Temporal Evolution of Diatoms in a Temporary Pond Situated in the Massif du Sancy Mountains (Massif Central, France) and Description of a New Pinnularia Species
Abstract
1. Introduction
2. Materials and Methods
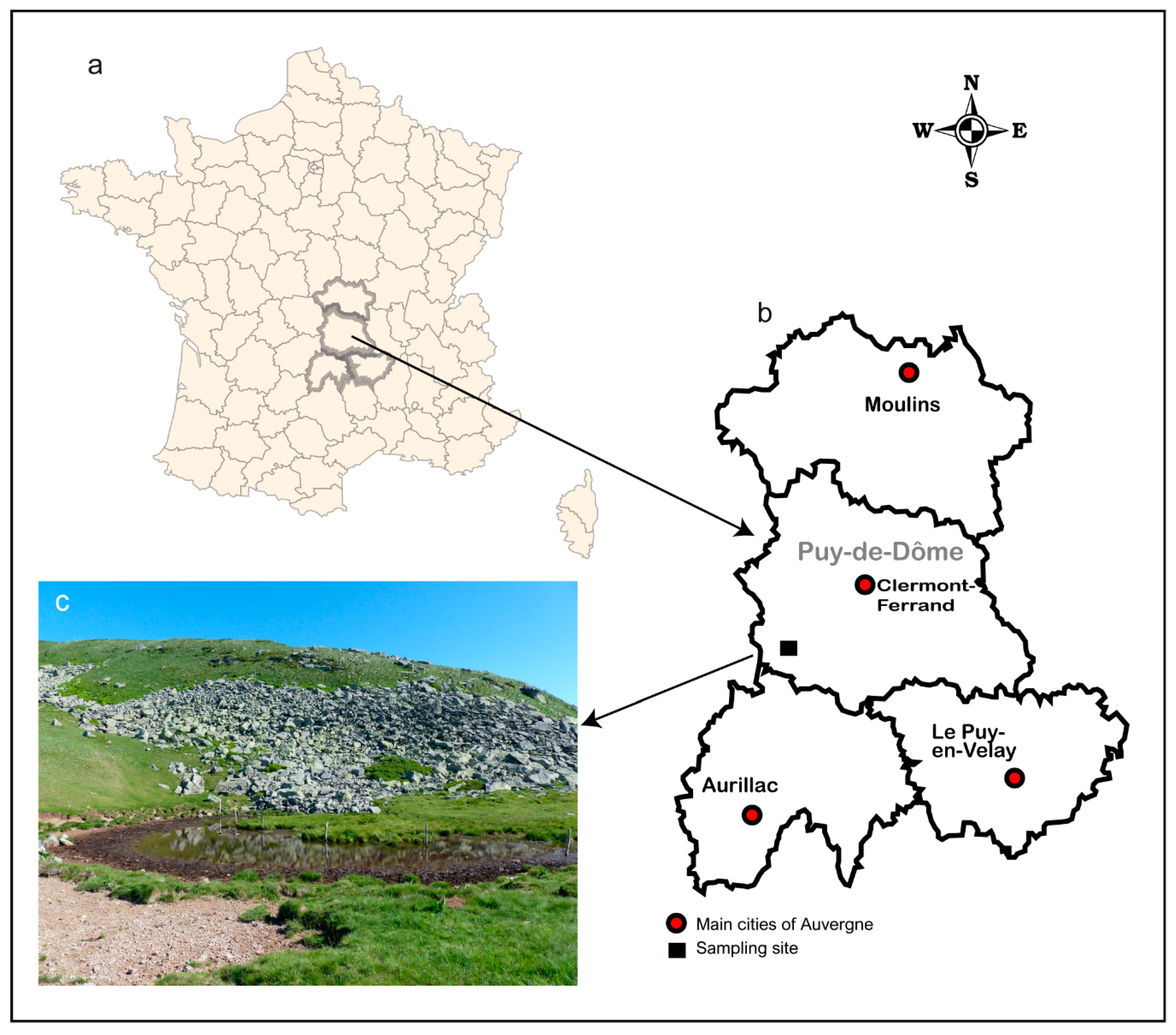
2.1. Study Site
2.2. Diatom Sampling and Physical and Chemical Analysis
2.3. Slide Preparation and Microscopy
2.4. Data Analysis
2.4.1. Statistical Analyses on the Physical and Chemical Variables
2.4.2. Diatoms Data Statistical Analyses
3. Results
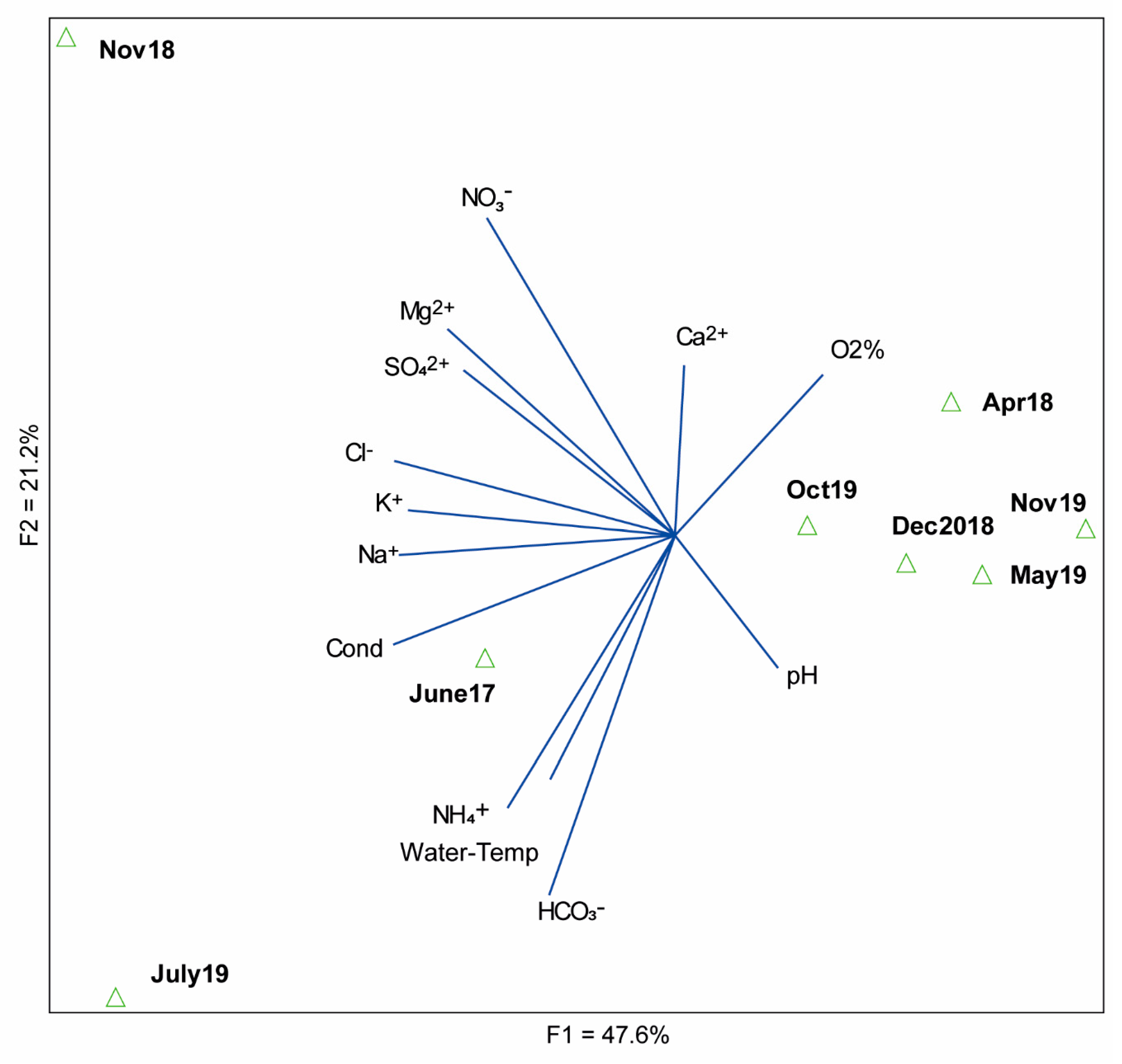
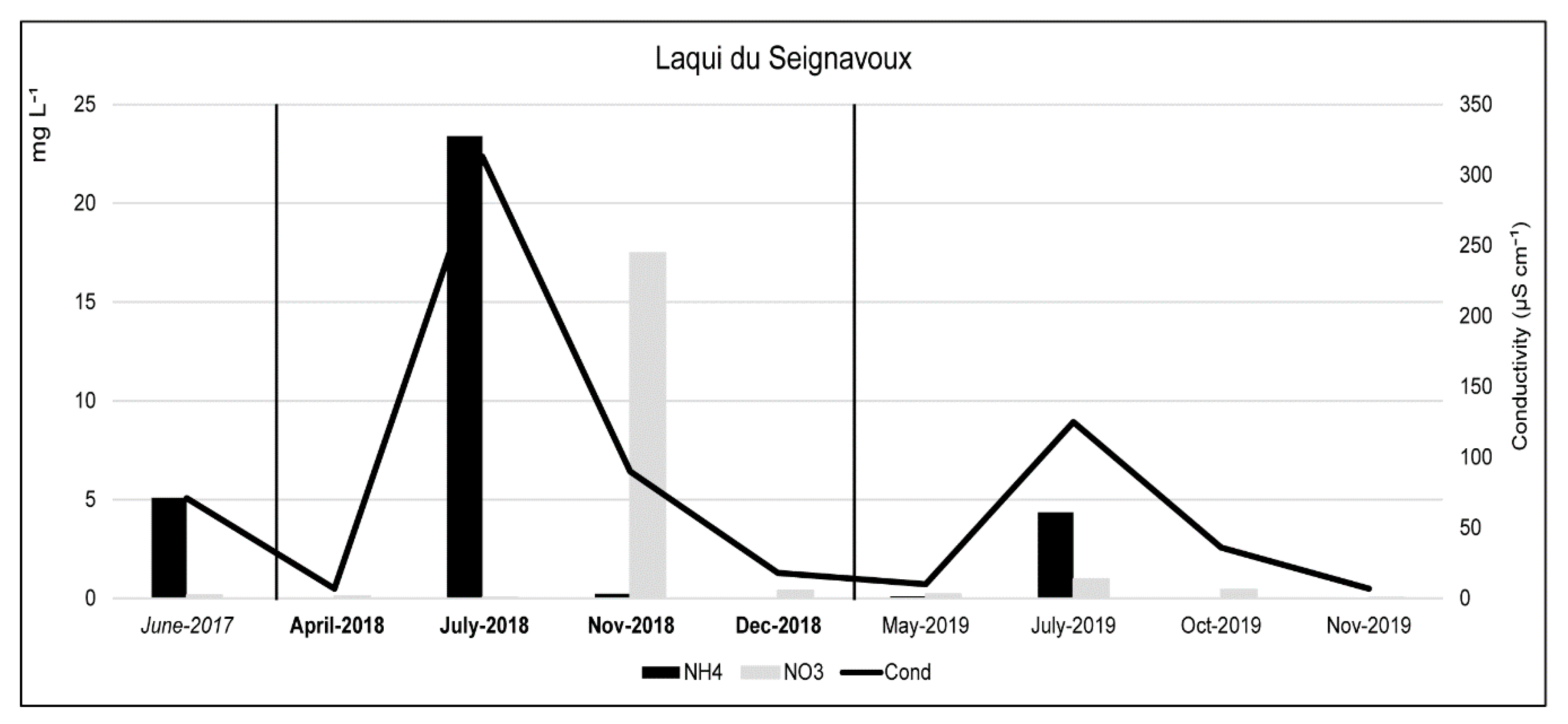
3.1. Physical and Chemical Characteristics
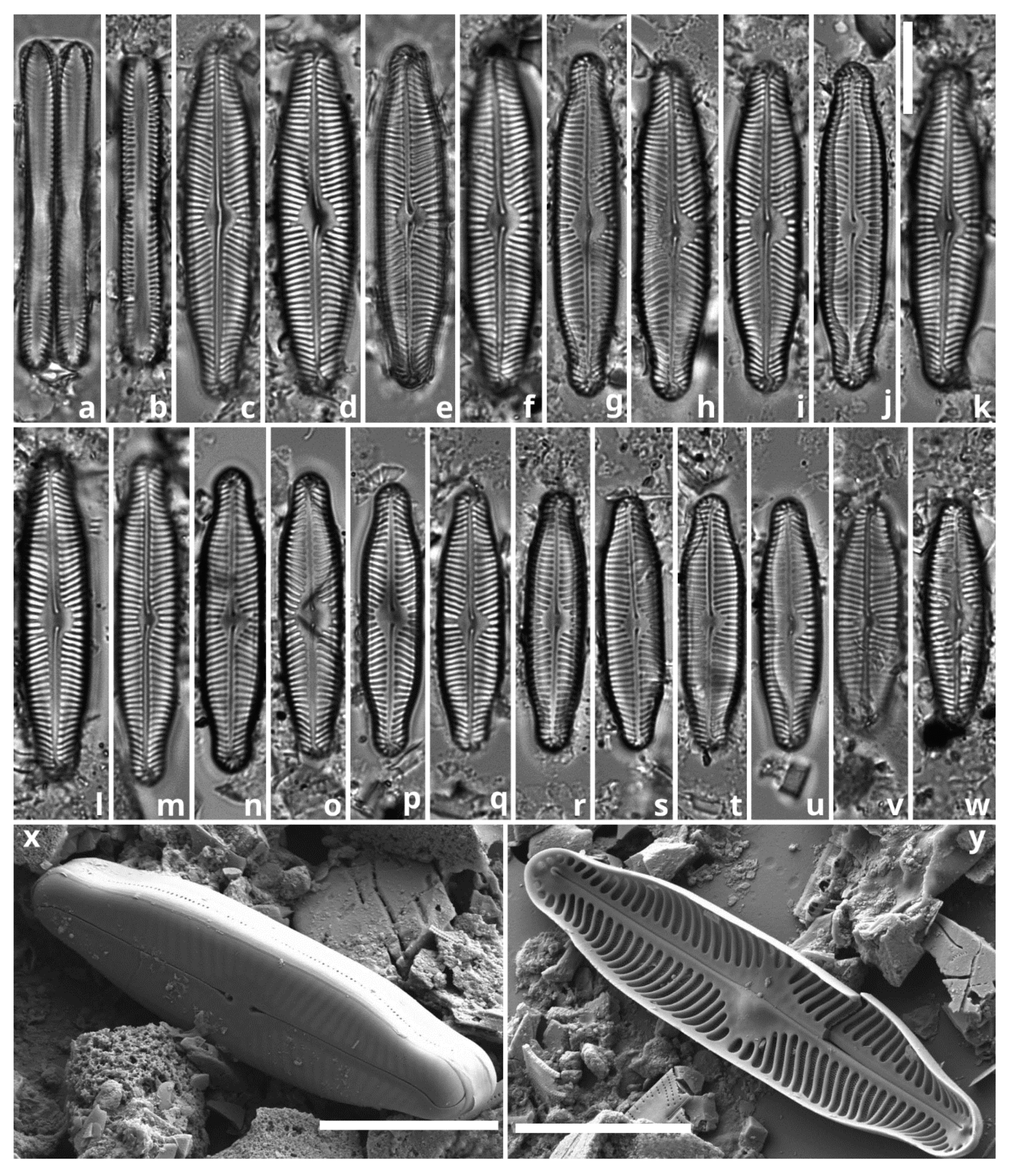
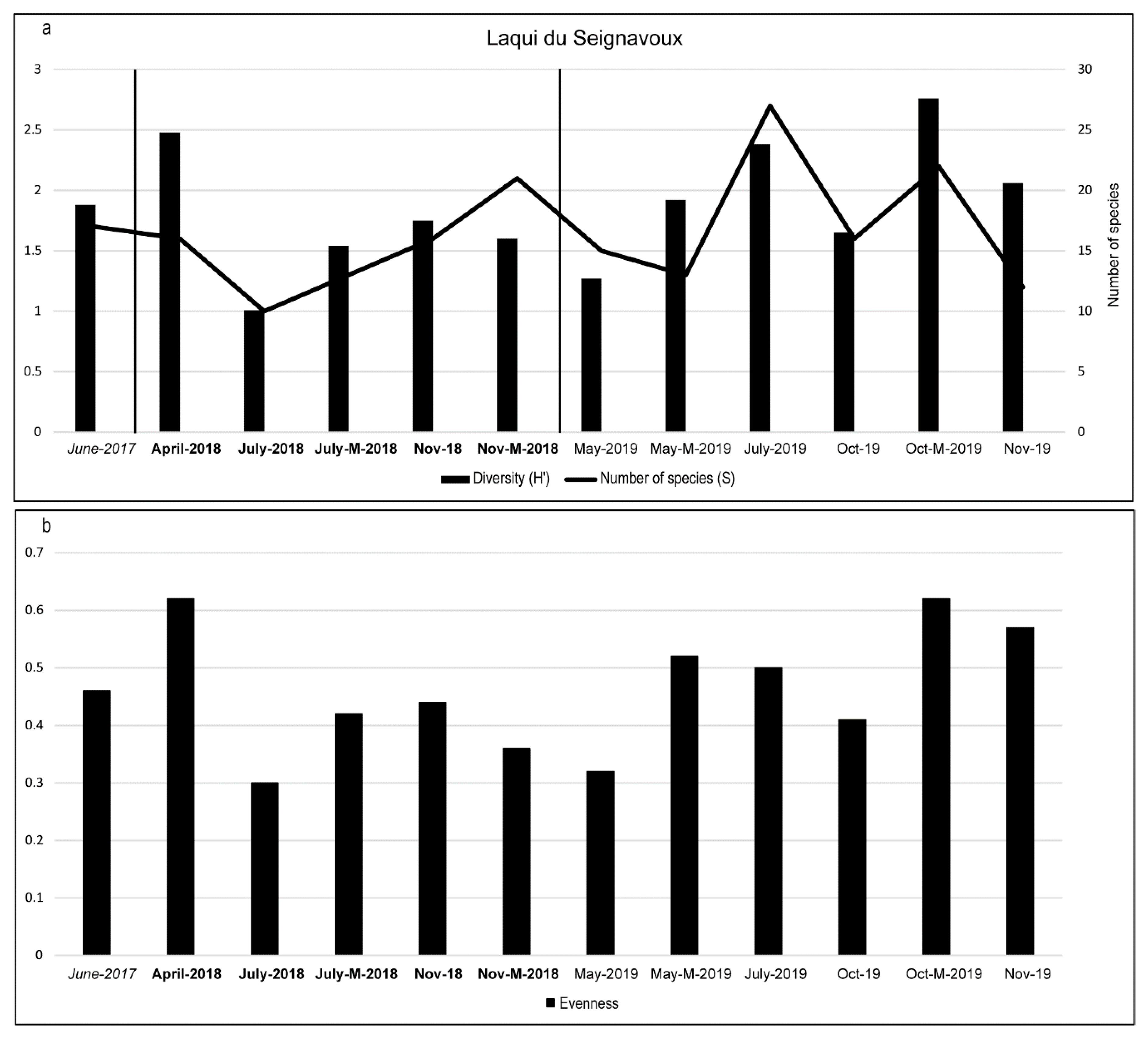
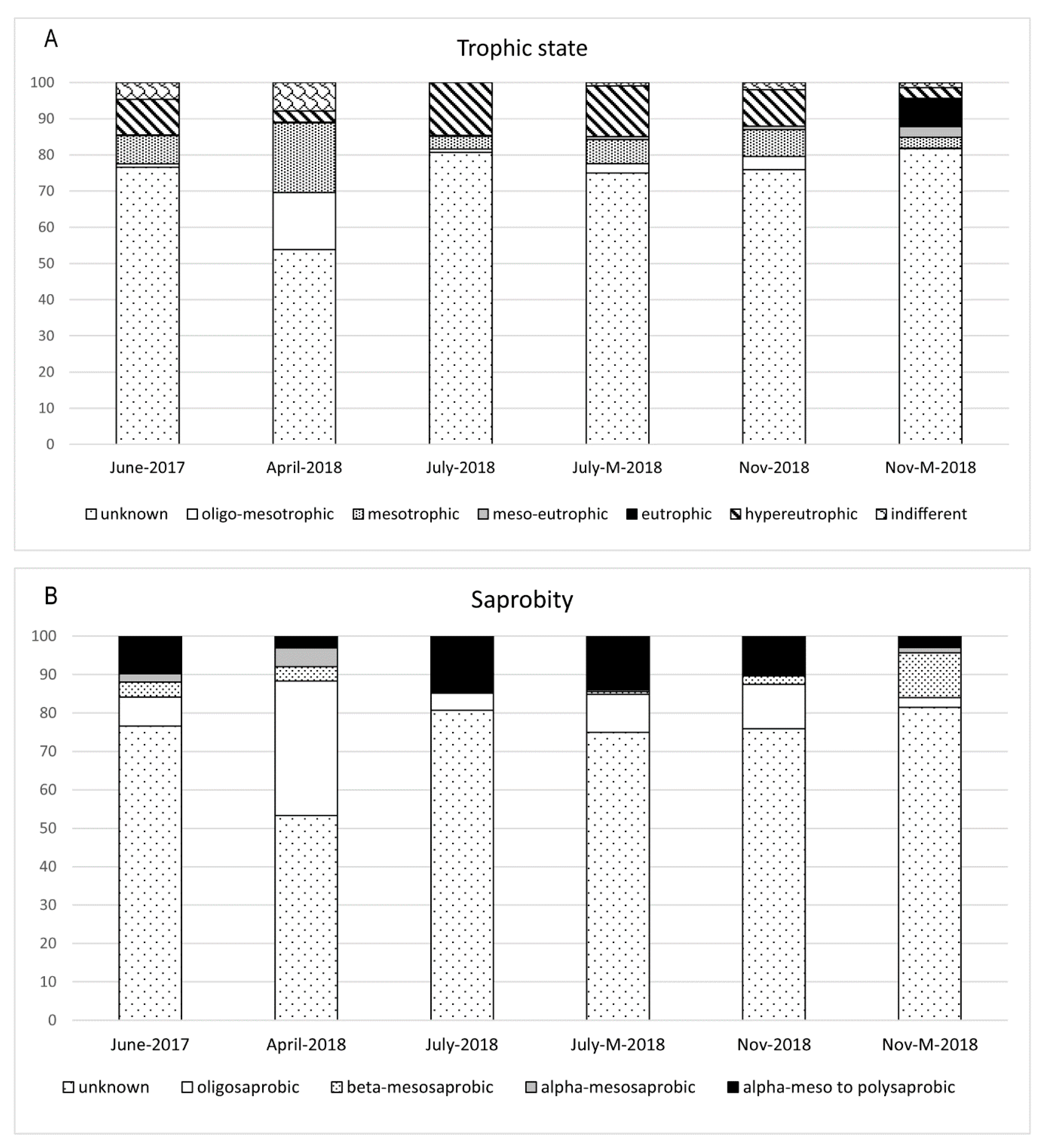
3.2. Diatom Composition and Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takaoka, S. Origin and geographical characteristics of ponds in a high mountain region of central Japan. Limnology 2015, 16, 103–112. [Google Scholar] [CrossRef]
- Biggs, J.; Williams, P.; Whitfield, M.; Nicolet, P.; Weatherby, A. 15 years of pond assessment in Britain: Results and lessons learned from the work of Pond Conservation. Aquat. Conserv. 2005, 15, 693–714. [Google Scholar] [CrossRef]
- Hinden, H.; Oertli, B.; Menetrey, N.; Sager, L.; Lachavanne, J.-B. Alpine pond biodiversity: What are the related environmental variables? Aquat. Conserv. 2005, 15, 613–624. [Google Scholar] [CrossRef]
- Füreder, L.; Ettinger, R.; Boggero, A.; Thaler, B.; Thies, H. Macroinvertebrate diversity in Alpine lakes: Effects of altitude and catchment properties. Hydrobiologia 2006, 562, 123–144. [Google Scholar] [CrossRef]
- Robinson, C.T.; Oertli, B. Long-term biomonitoring of alpine waters in the Swiss National Park. Eco. Mont 2009, 1, 23–34. [Google Scholar] [CrossRef]
- Martínez-Sanz, C.; Cenzano, C.S.S.; Fernández-Aláez, M.; García-Criado, F. Relative contribution of small mountain ponds to regional richness of littoral macroinvertebrates and the implications for conservation. Aquat. Conserv. 2012, 22, 155–164. [Google Scholar] [CrossRef]
- Ilg, C.; Oertli, B. How can we conserve cold stenotherm communities in warming Alpine ponds? Hydrobiologia 2014, 723, 53–62. [Google Scholar] [CrossRef]
- Pérez-Bilbao, A.; Benetti, C.J.; Garrido, J. Assessment of the effects of the dry period on the faunal composition of aquatic macroinvertebrate assemblages in two temporary ponds in NW Spain. J. Limnol. 2015, 74, 467–476. [Google Scholar] [CrossRef]
- Kopáček, J.; Stuchlík, E.; Vyhnálek, V.; Závodský, D. Concentration of nutrients in selected lakes in the High Tatra Mountains, Slovakia: Effect of season and watershed. Hydrobiologia 1996, 319, 47–55. [Google Scholar] [CrossRef]
- Zaharescu, D.G.; Hooda, P.S.; Burghelea, C.I.; Palanca-Soler, A. A multiscale framework for deconstructing the ecosystem physical template of high-altitude lakes. Ecosystems 2016, 19, 1064–1079. [Google Scholar] [CrossRef]
- Sabater, S.; Roca, J.R. Some factors affecting distribution of diatom assemblages in Pyrenean springs. Freshw. Biol. 1990, 24, 493–507. [Google Scholar] [CrossRef]
- Kilroy, C.; Biggs, B.J.F.; Vyverman, W.; Broady, P.A. Benthic diatom communities in subalpine pools in New Zealand: Relationships to environmental variables. Hydrobiologia 2006, 561, 95–110. [Google Scholar] [CrossRef]
- Kihara, Y.; Sahashi, Y.; Ohtsuka, T. Diatoms of Kojorougaike Pond in the Hira Mountain Range, west-central Japan. Diatom 2008, 24, 73–79. (In Japanese) [Google Scholar] [CrossRef]
- Ognjanova-Rumenova, N.; Botev, I.; Kernan, M. Benthic diatom flora in relation to chemical and physical factors in high mountain lakes in the Rila Mountains (southwestern Bulgaria). Adv. Limnol. 2009, 62, 153–166. [Google Scholar] [CrossRef]
- Cantonati, M.; Lange-Bertalot, H.; Decet, F.; Gabrieli, J. Diatoms in very-shallow pools of the site of community importance Danta di Cadore Mires (south-eastern Alps), and the potential contribution of these habitats to diatom biodiversity conservation. Nova Hedwig. 2011, 93, 475–507. [Google Scholar] [CrossRef]
- Şahin, B.; Akar, B.; Barinova, S. Algal flora and ecology of the high mountain lakes in the Artabel Lakes Nature Park (Gümüşhane, Turkey), I-Bacillariophyta. Int. J. Adv. Res. Bot. 2019, 5, 25–37. [Google Scholar] [CrossRef]
- Blanco, S.; Olenici, A.; Ortega, F.; Jiménez-Gómez, F.; Guerrero, F. Identifying environmental drivers of benthic diatom diversity: The case of Mediterranean mountain ponds. PeerJ 2020, 8, e8825. [Google Scholar] [CrossRef]
- Nomade, S.; Pastre, J.-F.; Nehlig, P.; Guillou, H.; Scao, V.; Scaillet, S. Tephrochronology of the Mont-Dore volcanic Massif (Massif Central, France): New 40Ar/39Ar constraints on the Late Pliocene and Early Pleistocene activity. Bull. Volcanol. 2014, 76, 798. [Google Scholar] [CrossRef]
- Gourgaud, A.; Maury, R.C. Magma mixing in alkaline series: An example from Sancy volcano (Mont-Dore, Massif Central, France). Bull. Volcanol. 1984, 47, 827–847. [Google Scholar] [CrossRef]
- Prygiel, J.; Coste, M. Guide Méthodologique Pour la Mise en Œuvre de L’indice Biologique Diatomées NF T 90-354; Etude Agences de l’Eau-Cemagref Bordeaux, Agences de l’Eau: Douai, France, 2000; p. 134. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süsswasserflora von Mitteleuropa, 2nd ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1997; Volume 2, p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa, 2nd ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1997; Volume 2, p. 611. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiae. In Süsswasserflora von Mitteleuropa, 2nd ed.; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 2000; Volume 2, p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 5. Teil: English and French translation of the keys. In Süsswasserflora von Mitteleuropa, 2nd ed.; Büdel, B., Gärtner, G., Krienitz, L., Lokhorst, G.M., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2000; Volume 2, p. 599. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae, kritische Ergänzungen zu Navicula (Lineoatae) und Gomphonema. In Süsswasserflora von Mitteleuropa, 2nd ed.; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2004; Volume 2, p. 468. [Google Scholar]
- Krammer, K. The genus Pinnularia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2000; Volume 1, p. 703. [Google Scholar]
- Krammer, K. Cymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2002; Volume 3, p. 584. [Google Scholar]
- Krammer, K. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2003; Volume 4, p. 530. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto, 10 Genera separated from Navicula sensu lato Frustulia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2001; Volume 2, p. 526. [Google Scholar]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Witkowski, A.; Dorofeyuk, N.I.; Genkal, S.I. Diatom assemblages from Sphagnum bogs of the World. Part I: Nur bog in northern Mongolia. Biblioth. Diatomol. 2010, 55, 1–326. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser-Benthos von Mitteleuropa. Bestimmungsflora Kieselalgen für die Ökologische Praxis. Über 700 der Häufigsten Arten und ihre Ökologie; A.R.G. Gantner Verlag Kommanditgesellschaft: Rugell, Liechtenstein; Koeltz Scientific Books: Königstein, Germany, 2011; p. 908. [Google Scholar]
- Lange-Bertalot, H.; Bąk, M.; Witkowski, A.; Tagliaventi, N. Eunotia and some related genera. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2011; Volume 6, p. 747. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; English edition with updated taxonomy and added species; Cantonati, M., Kelly, M.G., Lange-Bertalot, H., Eds.; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; p. 842. [Google Scholar]
- Wetzel, C.E.; Ector, L.; Van de Vijver, B.; Compère, P.; Mann, D.G. Morphology, typification and critical analysis of some ecologically important small naviculoid species (Bacillariophyta). Fottea 2015, 15, 203–234. [Google Scholar] [CrossRef]
- Levkov, Z.; Mitic-Kopanja, D.; Reichardt, E. The diatom genus Gomphonema from the Republic of Macedonia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2016; Volume 8, p. 552. [Google Scholar]
- Hill, M.O.; Gauch, H.G., Jr. Detrended correspondence analysis: An improved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Prentice, I.C. A theory of gradient analysis. Adv. Ecol. Res. 1988, 18, 271–317. [Google Scholar] [CrossRef]
- Birks, H.J.B. Quantitative palaeoenvironmental reconstructions. In Statistical Modelling of Quaternary Science Data; Maddy, D., Brew, J.J., Eds.; Quaternary Research Association: Cambridge, UK, 1995; pp. 161–254. [Google Scholar]
- van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Lecointe, C.; Coste, M.; Prygiel, J. “Omnidia”: Software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia 1993, 269, 509–513. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD. In Multivariate Analysis of Ecological Data. Version 7; MjM Software Design: Gleneden Beach, OR, USA, 2016. [Google Scholar]
- Beauger, A. Contribution of diatoms to monitoring the water quality of the RNN Chastreix-Sancy watershed from the spring areas. In Apport des Diatomées au Suivi de la Qualité de L’eau du bassin versant de la RNN Chastreix-Sancy à Partir des Zones de Sources; UMR GEOLAB: Clermont-Ferrand, France, 2018; p. 67. [Google Scholar]
- Souffreau, C.; Vanormelingen, P.; Verleyen, E.; Sabbe, K.; Vyverman, W. Tolerance of benthic diatoms from temperate aquatic and terrestrial habitats to experimental desiccation and temperature stress. Phycologia 2010, 49, 309–324. [Google Scholar] [CrossRef]
- van de Vijver, B.; Frenot, Y.; Beyens, L. Freshwater diatoms from Île de la Possession Crozet Archipelago, sub-Antarctica. Biblioth. Diatomol. 2002, 46, 1–412. [Google Scholar]
- Nováková, S. Algal flora of subalpine peat bog pools in the Krkonoše Mts. Preslia 2002, 74, 45–56. [Google Scholar]
- Rodier, J.; Legube, B.; Merlet, N.; Brunet, R. L’Analyse de L’eau, 9th ed.; Dunod: Paris, France, 2009; p. 1579. [Google Scholar]
- Beauger, A.; Wetzel, C.E.; Peiry, J.-P.; Voldoire, O.; Ector, L. Craticula widouensis, a new diatom (Bacillariophyta) species of a Sahelian temporary pond (North Senegal). Bot. Lett. 2019, 166, 254–267. [Google Scholar] [CrossRef]
- Brezonik, P.L.; Lee, G.F. Denitrification as a nitrogen sink in lake Mendota, Wisconsin. Environ. Sci. Technol. 1968, 2, 120–125. [Google Scholar] [CrossRef]
- Pavlov, A.; Levkov, Z. Diversity and distribution of taxa in the genus Eunotia Ehrenberg (Bacillariophyta) in Macedonia. Phytotaxa 2013, 86, 1–117. [Google Scholar] [CrossRef]
- Salomoni, S.E.; Rocha, O.; Callegaro, V.L.; Lobo, E.A. Epilithic diatoms as indicators of water quality in the Gravataí river, Rio Grande do Sul, Brazil. Hydrobiologia 2006, 559, 233–246. [Google Scholar] [CrossRef]
- Bere, T.; Tundisi, J.G. Influence of land-use patterns on benthic diatom communities and water quality in the tropical Monjolinho hydrological basin, São Carlos-SP, Brazil. Water SA 2011, 37, 93–102. [Google Scholar] [CrossRef]
- Bere, T.; Tundisi, J.G. The effects of substrate type on diatom-based multivariate water quality assessment. Water Air Soil Pollut. 2011, 216, 391–409. [Google Scholar] [CrossRef]
- Hecky, R.E.; Kilham, P. Nutrients limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 1988, 33, 796–822. [Google Scholar] [CrossRef]
- Masithah, E.D.; Nindarwi, D.D.; Rahma, T.; Satrya, R.R. Dynamic ratio correlation of N:P in relation to the diatom abundance in the intensive system of the Vannamei (Litopenaeus vannamei) shrimp pond. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012017. [Google Scholar] [CrossRef]
- Taylor, J.C.; Harding, W.R.; Archibald, C.G.M. An Illustrated Guide to Some Common Diatom Species from South Africa; WRC Report TT 282/07, pls 178; Water Research Commission: Pretoria, South Africa, 2007. [Google Scholar]
- Mora, D.; Carmona, J.; Jahn, R.; Zimmermann, J.; Abarca, N. Epilithic diatom communities of selected streams from the Lerma-Chapala Basin, Central Mexico, with the description of two new species. PhytoKeys 2017, 88, 39–69. [Google Scholar] [CrossRef]



| Sampling Dates | Diatom Sample | Water Sample | Substrate Collected |
|---|---|---|---|
| 06/19/2017 | Yes | Yes | Mud |
| 04/21/2018 | Yes | Yes | Mud |
| 07/12/2018 | Yes | Yes | Mud and macrophytes |
| 11/08/2018 | Yes | Yes | Mud and macrophytes |
| 12/12/2018 | No | Yes | Mud |
| 05/16/2019 | Yes | Yes | Mud and macrophytes |
| 07/10/2019 | Yes | Yes | Mud |
| 10/07/2019 | Yes | Yes | Mud and macrophytes |
| 11/29/2019 | Yes | Yes | Mud |
| Date | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | Cl− | NO3− | PO43- | SO42− | HCO3− | Cond | pH | O2% | Water-T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 06/19/2017 | 1.58 | 5.09 | 2.08 | 1.10 | 2.81 | 5.79 | 0.16 | <0.04 | 3.93 | 20.0 | 71 | 6.25 | 91 | 20.4 |
| 04/21/2018 | 0.50 | 0.02 | 0.39 | 0.31 | 4.22 | 2.69 | 0.12 | <0.04 | 0.92 | 8.3 | 7 | 6.03 | 65.4 | 1.4 |
| 07/12/2018 | 9.04 | 23.40 | 57.41 | 1.72 | 9.03 | 50.88 | 0.05 | <0.04 | 10.48 | 108.0 | 313 | 7.04 | 3.8 | 23.2 |
| 11/08/2018 | 5.24 | 0.23 | 21.55 | 1.50 | 2.92 | 19.23 | 17.47 | <0.04 | 5.16 | 3.2 | 90 | 6.34 | 69 | 4.8 |
| 12/12/2018 | 1.44 | 0.01 | 2.45 | 0.34 | 4.44 | 1.88 | 0.40 | 0.05 | 1.11 | 20.0 | 18 | 7.7 | 54.9 | 0.7 |
| 05/16/2019 | 0.68 | 0.11 | 1.62 | 0.24 | 1.01 | 0.92 | 0.22 | <0.04 | 0.39 | 7.5 | 10 | 6.7 | 93.1 | 13.6 |
| 07/10/2019 | 5.69 | 4.36 | 20.63 | 0.50 | 1.00 | 15.03 | 0.99 | <0.04 | 1.94 | 35.6 | 125 | 7.04 | 25.4 | 16.9 |
| 10/07/2019 | 1.48 | 0.01 | 5.03 | 0.44 | 2.32 | 2.51 | 0.43 | 0.06 | 3.66 | 13.2 | 36 | 7.57 | 96 | 12.1 |
| 11/29/2019 | 0.59 | 0.01 | 0.75 | 0.20 | 0.47 | 0.69 | 0.03 | <0.04 | 0.21 | 3.6 | 7 | 7.66 | 88 | 1.3 |
| Genus | Taxa | % of All Counted Valves |
|---|---|---|
| EUNOTIA | Eunotia bilunaris (Ehrenberg) Mills | 3.10 |
| Eunotia bilunaris (Ehrenberg) Mills abnormal form | 0.04 | |
| Eunotia cisalpina Lange-Bertalot & Cantonati in Cantonati & Lange-Bertalot | 0.06 | |
| Eunotia curtagrunowii Nörpel-Schempp & Lange-Bertalot | 0.02 | |
| Eunotia exigua (Brébisson ex Kützing) Rabenhorst | 1.77 | |
| Eunotia groenlandica (Grunow) Nörpel-Schempp & Lange-Bertalot | 0.10 | |
| Eunotia incisa W.Gregory | 0.02 | |
| Eunotia islandica Østrup | 0.28 | |
| Eunotia minor (Kützing) Grunow in Van Heurck | 0.14 | |
| Eunotia palatina Lange-Bertalot & W.Krüger in Werum & Lange-Bertalot | 0.02 | |
| Eunotia pseudogroenlandica Lange-Bertalot & Tagliaventi in Lange-Bertalot et al. | 7.24 | |
| Eunotia subarcuatoides Alles, Nörpel & Lange-Bertalot in Alles et al. | 0.06 | |
| Eunotia tenella (Grunow in Van Heurck) Hustedt in Schmidt et al. | 0.24 | |
| GOMPHONEMA | Gomphonema angustatum (Kützing) Rabenhorst | 0.04 |
| Gomphonema aff. acidoclinatum | 0.10 | |
| Gomphonema clavatum Ehrenberg | 0.02 | |
| Gomphonema drutelingense E.Reichardt | 0.02 | |
| Gomphonema hebridense W.Gregory | 0.06 | |
| Gomphonema aff. innocens | 6.51 | |
| Gomphonema aff. insigniforme | 0.02 | |
| Gomphonema aff. micropus | 0.04 | |
| Gomphonema lagenula Kützing | 0.06 | |
| Gomphonema parvulum Kützing | 0.06 | |
| Gomphonema sarcophagus W.Gregory | 0.02 | |
| Gomphonema varioreduncum Jüttner, Ector, E.Reichardt, Van de Vijver & E.J.Cox abnormal form | 0.02 | |
| PINNULARIA | Pinnularia sp. | 62.26 |
| Pinnularia borealis Ehrenberg | 0.30 | |
| Pinnularia aff. carminata | 0.04 | |
| Pinnularia cf. kuetzingii | 0.04 | |
| Pinnularia microstauron (Ehrenberg) Cleve | 0.04 | |
| Pinnularia persudetica Krammer | 0.06 | |
| Pinnularia saprophila Lange-Bertalot, H.Kobayasi & Krammer | 0.02 | |
| Pinnularia aff. saprophila | 0.08 | |
| Pinnularia sinistra Krammer | 0.08 | |
| Pinnularia viridiformis Krammer | 0.16 |
| Date | 2018 | 2019 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apr | July | July-M | Nov | Nov-M | May | May-M | July | Oct | Oct-M | Nov | |
| Pinnularia sp. | 47.7 | 79.4 | 72.3 | 69.8 | 75.2 | 81.8 | 56.2 | 58.7 | 73.2 | 45.0 | 22.0 |
| Nitzschia palea var. tenuirostris | 3.0 | 14.0 | 10.8 | 9.1 | 2.1 | 4.7 | 1.4 | 5.6 | 3.8 | 3.2 | 2.1 |
| Gomphonema aff. innocens | 5.3 | 0.0 | 2.3 | 6.1 | 0.7 | 2.2 | 22.7 | 1.9 | 5.5 | 14.4 | 3.6 |
| Psammothidium helveticum | 18.9 | 3.3 | 6.3 | 6.6 | 1.6 | 3.2 | 1.9 | 12.9 | 8.1 | 3.2 | 2.9 |
| Eunotia pseudogroenlandica | 10.1 | 0.0 | 1.6 | 0.0 | 0.0 | 2.0 | 2.1 | 5.8 | 1.9 | 9.5 | 53.9 |
| Eunotia exigua | 4.6 | 0.0 | 0.2 | 0.0 | 0.0 | 1.2 | 4.7 | 0.7 | 0.5 | 5.6 | 2.1 |
| Eunotia bilunaris | 3.2 | 0.0 | 0.0 | 1.5 | 0.2 | 0.5 | 8.4 | 0.7 | 1.4 | 9.5 | 9.5 |
| Cocconeis euglypta | 0.0 | 0.0 | 0.0 | 0.0 | 7.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Neidium alpinum | 1.8 | 0.7 | 0.9 | 0.5 | 0.0 | 1.5 | 0.7 | 1.7 | 2.2 | 1.0 | 0.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beauger, A.; Allain, E.; Voldoire, O.; Wetzel, C.E.; Ector, L.; Van de Vijver, B. Temporal Evolution of Diatoms in a Temporary Pond Situated in the Massif du Sancy Mountains (Massif Central, France) and Description of a New Pinnularia Species. Diversity 2020, 12, 367. https://doi.org/10.3390/d12100367
Beauger A, Allain E, Voldoire O, Wetzel CE, Ector L, Van de Vijver B. Temporal Evolution of Diatoms in a Temporary Pond Situated in the Massif du Sancy Mountains (Massif Central, France) and Description of a New Pinnularia Species. Diversity. 2020; 12(10):367. https://doi.org/10.3390/d12100367
Chicago/Turabian StyleBeauger, Aude, Elisabeth Allain, Olivier Voldoire, Carlos E. Wetzel, Luc Ector, and Bart Van de Vijver. 2020. "Temporal Evolution of Diatoms in a Temporary Pond Situated in the Massif du Sancy Mountains (Massif Central, France) and Description of a New Pinnularia Species" Diversity 12, no. 10: 367. https://doi.org/10.3390/d12100367
APA StyleBeauger, A., Allain, E., Voldoire, O., Wetzel, C. E., Ector, L., & Van de Vijver, B. (2020). Temporal Evolution of Diatoms in a Temporary Pond Situated in the Massif du Sancy Mountains (Massif Central, France) and Description of a New Pinnularia Species. Diversity, 12(10), 367. https://doi.org/10.3390/d12100367





