What is the Long-Term Effect of Bamboo Dominance on Adult Trees in the Araucaria Forest? A Comparative Analysis between Two Successional Stages in Southern Brazil
Abstract
1. Introduction
2. Materials and Methods
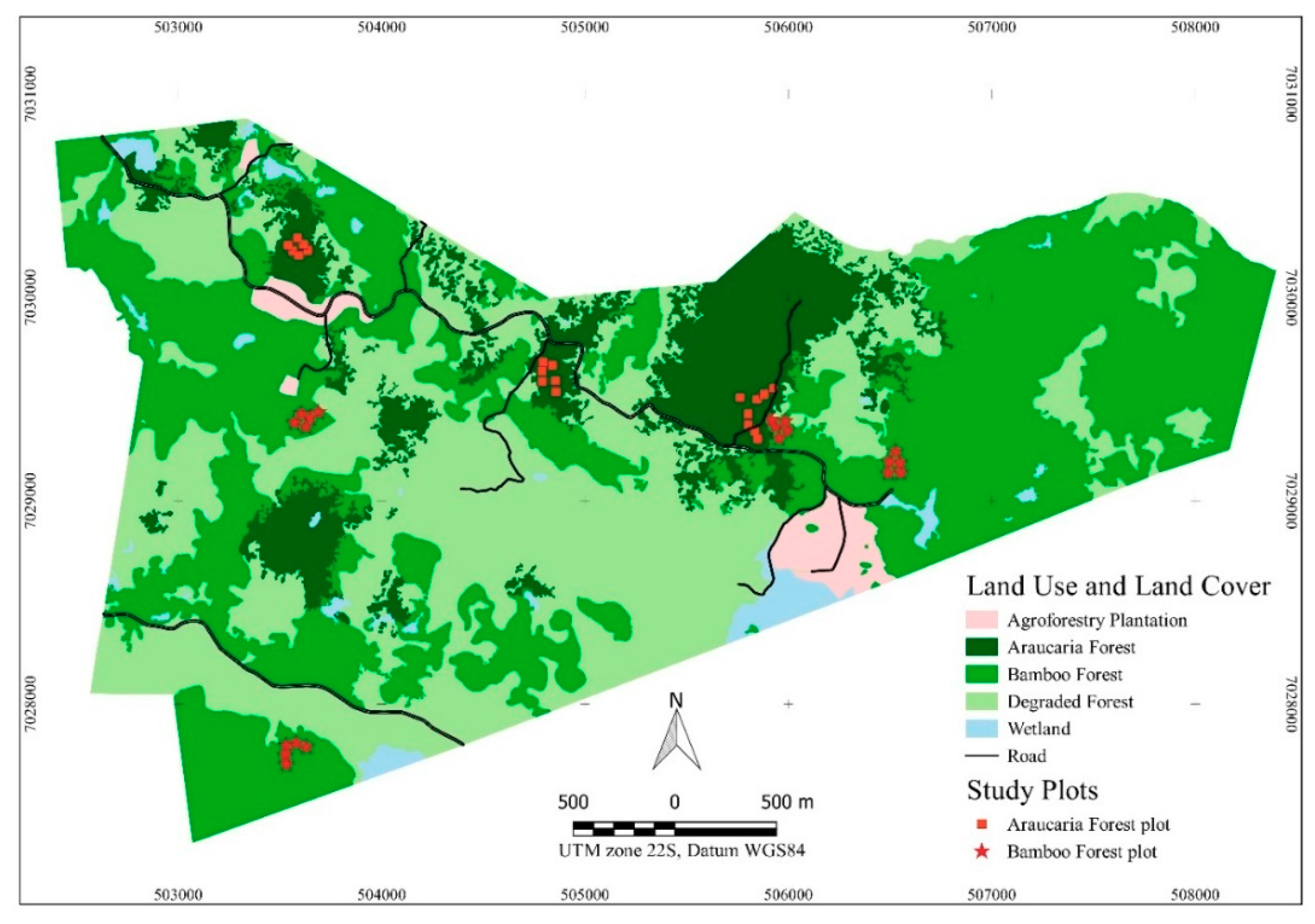
2.1. Study Area
2.2. Methods
2.2.1. Data Collection
2.2.2. Data Analysis
3. Results
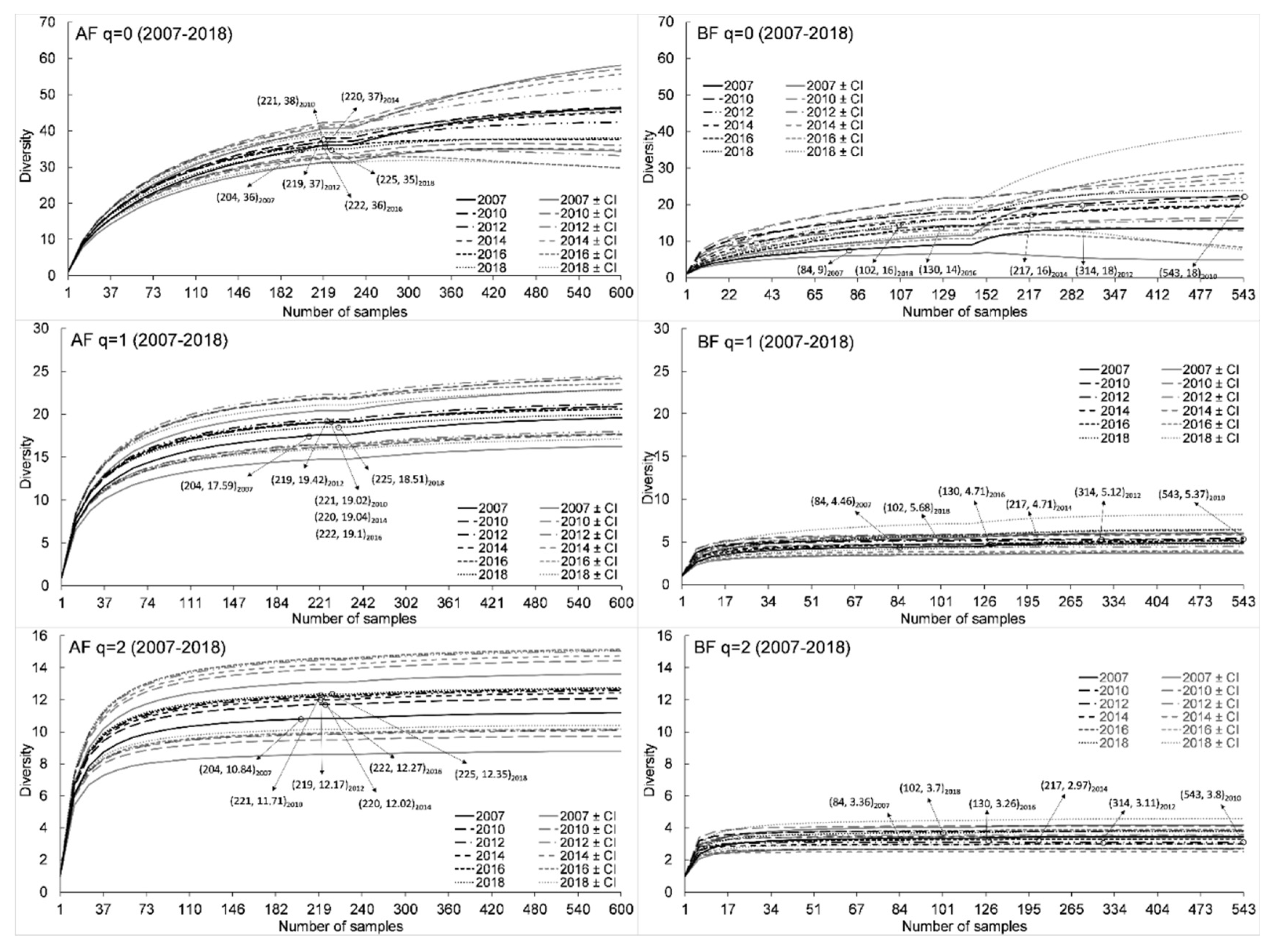
3.1. Species Diversity and Composition
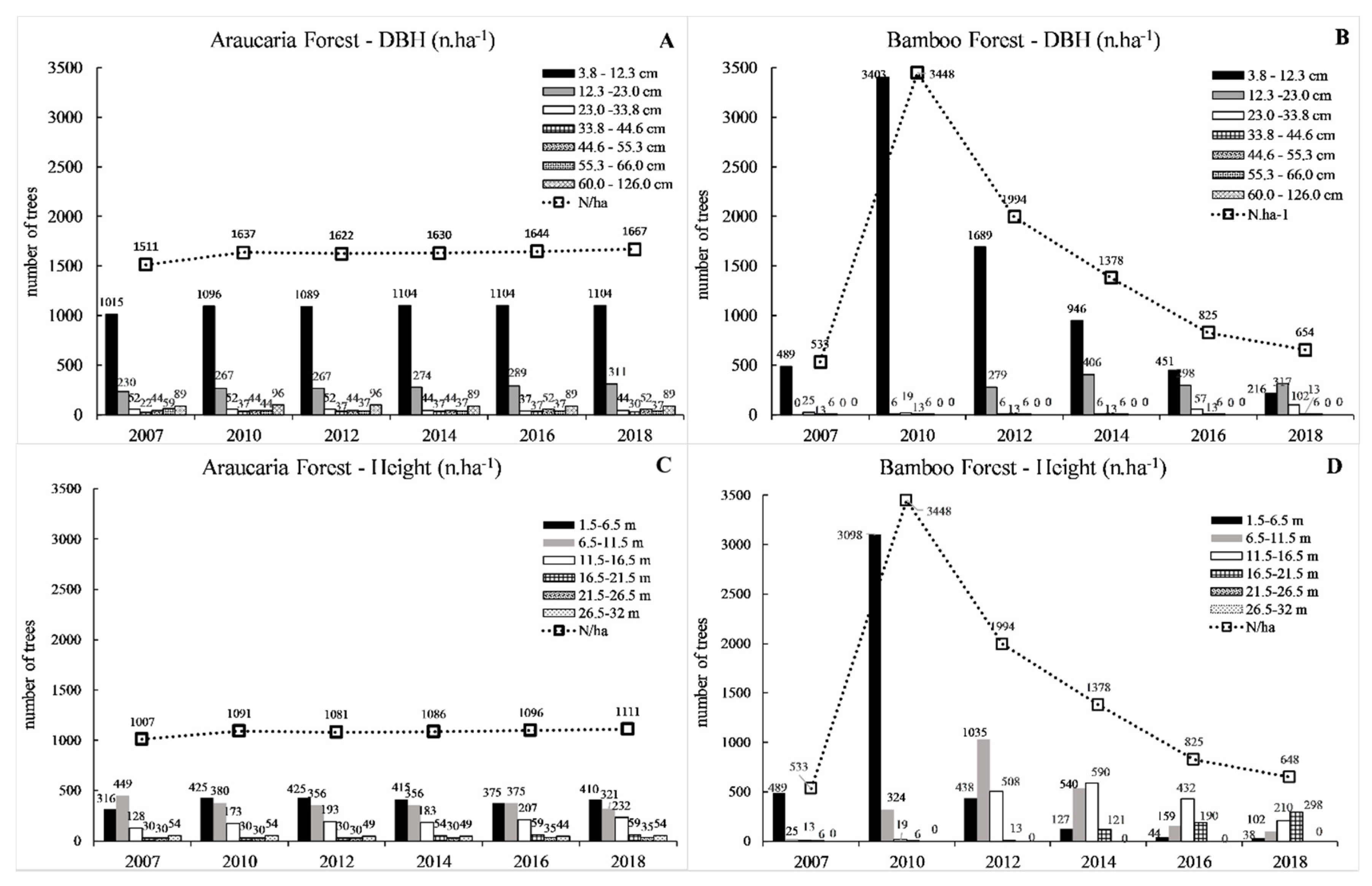
3.2. Forest Structure
4. Discussion
4.1. Species Diversity and Composition
4.2. Forest Structure
4.3. Implications for Conservation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix
| 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF | BF | AF | BF | AF | BF | AF | BF | AF | BF | AF | BF | |
| 0Dest (± CI) | 43.81 * (8.73) | 11.23 (4.53) | 47.5 * (13.52) | 21.29 (6.03) | 41.72 * (7.02) | 22.09 (6.45) | 42.95 * (7.52) | 16.84 (3.59) | 36.46 * (3.48) | 17.67 (5.8) | 35 * (3.58) | 22.41 (9.2) |
| 1Dest (± CI) | 19.12 * (3.45) | 4.67 (1.16) | 21.24 * (3.46) | 5.43 (0.54) | 20.98 * (3.14) | 5.24 (0.67) | 20.69 * (2.96) | 4.74 (0.94) | 19.27 * (2.65) | 4.95 (1.1) | 18.51 * (2.53) | 6.26 (1.68) |
| 2Dest (± CI) | 11.12 * (2.34) | 3.4 (0.69) | 12.15 * (2.33) | 3.81 (0.31) | 12.52 * (2.45) | 3.12 (0.44) | 12.35 * (2.28) | 2.98 (0.41) | 12.32 * (2.37) | 3.29 (0.56) | 12.35 * (1.99) | 3.76 (0.8) |
| coverage | 0.96 | 0.99 | 0.98 | 0.97 | 0.96 | 0.95 | ||||||
| Density (no. of tree ha−1) | |||||||||||||||
| Year | AF × BF | Araucaria Forest—AF | Bamboo Forest—BF | ||||||||||||
| 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | ||||
| 2007 | 0.77362 | 2007 | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 2007 | - | 0.00000 * | 0.00000 * | 0.00236 * | 1.00000 | 1.00000 |
| 2010 | 0.00000 * | 2010 | - | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 2010 | - | - | 0.00000 * | 0.00000 * | 0.00000 * | 0.00000 * |
| 2012 | 0.03229 * | 2012 | - | - | - | 1.00000 | 1.00000 | 1.00000 | 2012 | - | - | - | 0.09460 | 0.00000 * | 0.00000 * |
| 2014 | 1.00000 | 2014 | - | - | - | - | 1.00000 | 1.00000 | 2014 | - | - | - | - | 0.23120 | 0.01624 * |
| 2016 | 1.00000 | 2016 | - | - | - | - | - | 1.00000 | 2016 | - | - | - | - | - | 1.00000 |
| 2018 | 0.82397 | 2018 | - | - | - | - | - | - | 2018 | - | - | - | - | - | - |
| Basal Area (m2 ha−1) | |||||||||||||||
| Year | AF × BF | Araucaria Forest—AF | Bamboo Forest—BF | ||||||||||||
| 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | ||||
| 2007 | 0.00842 * | 2007 | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 2007 | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| 2010 | 0.01353 * | 2010 | - | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 2010 | - | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| 2012 | 0.01705 * | 2012 | - | - | - | 1.00000 | 1.00000 | 1.00000 | 2012 | - | - | - | 1.00000 | 1.00000 | 1.00000 |
| 2014 | 0.02122 * | 2014 | - | - | - | - | 1.00000 | 1.00000 | 2014 | - | - | - | - | 1.00000 | 1.00000 |
| 2016 | 0.01835 * | 2016 | - | - | - | - | - | 1.00000 | 2016 | - | - | - | - | - | 1.00000 |
| 2018 | 0.02033 * | 2018 | - | - | - | - | - | - | 2018 | - | - | - | - | - | - |
| Mean Height (m) | |||||||||||||||
| Year | AF × BF | Araucaria Forest—AF | Bamboo Forest—BF | ||||||||||||
| 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | ||||
| 2007 | 0.00000 * | 2007 | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 2007 | - | 1.00000 | 0.00000 * | 0.00000 * | 0.00000 * | 0.00000 * |
| 2010 | 0.00001 * | 2010 | - | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 2010 | - | - | 0.00000 * | 0.00000 * | 0.00000 * | 0.00000 * |
| 2012 | 1.00000 | 2012 | - | - | - | 1.00000 | 1.00000 | 1.00000 | 2012 | - | - | - | 0.00971 * | 0.00001 * | 0.00000 * |
| 2014 | 0.472731 | 2014 | - | - | - | - | 1.00000 | 1.00000 | 2014 | - | - | - | - | 0.04043 * | 0.00004 * |
| 2016 | 0.003594 * | 2016 | - | - | - | - | - | 1.00000 | 2016 | - | - | - | - | - | 0.04391 * |
| 2018 | 0.000002 * | 2018 | - | - | - | - | - | - | 2018 | - | - | - | - | - | - |
| MEAN DBH (cm) | |||||||||||||||
| Year | AF × BF | Araucaria Forest—AF | Bamboo Forest—BF | ||||||||||||
| 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | ||||
| 2007 | 0.000658 * | 2007 | - | 0.57393 | 0.24301 | 0.09949 | 1.00000 | 1.00000 | 2007 | - | 1.00000 | 0.11814 | 0.00777 * | 0.05967 | 0.00942 * |
| 2010 | 0.000910 * | 2010 | - | - | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 2010 | - | - | 0.08088 | 0.00635 * | 0.00174 * | 0.00861 * |
| 2012 | 0.002664 * | 2012 | - | - | - | 1.00000 | 1.00000 | 1.00000 | 2012 | - | - | - | 0.101770 | 0.01077 * | 0.00078 * |
| 2014 | 0.011045 * | 2014 | - | - | - | - | 1.00000 | 1.00000 | 2014 | - | - | - | - | 0.22477 | 0.00295 * |
| 2016 | 0.034718 * | 2016 | - | - | - | - | - | 1.00000 | 2016 | - | - | - | - | - | 0.01339 * |
| 2018 | 1.00000 | 2018 | - | - | - | - | - | - | 2018 | - | - | - | - | - | - |
References
- Montti, L.; Villagra, M.; Campanello, P.I.; Gatti, M.G.; Goldstein, G. Functional traits enhance invasiveness of bamboos over co-occurring tree saplings in the semideciduous Atlantic Forest. Acta Oecol. 2014, 54, 36–44. [Google Scholar] [CrossRef]
- Judziewicz, E.J.; Clark, L.G.; Londoño, X.; Stern, M.J. American Bamboos; Smithsonian Institution Press: Washington, DC, USA, 1999. [Google Scholar]
- Abe, M.; Izaki, J.; Miguchi, H.; Masaki, T.; Makita, A.; Nakashizuka, T. The effects of Sasa and canopy gap formation on tree regeneration in an old beech forest. J. Veg. Sci. 2002, 13, 565–574. [Google Scholar] [CrossRef]
- Holz, C.A.; Veblen, T.T. Tree regeneration responses to Chusquea montana bamboo die-off in a subalpine Nothofagus forest in the southern Andes. J. Veg. Sci. 2006, 17, 19. [Google Scholar] [CrossRef]
- Campanello, P.I.; Genoveva Gatti, M.; Ares, A.; Montti, L.; Goldstein, G. Tree regeneration and microclimate in a liana and bamboo-dominated semideciduous Atlantic Forest. For. Ecol. Manag. 2007, 252, 108–117. [Google Scholar] [CrossRef]
- Griscom, B.W.; Ashton, P.M.S. A self-perpetuating bamboo disturbance cycle in a neotropical forest. J. Trop. Ecol. 2006, 22, 587. [Google Scholar] [CrossRef]
- Kellermann, B.; Lacerda, A.E.B. Bambus nativos como espécies invasoras no sul do Brasil. In Bambus no Brasil: Da Biologia à Tecnologia; Drumond, P.M., Wiedman, G., Eds.; Embrapa: Brasília, Brazil, 2017; pp. 179–196. [Google Scholar]
- Kellermann, B.; Lacerda, A.E.B. Arrested development? Investigating the role of bamboo in Araucaria Forest succession in Southern Brazil. J. Plant Ecol. 2019. [Google Scholar] [CrossRef]
- Larpkern, P.; Moe, S.R.; Totland, Ø. Bamboo dominance reduces tree regeneration in a disturbed tropical forest. Oecologia 2011, 165, 161–168. [Google Scholar] [CrossRef]
- Budke, J.C.; Alberti, M.S.; Zanardi, C.; Baratto, C.; Zanin, E.M. Bamboo dieback and tree regeneration responses in a subtropical forest of South America. For. Ecol. Manag. 2010, 260, 1345–1349. [Google Scholar] [CrossRef]
- Kellermann, B. Monitoramento da Regeneração Natural em Fragmento de Floresta Ombrófila Mista e Morfoanatomia de Plântulas e Tirodendros de Piptocarpha Angustifolia Dusén ex Malme (Asteraceae); Federal University of Paraná: Curitiba, Brazil, 2011. [Google Scholar]
- Montti, L.; Campanello, P.I.; Gatti, M.G.; Blundo, C.; Austin, A.T.; Sala, O.E.; Goldstein, G. Understory bamboo flowering provides a very narrow light window of opportunity for canopy-tree recruitment in a neotropical forest of Misiones, Argentina. For. Ecol. Manag. 2011, 262, 1360–1369. [Google Scholar] [CrossRef]
- Klein, R.M. O aspeto dinâmico do pinheiro brasileiro. Sellowia 1960, 48, 17–51. [Google Scholar]
- Leite, P.F.; Klein, R.M. Vegetação. In Geografia do Brasil: Região Sul; IBGE: Rio de Janeiro, Brazil, 1990; pp. 113–150. [Google Scholar]
- Vibrans, A.C.; Sevegnani, L.; de Gasper, A.L.; Lingner, D.V. Floresta Ombrófila Mista; Edifurb: Blumenau, Brazil, 2013. [Google Scholar]
- Valéry, L.; Fritz, H.; Lefeuvre, J.C.; Simberloff, D. Invasive species can also be native. Trends Ecol. Evol. 2009, 24, 585. [Google Scholar] [CrossRef] [PubMed]
- Sendulsky, T. Merostachys multiramea (Poaceae: Bambusoideae: Bambuseae) and Similar Species from Brazil. Novon 1995, 5, 76–96. [Google Scholar] [CrossRef]
- Pereira, C. Sobre as ‘ratadas’ no sul do Brasil e o ciclo vegetativo das taquaras. Arq. Inst. Biol. São Paulo 1941, 12, 175–195. [Google Scholar]
- M, G.; Vellozo, L.; Kubiak, G. Sobre as “ratadas” do primeiro planalto paranaense. Arq. Biol. Tecnol. 1946, 1, 185–195. [Google Scholar]
- IBGE. Manual Técnico da Vegetação Brasileira; IBGE: Rio de Janeiro, Brazil, 2012.
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; van Dijk, P.P.; Rhodin, A.G.J.; Nash, S.D. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Ecoregions; CEMEX: Mexico City, Mexico, 2004. [Google Scholar]
- Lacerda, A.E.B. Conservation strategies for Araucaria Forests in Southern Brazil: Assessing current and alternative approaches. Biotropica 2016, 48, 537–544. [Google Scholar] [CrossRef]
- Rosot, M.A.D.; De Oliveira, Y.M.M.; De Mattos, P.P.; Garrastazu, M.C.; Shimizu, J.Y. Monitoramento na Reserva Florestal da Embrapa/Epagri (RFEE) em Caçador, SC. Documentos 2007, 158, 35. [Google Scholar]
- Brasil. Portaria do Ministério do Meio Ambiente no. 443, de 17 de dezembro de 2014. Diário Of. União 2014, 245, 110–121. [Google Scholar]
- Klein, R.M. Aspectos Dinâmicos da Vegetação do Sul do Brasil. Sellowia 1984, 36, 5–54. [Google Scholar]
- Lacerda, A.E.B.; Rosot, M.A.D.; Filho, A.F.; Garrastazú, M.C.; Nimmo, E.R.; Kellermann, B.; Radomski, M.I.; Beimgraben, T.; Mattos, P.P.; Oliveira, Y.M.M. Sustainable Forest Management in Rural Southern Brazil: Exploring Participatory Forest Management Planning. In Sustainable Forest Management—Case Studies; Martin-Garcia, J., Diez, J.J., Eds.; InTech: London, UK, 2012. [Google Scholar]
- FAO Assessing Forest Degradation. Towards the Development of Globally Applicable Guidelines—Forest Resources Assessment Working Paper 177; FAO: Roma, Italy, 2011. [Google Scholar]
- Valéry, L.; Fritz, H.; Lefeuvre, J.C.; Simberloff, D. In search of a real definition of the biological invasion phenomenon itself. Biol. Invasions 2008, 10, 1345–1351. [Google Scholar] [CrossRef]
- Budowski, G. Distribution of tropical american rain Forest species in the light of sucessional processes. Turrialba 1965, 15, 40–42. [Google Scholar]
- Carvalho, P.E.R. Espécies arbóreas brasileiras; Embrapa Informação Tecnológica: Brasília, Brazil, 2003. [Google Scholar]
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT (iNterpolation and EXTrapolation Online, Hsin-Chu, Taiwan. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/2016 (accessed on 10 April 2019).
- Chao, A.; Otelli, N.I.J.G.; Sieh, T.C.H.; Ander, E.L.L.S.; Olwell, R.O.K.C. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chiu, C.H. Nonparametric Estimation and Comparison of Species Richness. eLS 2016, 1–11. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data, version 7.06; Wild Blueberry Media LLC: Corvallis, OR, USA, 2018. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- NCSS. 2019 Statistical Software; NCSS, LLC: Kaysville, UT, USA, 2019. [Google Scholar]
- Cientec. Software Mata Nativa 4: Sistema para análise fitossociológica, elaboração de inventários e planos de manejo de florestas nativas; Cientec: Viçosa, Brazil, 2016. [Google Scholar]
- Gómez-Pompa, A.; Vázquez-Yanes, C. Successional Studies of a Rain Forest in Mexico. In Forest Succession, Concepts and Application; West, D.C., Shugart, H.H., Botkin, D.B., Eds.; Springer: New York, NY, USA, 1981; pp. 246–266. [Google Scholar]
- Van Breugel, M.; Bongers, F.; Martínez-Ramos, M. Species Dynamics During Early Secondary Forest Succession: Recruitment, Mortality and Species Turnover. Biotropica 2007, 39, 610–619. [Google Scholar] [CrossRef]
- Montti, L.; Campanello, P.I.; Goldstein, G. Flowering, die-back and recovery of a semelparous woody bamboo in the Atlantic Forest. Acta Oecol. 2011, 37, 361–368. [Google Scholar] [CrossRef]
- González, M.E.; Veblen, T.T.; Donoso, C.; Valeria, L. Tree regeneration responses in a lowland Nothofagus-dominated forest after bamboo die-off in South-Central Chile. Plant Ecol. 2002, 161, 59–73. [Google Scholar] [CrossRef]
- Greig, C.; Robertson, C.; Lacerda, A.E.B. Spectral-temporal modelling of bamboo-dominated forest succession in the Atlantic Forest of Southern Brazil. Ecol. Model. 2018, 384, 316–332. [Google Scholar] [CrossRef]
- Taylor, A.H.; Jinyan, H.; ShiQiang, Z. Canopy tree development and undergrowth bamboo dynamics in old-growth Abies-Betula forests in southwestern China: A 12-year study. For. Ecol. Manag. 2004, 200, 347–360. [Google Scholar] [CrossRef]
- Noguchi, M.; Yoshida, T. Factors influencing the distribution of two co-occurring dwarf bamboo species (Sasa kurilensis and S. senanensis) in a conifer-broadleaved mixed stand in northern Hokkaido. Ecol. Res. 2005, 20, 25–30. [Google Scholar] [CrossRef]
- Walker, L.R.; del Moral, R. Primary Succession and Ecosystem Rehabilitation; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
| Year | Araucaria Forest | Bamboo Forest | Summary | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0D | 1D | 2D | Sexcl | 0D | 1D | 2D | Sexcl | Sshared | Stotal | |
| 2007 | 36 | 17.6 | 10.8 | 34 | 9 * | 4.2 * | 3.4 * | 7 | 2 | 43 |
| 2010 | 38 | 19 | 11.7 | 34 | 18 * | 5.4 * | 3.8 * | 14 | 4 | 52 |
| 2012 | 37 | 19.4 | 12.2 | 32 | 18 * | 5.1 * | 3.1 * | 13 | 5 | 50 |
| 2014 | 37 | 19 | 12 | 32 | 16 * | 4.7 * | 3 * | 11 | 5 | 48 |
| 2016 | 36 | 19.1 | 12.3 | 31 | 14 * | 4.7 * | 3.3 * | 9 | 5 | 45 |
| 2018 | 35 | 18.5 | 12.3 | 28 | 16 * | 5.7 * | 3.7 * | 8 | 8 | 43 |
| Araucaria Forest | |||||||
| 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | Coverage | |
| 0Dest (± CI) | 45.36 (10.55) | 45.25 (8.83) | 40.93 (6.43) | 43.91 (8.81) | 37.31 (4.59) | 36.93 (5.07) | 0.97 |
| 1Dest (± CI) | 19.03 (3.26) | 20.59 (3.18) | 20.54 (3.09) | 20.57 (3.24) | 19.55 (2.76) | 19.04 (2.81) | 0.97 |
| 2Dest (± CI) | 11.16 (2.41) | 12.04 (2.34) | 12.44 (2.41) | 12.36 (2.31) | 12.36 (2.37) | 12.52 (2.56) | 0.97 |
| Bamboo Forest | |||||||
| 2007 | 2010 | 2012 | 2014 | 2016 | 2018 | Coverage | |
| 0Dest (± CI) | 12.45 (6.63) | 18 (3.68) | 21.41 (5.89) | 18.63 (5.4) | 19.35 (9.48) | 23.66 (12.39) | 0.98 |
| 1Dest (± CI) | 4.76 (1.12) | 5.37 (0.49) | 5.23 (0.76) | 4.84 (0.86) | 5.08 (1.14) | 6.42 (1.70) | 0.98 |
| 2Dest (± CI) | 3.43 (0.76) | 3.8 (0.3) | 3.12 (0.48) | 2.98 (0.46) | 3.3 (0.56) | 3.78 (0.82) | 0.98 |
| Year | MRPP | Indicator Species Analysis (ISA) | ||
|---|---|---|---|---|
| A | p-Value | AF Indicator Species | BF Indicator Species | |
| 2007 | 0.10211 | 0.000151 * | Araucaria angustifolia; Casearia decandra; Cupania vernalis; Myrceugenia miersiana; Ocotea porosa; Trichilia elegans | Aegiphila riedeliana; Piptocarpha angustifolia |
| 2010 | 0.286611 | 0.000027 * | Araucaria angustifolia; Casearia decandra; Cupania vernalis; Myrceugenia miersiana; Ocotea porosa; Trichilia elegans | Aegiphila riedeliana; Piptocarpha angustifolia; Mimosa scabrella; Solanum erianthum; Ocotea puberula; Vernonanthura discolor |
| 2012 | 0.310298 | 0.000201 * | Araucaria angustifolia; Casearia decandra; Cupania vernalis; Myrceugenia miersiana; Ocotea porosa; Trichilia elegans | Piptocarpha angustifolia; Mimosa scabrella; Ocotea puberula; Solanum erianthum; Vernonanthura discolor |
| 2014 | 0.276813 | 0.000168 * | Araucaria angustifolia; Casearia decandra; Cupania vernalis; Myrceugenia miersiana; Ocotea porosa; Trichilia elegans | Piptocarpha angustifolia; Mimosa scabrella; Vernonanthura discolor |
| 2016 | 0.220812 | 0.00005 * | Araucaria angustifolia; Casearia decandra; Cupania vernalis; Myrceugenia miersiana; Ocotea porosa; Trichilia elegans | Piptocarpha angustifolia; Mimosa scabrella; Vernonanthura discolor |
| 2018 | 0.190865 | 0.000039 * | Araucaria angustifolia; Casearia decandra; Cupania vernalis; Myrceugenia miersiana; Ocotea porosa; Trichilia elegans; Nectandra megapotamica | Piptocarpha angustifolia; Mimosa scabrella; Vernonanthura discolor |
| Araucaria Forest—AF | Bamboo Forest—BF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0D (%) | N (%) | BA (%) | Height Mean (SD) | DBH Mean (SD) | 0D (%) | N (%) | BA (%) | Height Mean (SD) | DBH Mean (SD) | |
| 2007 | ||||||||||
| Pioneer | 2 (5.6) | 10 (1.0) | 0.1 (0.1) | 7.25 (1.8) | 9.5 (7.3) | 6 (66.6) | 514 (96.4) | 2.9 (56.1) | 4.1 (2.3) | 5.4 (6.4) |
| Secondary | 32 (88.9) | 957 (95.1) | 57.5 (86.6) | 10 (6.6) | 16.5 (22.5) | 3 (33.3) | 19 (3.6) | 2.2 (43.9) | 9.9 (3.3) | 34 (22.4) |
| Late suc. | 2 (5.6) | 40 (3.9) | 8.5 (13.2) | 14.3 (3.3) | 48.4 (23.1) | - | - | - | - | - |
| Total | 36 | 1007 | 66.1 | 10.2 (6.6) | 17.7 (23) | 9 | 533 | 5.1 | 4.3 (2.5) | 6.4 (9) |
| 2010 | ||||||||||
| Pioneer | 3 (7.9) | 20 (1.8) | 0.1 (0.2) | 5.6 (2.3) | 6.8 (5.4) | 12 (66.6) | 3365 (97.6) | 11.0 (82.4) | 5 (1.5) | 5.8 (2.9) |
| Secondary | 33 (86.8) | 1031 (94.5) | 59 (87.7) | 9.7 (6.7) | 15.9 (21.8) | 6 (33.3) | 83 (2.4) | 2.6 (17.6) | 6.2 (3.9) | 11 (16.2) |
| Late suc. | 2 (5.3) | 40 (3.6) | 8.1 (12.1) | 14.3 (3.5) | 46.5 (22.9) | - | - | - | - | - |
| Total | 38 | 1091 | 67.2 | 9.8 (6.6) | 16.9 (22.4) | 18 | 3448 | 13.6 | 5 (1.6) | 5.9 (3.8) |
| 2012 | ||||||||||
| Pioneer | 3 (8.1) | 30 (2.7) | 0.1 (0.2) | 5.7 (1.8) | 6.3 (4.5) | 11(61.1) | 1880 (94.3) | 12.5 (83.3) | 9.5 (3.2) | 8.2 (4.2) |
| Secondary | 32 (86.5) | 1016 (93.6) | 57.2 (87.2) | 9.7 (6.6) | 16 (21.6) | 7 (38.9) | 114 (5.7) | 2.5 (16.7) | 7.2 (3.4) | 10 (13.8) |
| Late suc. | 2 (5.4) | 40 (3.7) | 8.3 (12.6) | 14.3 (3.5) | 46.9 (23.1) | - | - | - | - | - |
| Total | 37 | 1086 | 65.6 | 9.7 (6.5) | 16.9 (22.1) | 18 | 1994 | 15.0 | 9.4 (3.2) | 8.3 (6.6) |
| 2014 | ||||||||||
| Pioneer | 3 (8.1) | 30 (2.7) | 0.1 (0.2) | 6 (2.2) | 6.9 (4.3) | 9 (56.2) | 1295 (94.0) | 13.6 (84.5) | 11.7 (3.6) | 10.5 (4.9) |
| Secondary | 32 (86.5) | 1016 (93.6) | 55.2 (86.5) | 9.9 (6.5) | 15.5 | 7 (43.8) | 83 (6.0) | 2.5 (15.5) | 8 (4) | 12.5 (15.8) |
| Late suc. | 2 (5.4) | 40 (3.6) | 8.5 (13.3) | 15.6 (3.7) | 47.6 (23.2) | - | - | - | - | - |
| Total | 37 | 1086 | 63.8 | 10 (6.5) | 16.5 (21.9) | 16 | 1378 | 16.1 | 11.5 (3.7) | 10.6 (6) |
| 2016 | ||||||||||
| Pioneer | 3 (8.3) | 35 (3.2) | 0.2 (0.3) | 6.7 (2.1) | 6.8 (4.1) | 8 (57.1) | 768 (93.1) | 12.3 (83.5) | 13.9 (3.4) | 12.6 (6.8) |
| Secondary | 31 (86.1) | 1021 (93.2) | 55.3 (86.1) | 10.1 (6.6) | 15.6 (21.2) | 6 (42.9) | 57 (6.9) | 2.4 (16.5) | 9.4 (4.2) | 15.5 (18.5) |
| Late suc. | 2 (5.6) | 40 (3.6) | 8.8 (13.6) | 16.5 (4.1) | 48.3 (23.7) | - | - | - | - | - |
| Total | 36 | 1096 | 64.3 | 10.2 (6.5) | 16.5 (21.8) | 14 | 825 | 14.7 | 13.6 (3.6) | 12.8 (8) |
| 2018 | ||||||||||
| Pioneer | 4 (11.4) | 35 (3.1) | 0.2 (0.4) | 6.1 (3.3) | 7.1 (5.6) | 7 (43.7) | 565 (87.3) | 14.9 (85.3) | 16.1 (3.3) | 17.2 (6.6) |
| Secondary | 29 (82.9) | 1036 (93.3) | 56.9 (86.4) | 10 (6.8) | 15.7 (21.3) | 9 (56.3) | 83 (12.7) | 2.6 (14.7) | 8.2 (4.8) | 12.7 (16) |
| Late suc. | 2 (5.7) | 40 (3.6) | 8.7 (13.2) | 17.8 (5.8) | 48.3 (23.2) | - | - | - | - | - |
| Total | 35 | 1111 | 65.8 | 10.1 (6.9) | 16.6 (21.9) | 16 | 648 | 17.5 | 15 (4.4) | 16.6 (8.4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacerda, A.E.B.; Kellermann, B. What is the Long-Term Effect of Bamboo Dominance on Adult Trees in the Araucaria Forest? A Comparative Analysis between Two Successional Stages in Southern Brazil. Diversity 2019, 11, 165. https://doi.org/10.3390/d11090165
Lacerda AEB, Kellermann B. What is the Long-Term Effect of Bamboo Dominance on Adult Trees in the Araucaria Forest? A Comparative Analysis between Two Successional Stages in Southern Brazil. Diversity. 2019; 11(9):165. https://doi.org/10.3390/d11090165
Chicago/Turabian StyleLacerda, André Eduardo Biscaia, and Betina Kellermann. 2019. "What is the Long-Term Effect of Bamboo Dominance on Adult Trees in the Araucaria Forest? A Comparative Analysis between Two Successional Stages in Southern Brazil" Diversity 11, no. 9: 165. https://doi.org/10.3390/d11090165
APA StyleLacerda, A. E. B., & Kellermann, B. (2019). What is the Long-Term Effect of Bamboo Dominance on Adult Trees in the Araucaria Forest? A Comparative Analysis between Two Successional Stages in Southern Brazil. Diversity, 11(9), 165. https://doi.org/10.3390/d11090165





