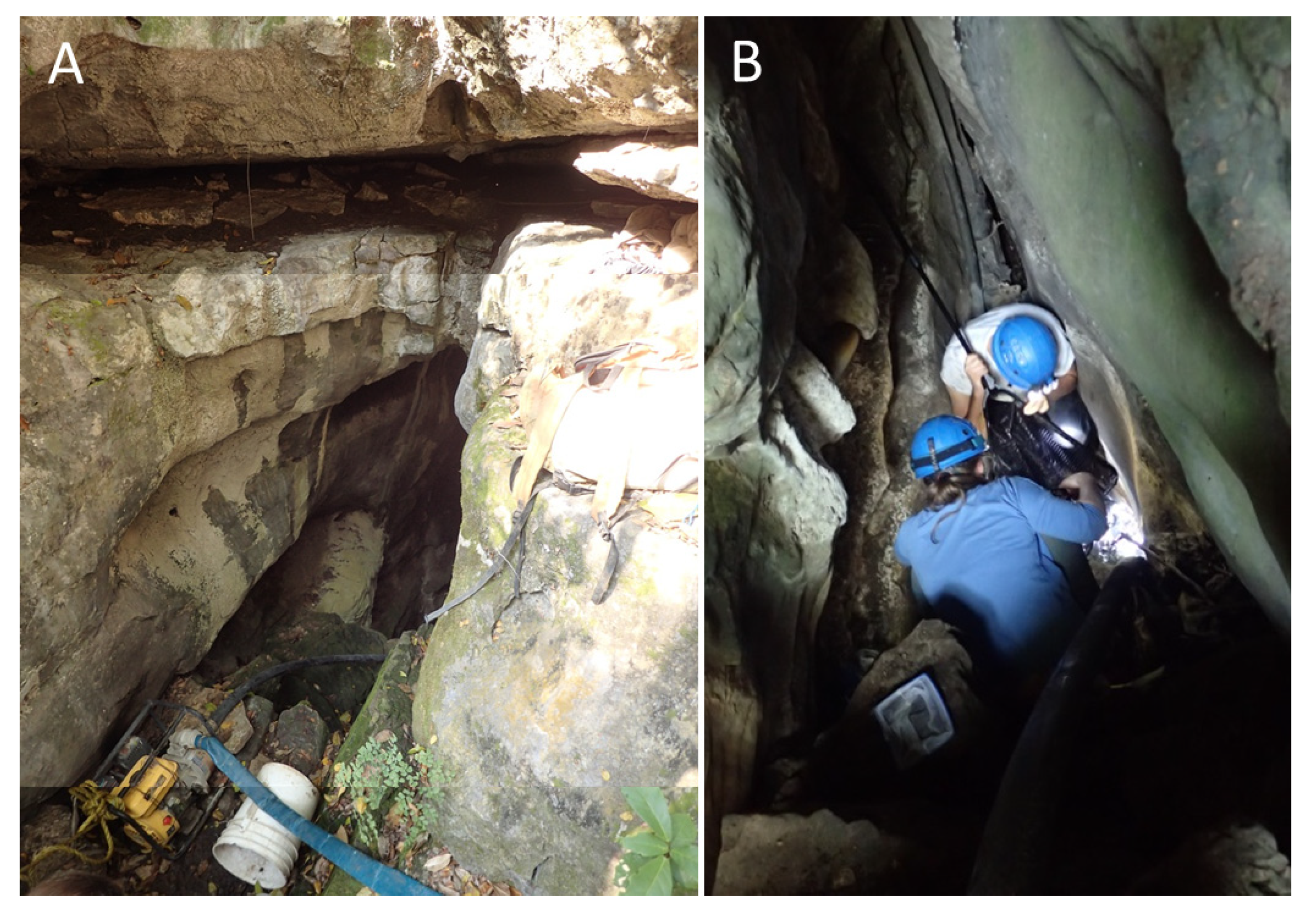
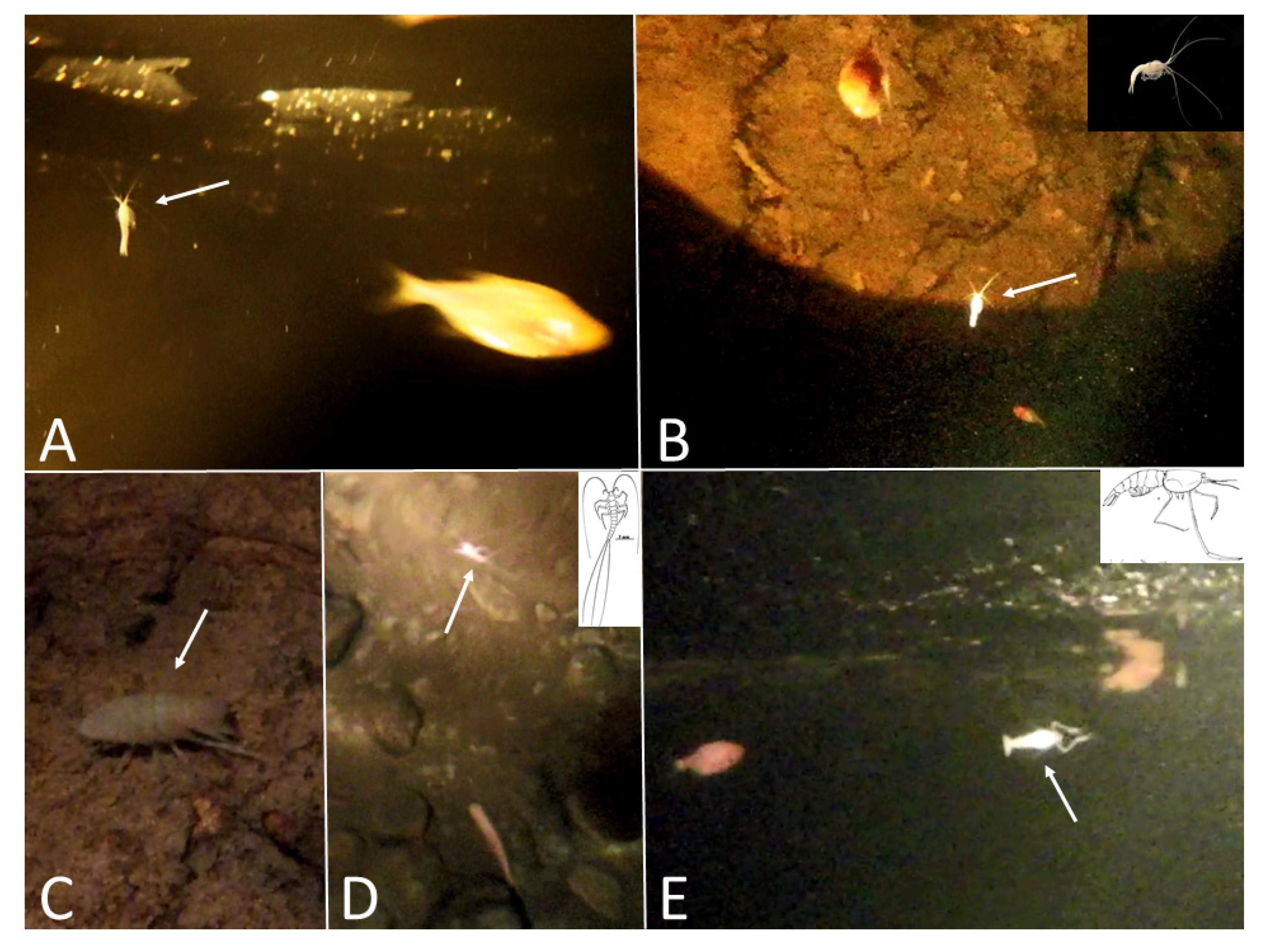
Sótano del Toro #2 consists of a vertical pit of about 12 m. For the first 6 m the pit is very narrow, just 1 m wide. Then it opens into the roof of a large chamber that is 20 m long. In this chamber there is a 10 m diameter pool with fish (
Figure 3 and
Figure 4). This pool is a sump heading in the general direction of Sótano del Toro. From the lake room several short dry galleries bifurcate, one of them being a 30 m crawl that reaches a low ceiling gallery with water, also inhabited by fish (
Figure 5). This water passage heads in a southerly direction for another 20 m until a sump is reached, at which point it appears to continue in a direction that would suggest a possible connection with Sótano de La Calera. It is noteworthy that the walls near the sump are covered with fine mud inhabited by scores of nicoletiid insects,
Anelpistina quinterensis (Paclt, 1979).
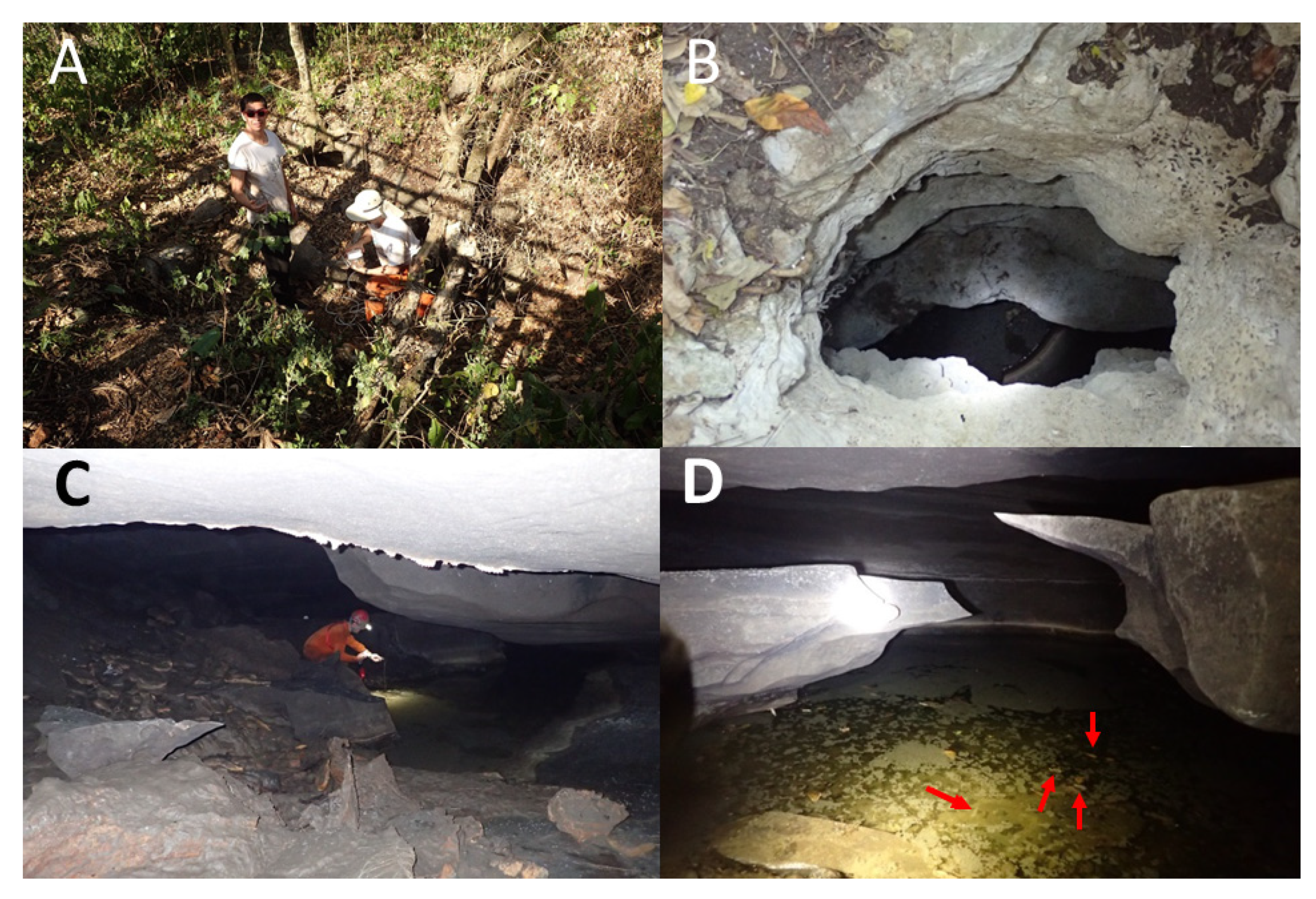
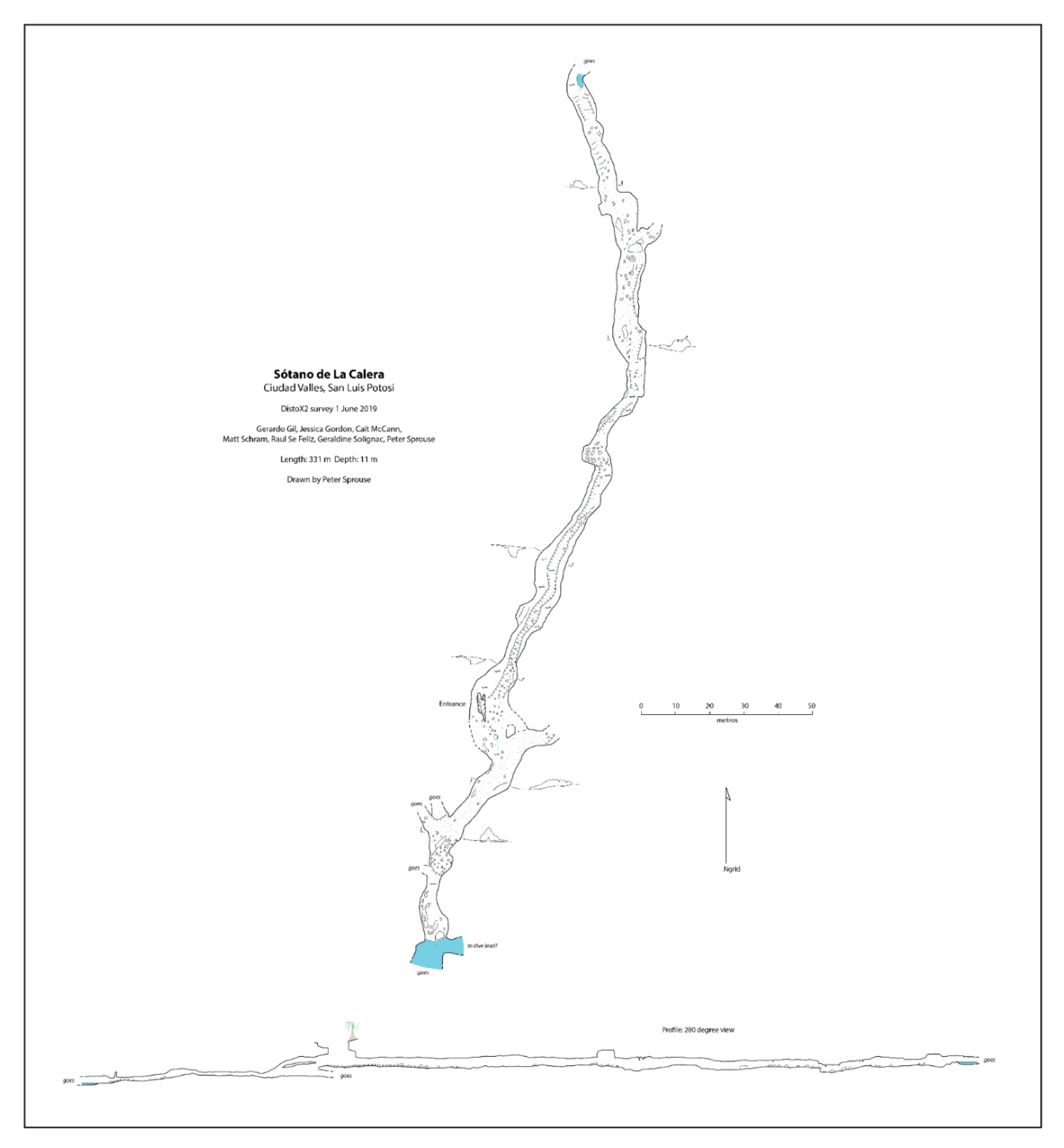
Sótano de La Calera has a 6 m pit entrance (
Figure 6A). During the dry season when the cave was explored, galleries were interspersed with many pools varying from just 0.5 m long to over 10 m, some inhabited by
Astyanax fish. Apart from the
Astyanax and the nicoletiid insects, this cave system is inhabited by the mysid shrimp
Spelaeomysis quinterensis, as well as
Speocirolana isopods. The downstream gallery of Sótano de La Calera heads south for about 77 m via low ceiling crawlways until a sump is reached. This sump has not been explored by divers, and could lead to a significant extension of the system to the south. The upstream gallery has been mapped for 186 m and heads north, in the direction of Sótano del Toro #2 (
Figure 7). Beyond the mapped limit, another 15 m have been explored until a crawlway is reached that is covered with fine mud and inhabited by scores of nicoletiids. There are also several unexplored side passages in Calera.
The entrances to Sótano del Toro #2 and Sótano de La Calera are only 239 m from each other. Considering that Calera extends north for about 200 m, heading directly towards Sótano del Toro #2, and that Sótano del Toro #2 extends south for about 40 m south, it is likely that the muddy sections inhabited by nicoletiids are the opposite sides of a short sump. We suggest that both caves are a single cave system. These two caves also may be connected to Sótano del Toro, only 68 m away. It probably is a single hydrologic system with three entrances (
Figure 8). During the rainy season, fish can probably travel easily from one section to the other.
From the point of view of the
Astyanax biology in the Toro system, our observations differed significantly from previous reports [
11] of a cave inhabited exclusively by troglomorphic fish. Instead, we found that the population is a mixed population (
Figure 9). Some were highly depigmented, with characteristic pinkish-white coloration and no external eyes. Other individuals were identical to surface fish, fully pigmented, with large eyes, and responsive to light. But many individuals had high variability in the eyes, with individuals having phenotypes such as small eye size (
Figure 9), closed pupil, embedded eye (
Figure 6D), and eye mostly absent. Introgression between the surface morph and the cave morph is suggested by the presence of individuals that are highly depigmented, but with eyes (
Figure 9G,H,K) or individuals with pigment, but reduced eyes (
Figure 9J).
None of these three caves has a large bat population. No large streams enter them. While the entrances may provide debris that can serve as food for the fish, large amounts of food are likely only in the pools directly under the entrances, and not throughout the cave system. Troglomorphic, epigeomorphic and presumed hybrids are found throughout the caves. Future studies may resolve if there are significant different ecological pressures in the different pools to promote intraspecific variability, although since during the rainy season fish from all pools are intermixed, we doubt it will have a large impact in the overall population.
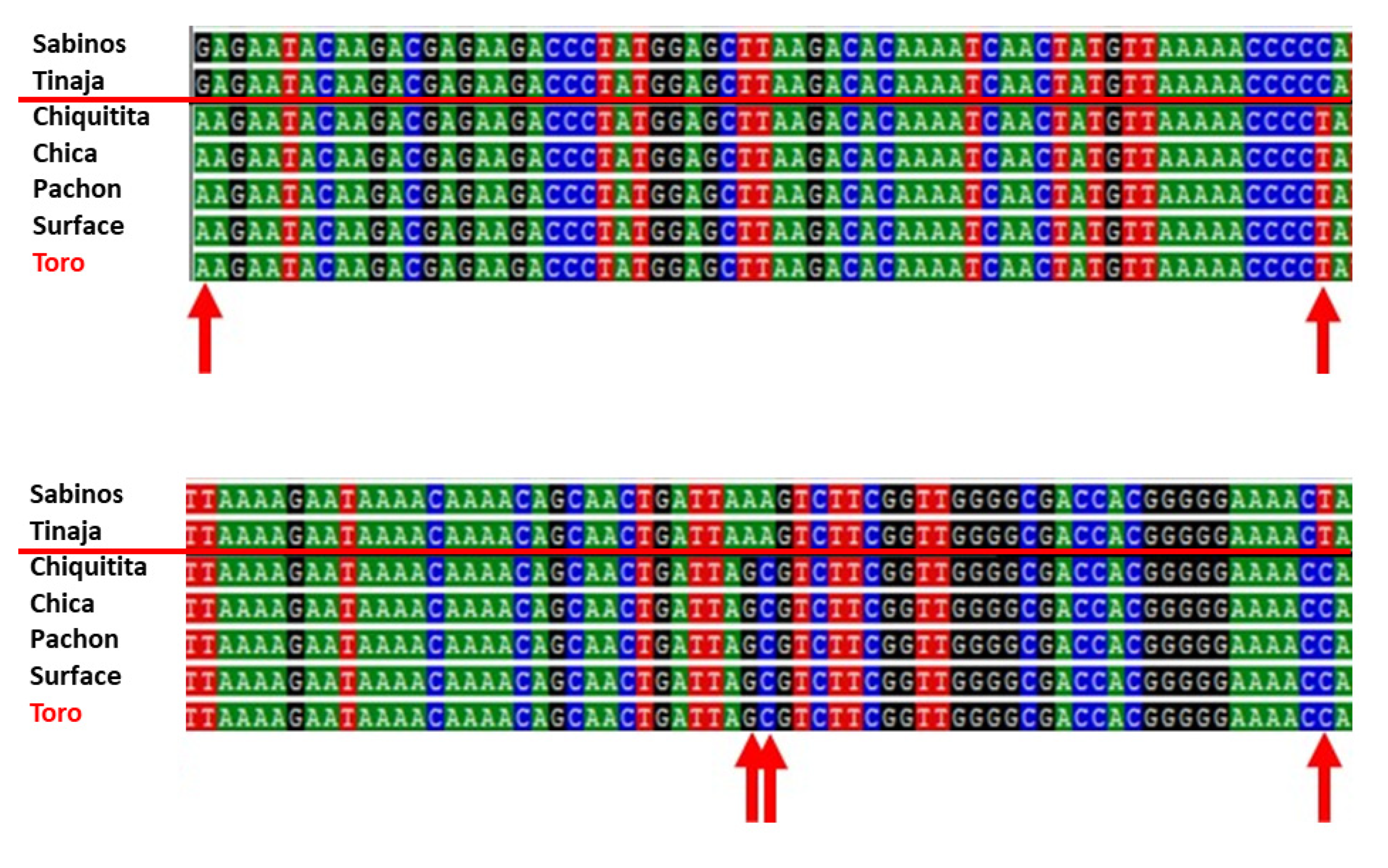
Five troglomorphic fish from the Toro system were genotyped. DNA amplification of mitochondrial 16S rRNA produced a 572 bp sequence. All specimens presented a haplotype A (GenBank# AP011982.1) identical in sequence to fish from Pachón, Chica, and Chiquitita caves and from the local surface
Astyanax (
Figure 10). When compared to the mitochondrial haplotypes B of fish from Rascón and Tamasopo surface streams, Toro specimens differed by 2–3 bp. When compared to the mitochondrial haplotypes B of cavefish from Sabinos, Tinaja, and Curva, they differed by 5 bp (red arrows in
Figure 10). It is thus supported that
Astyanax Toro specimens have a mitochondrial DNA most similar to haplotypes A, with specimens being identical to individuals from other southern El Abra caves like Chica and Chiquitita.
In the mysid shrimp from the Toro system, the H3 fragment was 328 bp long. Two clades or haplotypes (GenBank # MH422492, MH422494) were found, in support of Kopp et al. [
13]. The two mysid shrimp haplotypes differed by 36 bp (10.9%). The first clade A included specimens from Pachón, Toro #2, and Chiquitita. The clade B included Tinaja and Sabinos. Thus, similarity of sequences among populations did not follow geographical proximity between caves. Specimens from the northernmost (Pachón) and the southernmost (Toro #2 and Chiquitita) portions of the Sierra de El Abra were identical. Likewise, the specimens from the central Sierra de El Abra (Sabinos and Tinaja) were identical. It is thus supported that the mysid shrimp from Toro #2 cave has an H3 DNA most closely related to the clade A, with their sequence being identical to individuals from other southern El Abra caves such as Cueva Chiquitita.
3.1.3. Discussion: Southern Sierra de El Abra
Our results revealed some interesting aspects of the cave populations in the southern Sierra de El Abra. Previous reports have described Sótano del Toro as a small cave with a population composed of only troglomorphic Astyanax. Instead, we have found this to be a cave system with over 350 m of passages, three different entrances, and many environmentally variable pools inhabited by a mixed population of troglomorphic, epigeomorphic and presumably hybrid fish, similar to those found in Chica, Cuates and Chiquitita caves. Toro system cavefish also have the mitochondrial A haplotype found in the aforementioned southern Sierra de El Abra cave populations, and in surface fish from the streams that surround Sierra de El Abra. They are distinct from the cavefish populations of the central Sierra de El Abra, and the surface fish from Rascón.
What is the origin of the surface fish in the Toro system? The cave is heading directly south, towards Chica, Cuates, and the resurgence of Chiquitita. The Chiquitita spring has been hypothesized to be the source and entrance point of surface fish to these caves [
8]. The Chiquitita resurgence is about 5.6 km to the south. Another alternative is that the cave system changes its direction and veers to the west to a spring near the town of “Ojo de Agua”, about 1.3 km away. Regardless of the surface fish’s source, the Toro system shows that they have the potential to penetrate deep into the cave systems. These surface fish are swimming upstream starting at a spring. They are not washed in. Furthermore, surface fish were found in galleries in complete darkness late in the dry season, so they must have been there for many months. Their presence in large numbers in a cave far from any surface stream input implies that, to a certain extent, they can survive and reproduce alongside the cave morph. While they may be slimmer than troglomorphic fish, they are somewhat successful in reproducing, as evidenced by the high number of apparent hybrids observed.
It has been argued that surface fish at Cueva Chica have been able to survive and successfully reproduce because of the mild selective conditions due to an ample food supply from the large bat colony in this cave [
6]. The Toro system shows that even without a large input of food, surface fish can still be part of the reproductive community over the span of six months or more. This contrasts drastically with the view that surface fish are to be considered accidentals, destined to die soon after, such as we have personally witnessed in the Río Subterráneo cave in the Micos area. This result is corroborated by the Cuates cave population, also in the southern El Abra, where fish of both morphs cohabitate.
A recent study has estimated that all cavefish populations are probably recent, less than 20,000 years old [
18]. It has been argued that the incongruence of the mitochondrial DNA phylogeny with phylogenies obtained with several independent nuclear loci does not support the existence of two cavefish lineages [
18]. Genomic studies have further shown a much more complex evolutionary history with reticulation, with recent and historical gene flow both within and between cave and surface populations where “a simple bifurcating tree does not fully capture the history of these populations” [
19]. Regardless of whether or not there are cave-adapted fish lineages, results show that caves harboring populations with haplotype B mitochondrial DNA (Sabinos, Tinaja, Piedras and Curva) are restricted to a distinct area in the central Sierra de El Abra [
13]. The Toro system is now recognized as belonging to the southern Sierra de El Abra, which harbors
Astyanax mitochondrial haplotype A. A consistent pattern was corroborated with the stygobitic mysid shrimp, also suggesting that those in the central Sierra de El Abra (Sabinos and Tinaja) harbor a separate haplotype different from the rest of the Sierra de El Abra populations (Pachón, Toro #2, and Cueva Chiquitita).
Phylogeographic results obtained from the mysid Shrimp,
Spelaeomysis quinterensis, are consistent with the results of mitochondrial studies in
Astyanax. This suggests that the geographic distribution of mitochondrial haplotypes in
Astyanax is not stochastic. It has been suggested that northern populations like Pachón, Molino, and Caballo Moro and the southern Sierra de El Abra populations have haplotype A, while the central populations have haplotype B because they represent different colorizations and/or because they have been affected by recent introgression from surface populations [
20]. It has also been suggested that the disparity between nuclear and mitochondrial DNA could be explained by linkage to paternally or maternally inherited adaptive characters of distinct populations [
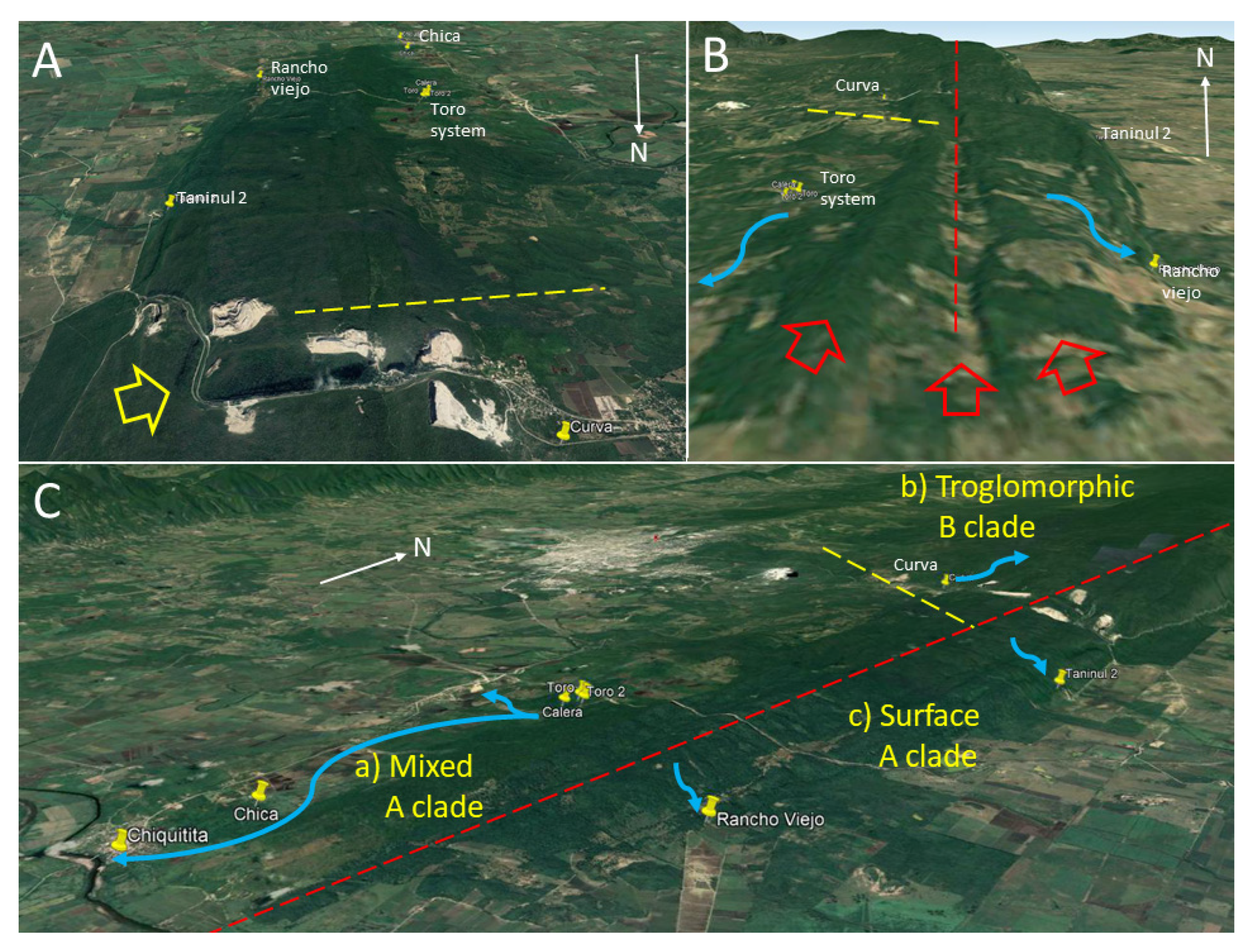
20]. The present data also support that the Sierra de El Abra has distinct biogeographic areas, with partial barriers that affect evolutionary histories and generate evolutionarily significant units across different species of the aquatic cave community. Since the Toro system is the northernmost cave of the southern Sierra de El Abra group and Curva cave is the southern-most cave of the central group, it is now recognized that the border between these two groups is probably between 21°58′ N and 21°54′ N.
We propose a model to explain the biogeography of the Sierra de El Abra, in which its low-lying southern portion is like a sponge or sieve where conduits have few barriers for surface fish entering at resurgences along the Río Tampaón (28.5 m above sea level masl) or into the Río Valles (48 masl). Chiquitita’s bottom is at 27 masl., Chica at 30, Cuates at 37, and Toro at 88 [
11]. All these caves have mixed populations with epigeomorphic fish and troglomorphic fish with mitochondrial A haplotypes. In the central Sierra de El Abra caves, populations are highly troglomorphic, with little incidence of hybridization, and harboring mitochondrial B haplotypes. Kopp et al. [
13] have proposed a biogeographic divide that isolates both
Astyanax cavefish and mysid cave shrimps in the central Sierra de El Abra caves from those in the southern Sierra de El Abra. The southernmost cave population of the central Sierra de El Abra group is Curva, whose bottom is at 113 masl. The central and southern portions of the Sierra de El Abra are separated by distance and altitude barriers, plus there is the remnant of a relict canyon where the Río Tampaon/Valles crossed farther north before plate tectonics elevated the southern portion [
10]. This relict canyon is used by the highway that connects Ciudad Valles with Tamuin (
Figure 11A). It has been estimated that the Río Tampaon/Valles changed its northern course to its current locality about 2.78–0.69 mya [
10]. The authors argued that the southern portion of El Abra was elevated, and cave erosion could not start until after that time [
10]. Caves north of this southern, low-lying sponge may effectively be isolated from surface fish immigrants, with the exception of Sótano de Yerbaniz and Sótano de Matapalma, where surface fish are flushed into entrance pits [
11].
A restriction to this low-lying sponge model of easy fish dispersal lies to the east. Both Cueva del Rancho Viejo and Taninul # 2 have a bottom at about 50 masl. Neither of them has evidence of cavefish. Only surface fish that derived from the nearby local surface streams inhabit them. Barriers on the east flank of the Sierra de El Abra for
Astyanax cavefish dispersal seem to be prevalent throughout the range. None of the caves or resurgences on the eastern side have troglomorphic
Astyanax. Along the southern Sierra de El Abra ridge there are two crests divided by a valley. This is probably linked to a synclinal and two anticlinal folds that comprise the southern El Abra limestone ridge (
Figure 11B). We suggest that these ridges and valley may create a divide for cave development that effectively subdivides the hydrologic systems: A western one that drains toward the Río Valles at the town of Ojo de Agua and/or to Río Tampaon at Chiquitita resurgence, and an eastern one that drains to Cueva del Rancho Viejo, Nacimiento del Río Choy, and/or other nacimientos on the eastern escarpment.
In conclusion, we suggest that the combination of tectonics, altitude difference, ancient canyons, ridges, and valleys has affected the karst development of caves in the central and southern Sierra de El Abra. There are barriers for cave-to-cave and surface-to-cave migration and gene flow. Such barriers have affected whole stygobitic communities, as evidenced by the congruence of phyletic markers in both cavefish and mysid shrimp. For
Astyanax, this has resulted in a group of caves in the central Sierra de El Abra with few instances of hybridization with surface fish and troglomorphic fish harboring mitochondrial haplotype B. In the southwest, there is a population of mixed troglomorphic, epigeomorphic and assumed hybrids which harbor haplotype A, and to the east there are caves with strictly epigeomorphic fish which also harbor haplotype A (
Figure 11C). Finally, the central Sierra de El Abra is isolated from the northernmost Pachón cave which harbors the same A mitochondrial haplotype as the southernmost caves studied here. Mitchell et al. [
6] proposed a drainage divide, in which the northern Sierra de El Abra drained to Nacimiento del Río Mante and the central and southern portions drained to Nacimiento del Río Choy. The exact location of this barrier is close to Sótano del Venadito, which as of today remains to be genotyped.