Bacterial Communities from Extreme Environments: Vulcano Island
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area: Vulcano Island
Sampling Areas
2.2. Interstitial Soil Gas Sampling and Analyses
2.3. Biological Sample Collection
2.4. Extraction of Genomic DNA and Next Generation Sequencing
2.5. Sequence Analysis
2.6. Statistical Testing
2.7. Isolation of Culturable Bacterial Strains
2.8. Random Amplified Polymorphic DNA (RAPD) Analysis
2.9. Taxonomical Characterization of Culturable Bacterial Strains
3. Results
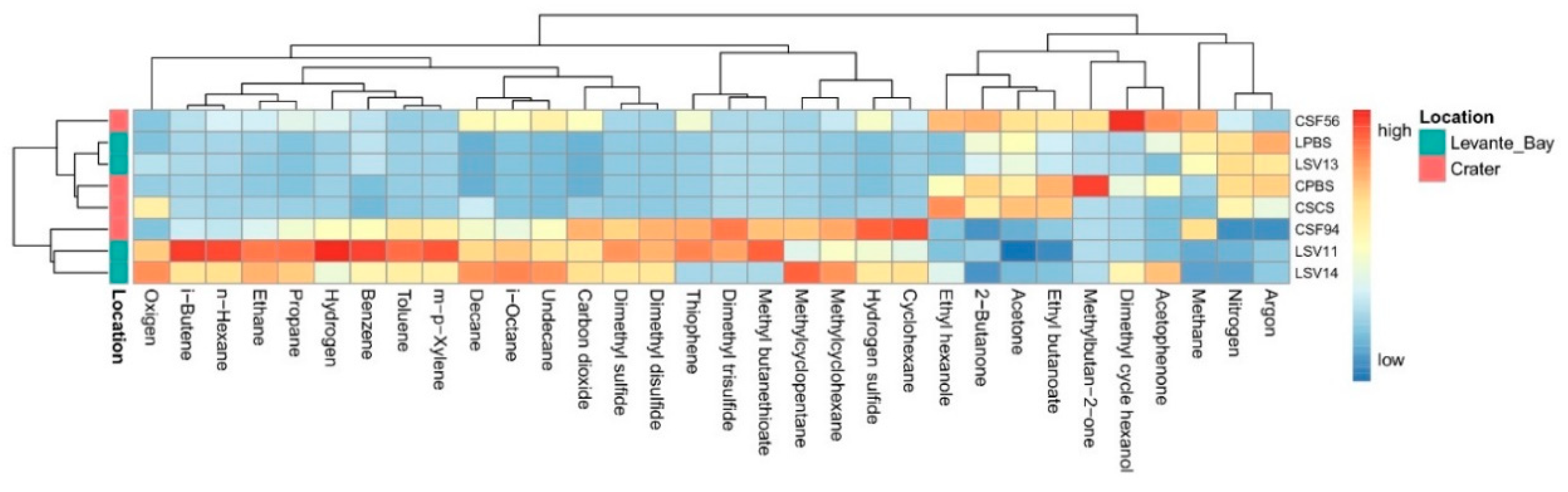
3.1. Geochemical Features of Interstitial Soil Gases
3.1.1. La Fossa Crater
3.1.2. Levante Bay
3.1.3. Pioneer Plant Sites
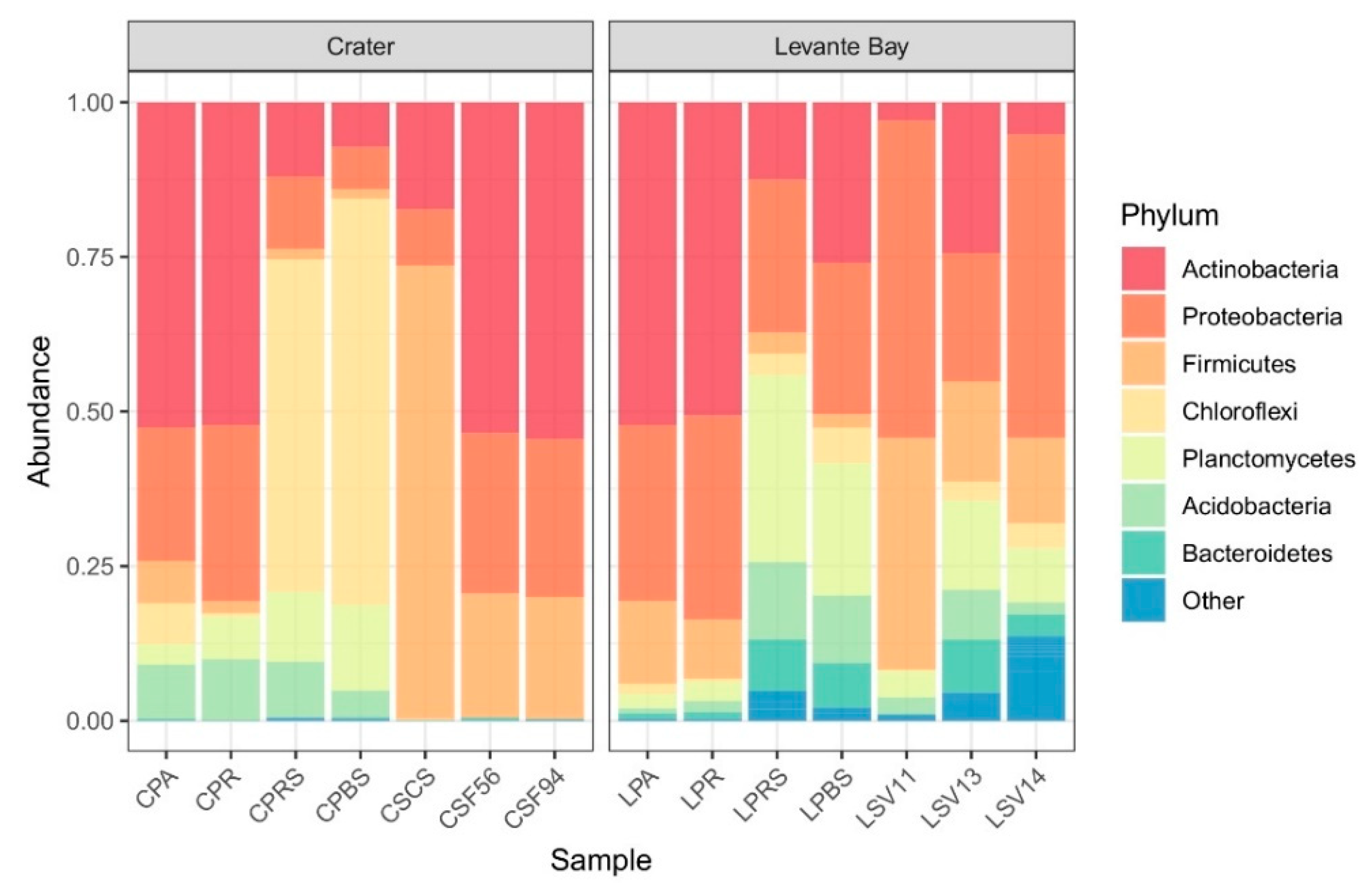
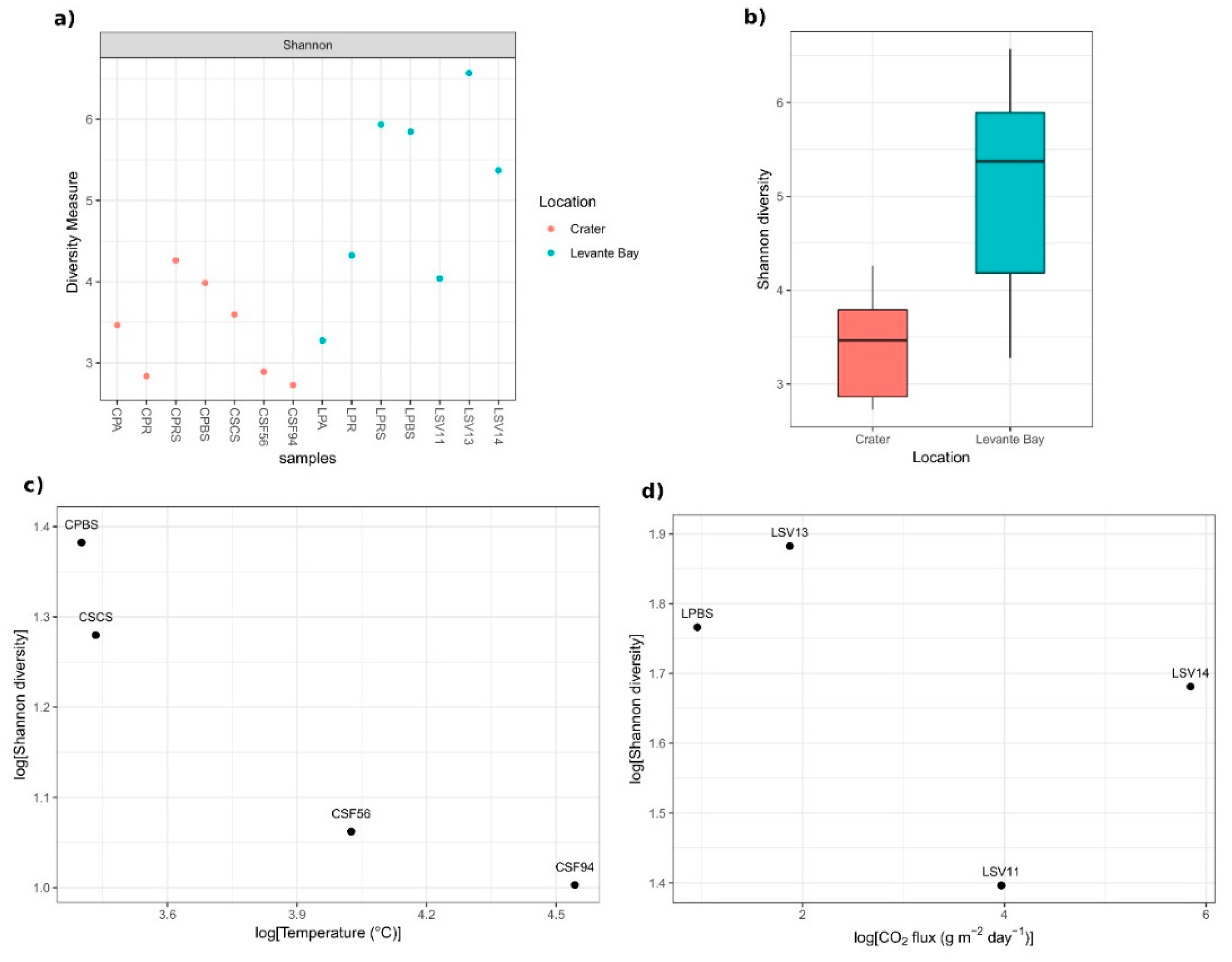
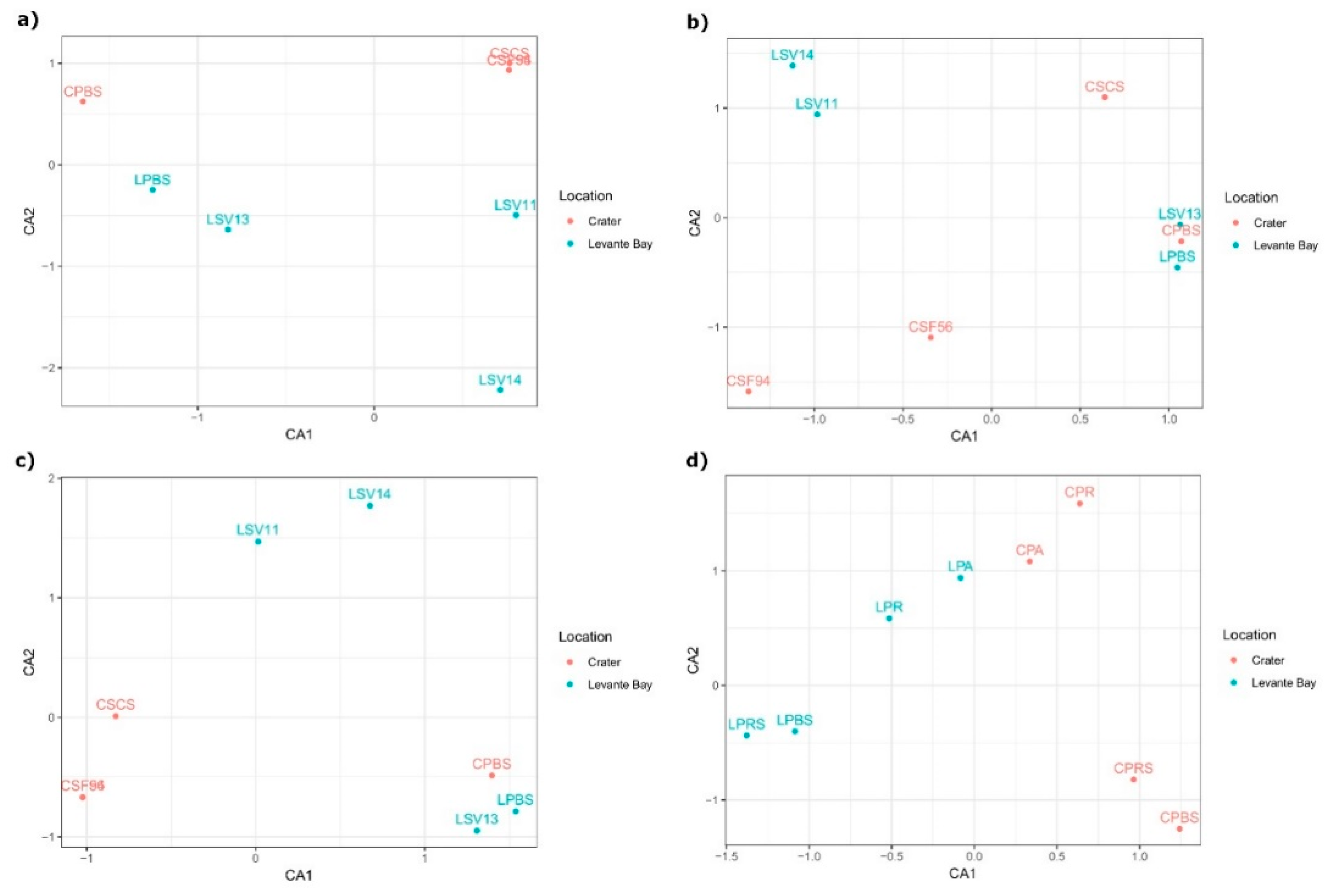
3.2. Molecular Characterization of Bacterial Communities
Comparison between La Fossa Crater and Levante Bay Soil and Plant Samples
3.3. Culturable Bacterial Community Analysis
4. Discussion
4.1. Bacterial Communities Study
4.1.1. Soil Samples Analysis
4.1.2. Plant Samples Analysis
4.2. Cultivable Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.J.; Hua, Z.S.; Huang, L.N.; Li, J.; Shi, S.H.; Chen, L.X.; Kuang, J.L.; Liu, J.; Hu, M.; Shu, W.S. Microbial communities evolve faster in extreme environments. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, C.; Miceli, E.; Bacci, G.; Fagorzi, C.; Coppini, E.; Fibbi, D.; Bianconi, G.; Mengoni, A.; Canganella, F.; Fani, R. Spatial structuring of bacterial communities in epilithic biofilms in the Acquarossa river (Italy). FEMS Microbiol. Ecol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Canfora, L.; Lo Papa, G.; Vittori Antisari, L.; Bazan, G.; Dazzi, C.; Benedetti, A. Spatial microbial community structure and biodiversity analysis in “extreme” hypersaline soils of a semiarid Mediterranean area. Appl. Soil Ecol. 2015. [Google Scholar] [CrossRef]
- Yadav, A.N.; Verma, P.; Kumar, M.; Pal, K.K.; Dey, R.; Gupta, A.; Padaria, J.C.; Gujar, G.T.; Kumar, S.; Suman, A.; et al. Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann. Microbiol. 2015, 65, 611–629. [Google Scholar] [CrossRef]
- Van Kranendonk, M.J.; Pirajno, F. Geochemistry of metabasalts and hydrothermal alteration zones associated with c. 3.45 Ga chert and barite deposits: Implications for the geological setting of the Warrawoona Group, Pilbara Craton, Australia. Geochem. Explor. Environ. Anal. 2005, 4, 253–278. [Google Scholar] [CrossRef]
- Herrera, A.; Cockell, C.S. Exploring microbial diversity in volcanic environments: A review of methods in DNA extraction. J. Microbiol. Methods 2007, 70, 1–12. [Google Scholar] [CrossRef]
- Stott, M.B.; Crowe, M.A.; Mountain, B.W.; Smirnova, A.V.; Hou, S.; Alam, M.; Dunfield, P.F. Isolation of novel bacteria, including a candidate division, from geothermal soils in New Zealand. Environ. Microbiol. 2008, 10, 2030–2041. [Google Scholar] [CrossRef]
- Crisci, G.M.; De Rosa, R.; Esperan, S.; Mazzuoli, R.; Sonnino, M.; Scienze, D.; Calabria, U. Temporal evolution of a three component system: The island of Lipari (Aeolian Arc, southern Italy) GM. Bull. Volcanol. 1991, 53, 207–221. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Lentini, V.; Gugliandolo, C.; Italiano, F.; Cousin, S.; Stackebrandt, E. Bacterial and archaeal populations at two shallow hydrothermal vents off Panarea Island (Eolian Islands, Italy). Extremophiles 2009, 13, 199–212. [Google Scholar] [CrossRef]
- Amend, J.P.; Rogers, K.L.; Shock, E.L.; Gurrieri, S.; Inguaggiato, S. Energetics of chemolithoautotrophy in the hydrothermal system of Vulcano Island, southern Italy. Geobiology 2003, 1, 37–58. [Google Scholar] [CrossRef]
- Caccamo, D.; Gugliandolo, C.; Stackebrandt, E.; Maugeri, T.L. Bacillus vulcani sp. nov., a novel thermophilic species isolated from a shallow marine hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2000, 50, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. A polyphasic taxonomic study of thermophilic bacilli from shallow, marine vents. Syst. Appl. Microbiol. 2001, 24, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. Three novel halotolerant and thermophilic Geobacillus strains from shallow marine vents. Syst. Appl. Microbiol. 2002, 25, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, C.; Maugeri, T.L.; Caccamo, D.; Stackebrandt, E. Bacillus aeolius sp. nov. a novel thermophilic, halophilic marine Bacillus species from Eolian Islands (Italy). Syst. Appl. Microbiol. 2003, 26, 172–176. [Google Scholar] [CrossRef]
- Insam, H.; Seewald, M.S.A. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 2010, 46, 199–213. [Google Scholar] [CrossRef]
- Mackie, A.E.; Wheatley, R.E. Effects and incidence of volatile organic compound interactions between soil bacterial and fungal isolates. Soil Biol. Biochem. 1999. [Google Scholar] [CrossRef]
- Bending, G.D.; Lincoln, S.D. Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products. Soil Biol. Biochem. 2000. [Google Scholar] [CrossRef]
- Orlandini, V.; Maida, I.; Fondi, M.; Perrin, E.; Papaleo, M.C.; Bosi, E.; de Pascale, D.; Tutino, M.L.; Michaud, L.; Lo Giudice, A.; et al. Genomic analysis of three sponge-associated Arthrobacter Antarctic strains, inhibiting the growth of Burkholderia cepacia complex bacteria by synthesizing volatile organic compounds. Microbiol. Res. 2014, 169, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef]
- Asensio, D.; Peñuelas, J.; Filella, I.; Llusià, J. On-line screening of soil VOCs exchange responses to moisture, temperature and root presence. Plant Soil 2007, 291, 249–261. [Google Scholar] [CrossRef]
- Gagliano, A.L.; Tagliavia, M.; D’Alessandro, W.; Franzetti, A.; Parello, F.; Quatrini, P. So close, so different: Geothermal flux shapes divergent soil microbial communities at neighbouring sites. Geobiology 2016. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Rosenblueth, M.; Martínez-Romero, E. Bacterial Endophytes and Their Interactions with Hosts. Mol. Plant-Microbe Interact. 2007, 19, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Glick, B.R. The Role of Plant Growth-Promoting Bacteria in Metal Phytoremediation, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 71, ISBN 0065-2911. [Google Scholar]
- Verma, P.; Yadav, A.N.; Kumar, V.; Singh, D.P.; Saxena, A.K. Beneficial plant-microbes interactions: Biodiversity of microbes from diverse extreme environments and its impact for crop improvement. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; ISBN 9789811065934. [Google Scholar]
- Fridriksson, S. Plant Colonization of a Volcanic Island, Surtsey, Iceland. Arct. Alp. Res. 2006, 19, 425. [Google Scholar] [CrossRef]
- Korablev, A.P.; Smirnov, V.E.; Neshataeva, V.Y.; Khanina, L.G. Plant Life-Forms and Environmental Filtering during Primary Succession on Loose Volcanic Substrata (Kamchatka, Russia). Biol. Bull. 2018, 45, 255–264. [Google Scholar] [CrossRef]
- Moral, R.; Wood, D.M. Early primary succession on the volcano Mount St. Helens. J. Veg. Sci. 2006. [Google Scholar] [CrossRef]
- Clarkson, B.D.; Clarkson, B.R.; Juvik, J.O. Pattern and process of vegetation change (succession) on two northern New Zealand island volcanoes. Surtsey Res. 2015, 45–48. [Google Scholar]
- Gabbianelli, G. Submarine morphology and tectonics of Vulcano (Aeolian Islands, Southern Tyrrhenian Sea). Acta Vulcanol. 1991, 1, 135–141. [Google Scholar]
- Calanchi, N.; Peccerillo, A.; Tranne, C.A.; Lucchini, F.; Rossi, P.L.; Kempton, P.; Barbieri, M.; Wu, T.W. Petrology and geochemistry of volcanic rocks from the island of Panarea: Implications for mantle evolution beneath the Aeolian island arc (Southern Tyrrhenian sea). J. Volcanol. Geotherm. Res. 2002, 115, 367–395. [Google Scholar] [CrossRef]
- Capasso, G.; Favara, R.; Inguaggiato, S. Chemical features and isotopic composition of gaseous manifestations on Vulcano Island, Aeolian Islands, Italy: An interpretative model of fluid circulation. Geochim. Cosmochim. Acta 1997. [Google Scholar] [CrossRef]
- Inguaggiato, S.; Mazot, A.; Diliberto, I.S.; Inguaggiato, C.; Madonia, P.; Rouwet, D.; Vita, F. Total CO2 output from Vulcano island (Aeolian Islands, Italy). Geochem. Geophys. Geosyst. 2012, 13, 1–19. [Google Scholar] [CrossRef]
- Garavelli, A.; LAviano, R.; Vurro, F. Sublimate deposition from hydrothermal fluids at the Fossa. Eur. J. Mineral. 1997, 9, 423–432. [Google Scholar] [CrossRef]
- Cheynet, B.; Dall’Aglio, M.; Garavelli, A.; Grasso, M.F.; Vurro, F. Trace elements from fumaroles at Vulcano Island (Italy): Rates of transport and a thermochemical model. J. Volcanol. Geotherm. Res. 2000. [Google Scholar] [CrossRef]
- Pinto, D.; Garavelli, A.; Mitolo, D. Baliczunicite, Bi2O(SO4)(2), a new fumarole mineral from La Fossa crater, Vulcano, Aeolian Islands, Italy. Mineral. Mag. 2014. [Google Scholar] [CrossRef]
- Demartin, F.; Campostrini, I.; Castellano, C.; Gramaccioli, C.M.; Russo, M. D’ansite-(Mn), Na21Mn2+(SO4)10Cl3 and d’ansite-(Fe), Na21Fe2+(SO4)10Cl3, two new minerals from volcanic fumaroles. Mineral. Mag. 2012. [Google Scholar] [CrossRef]
- Boatta, F.; D’Alessandro, W.; Gagliano, A.L.; Liotta, M.; Milazzo, M.; Rodolfo-Metalpa, R.; Hall-Spencer, J.M.; Parello, F. Geochemical survey of Levante Bay, Vulcano Island (Italy), a natural laboratory for the study of ocean acidification. Mar. Pollut. Bull. 2013, 73, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, G.; Cioni, R.; Guidi, M.; Raco, B.; Marini, L.; Chiodini, G.; Cioni, R.; Guidi, M.; Raco, B.; Marini, L. Soil CO2 flux measurements in volcanic and geothermal areas. Appl. Geochem. 1998, 13, 543–552. [Google Scholar] [CrossRef]
- Venturi, S.; Tassi, F.; Cabassi, J.; Vaselli, O.; Minardi, I.; Neri, S.; Caponi, C.; Capasso, G.; Di Martino, R.M.R.; Ricci, A.; et al. A multi-instrumental geochemical approach to assess the environmental impact of CO2-rich gas emissions in a densely populated area: The case of Cava dei Selci (Latium, Italy). Appl. Geochem. 2019, 101, 109–126. [Google Scholar] [CrossRef]
- Tassi, F.; Venturi, S.; Cabassi, J.; Capecchiacci, F.; Nisi, B.; Vaselli, O. Volatile organic compounds (VOCs) in soil gases from Solfatara crater (Campi Flegrei, Southern Italy): Geogenic source(s) vs. biogeochemical processes. Appl. Geochem. 2015, 56, 37–49. [Google Scholar] [CrossRef]
- Tassi, F.; Venturi, S.; Cabassi, J.; Vaselli, O.; Gelli, I.; Cinti, D.; Capecchiacci, F. Organic Geochemistry Biodegradation of CO2, CH4 and volatile organic compounds (VOCs) in soil gas from the Vicano—Cimino hydrothermal system (central Italy). Org. Geochem. 2015, 86, 81–93. [Google Scholar] [CrossRef]
- Venturi, S.; Tassi, F.; Magi, F.; Cabassi, J.; Ricci, A.; Capecchiacci, F.; Caponi, C.; Nisi, B.; Vaselli, O. Carbon isotopic signature of interstitial soil gases reveals the potential role of ecosystems in mitigating geogenic greenhouse gas emissions: Case studies from hydrothermal systems in Italy. Sci. Total Environ. 2019, 655, 887–898. [Google Scholar] [CrossRef]
- Chakravorty, S.; Helb, D.; Burday, M.; Connell, N.; Alland, D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J. Microbiol. Methods 2007. [Google Scholar] [CrossRef]
- Petrosino, J.F.; Highlander, S.; Luna, R.A.; Gibbs, R.A.; Versalovic, J. Metagenomic pyrosequencing and microbial identification. Clin. Chem. 2009, 55, 856–866. [Google Scholar] [CrossRef]
- Huse, S.M.; Dethlefsen, L.; Huber, J.A.; Welch, D.M.; Relman, D.A.; Sogin, M.L. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet. 2008. [Google Scholar] [CrossRef]
- Herlemann, D.P.R.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 1994, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Dada, S.P.H. High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2014. Available online: http//www.R-project.org/ (accessed on 20 August 2019).
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011. [Google Scholar] [CrossRef]
- Good, I.J. The Population Frequencies of Species and the Estimation of Population Parafeters. Biometrika 1953, 40, 237–264. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973. [Google Scholar] [CrossRef]
- Chiellini, C.; Maida, I.; Emiliani, G.; Mengoni, A.; Mocali, S.; Fabiani, A.; Biffi, S.; Maggini, V.; Gori, L.; Vannacci, A.; et al. Endophytic and rhizospheric bacterial communities isolated from the medicinal plants echinacea purpurea and echinacea angustifolia. Int. Microbiol. 2015, 17, 165–174. [Google Scholar] [CrossRef]
- Williams, J.G.K.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990. [Google Scholar] [CrossRef]
- Mori, E.; Liò, P.; Daly, S.; Damiani, G.; Perito, B.; Fani, R. Molecular nature of RAPD markers from Haemophilus influenzae Rd genome. Res. Microbiol. 1999. [Google Scholar] [CrossRef]
- Di Cello, F.; Fani, R. A molecular strategy for the study of natural bacterial communities by PCR-based techniques. Minerva Biotecnol. 1996, 8, 126–134. [Google Scholar]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014. [Google Scholar] [CrossRef]
- Capaccioni, B.; Tassi, F.; Vaselli, O. Organic and inorganic geochemistry of low temperature gas discharges at the Baia di Levante beach, Vulcano Island, Italy. J. Volcanol. Geotherm. Res. 2001, 108, 173–185. [Google Scholar] [CrossRef]
- Bacci, G.; Cerri, M.; Lastrucci, L.; Ferranti, F.; Ferri, V.; Foggi, B.; Gigante, D.; Venanzoni, R.; Viciani, D.; Mengoni, A.; et al. Applying predictive models to decipher rhizobacterial modifications in common reed die-back affected populations. Sci. Total Environ. 2018. [Google Scholar] [CrossRef]
- Chan, C.S.; Chan, K.G.; Ee, R.; Hong, K.W.; Urbieta, M.S.; Donati, E.R.; Shamsir, M.S.; Goh, K.M. Effects of physiochemical factors on prokaryotic Biodiversity in Malaysian circumneutral hot springs. Front. Microbiol. 2017. [Google Scholar] [CrossRef]
- Medrano-Santillana, M.; Souza-Brito, E.M.; Duran, R.; Gutierrez-Corona, F.; Reyna-López, G.E. Bacterial diversity in fumarole environments of the Paricutín volcano, Michoacán (Mexico). Extremophiles 2017. [Google Scholar] [CrossRef]
- Lanzén, A. Analysis of Sequencing Data in Environmental Genomics Exploring the Diversity of the Microbial Biosphere. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2013. [Google Scholar]
- Neilson, J.W.; Quade, J.; Ortiz, M.; Nelson, W.M.; Legatzki, A.; Tian, F.; LaComb, M.; Betancourt, J.L.; Wing, R.A.; Soderlund, C.A.; et al. Life at the hyperarid margin: Novel bacterial diversity in arid soils of the Atacama Desert, Chile. Extremophiles 2012, 16, 553–566. [Google Scholar] [CrossRef]
| No. | Code | Site | Sample |
|---|---|---|---|
| 1 | CPA | La Fossa Crater | Plant Aerial Part |
| 2 | CPR | La Fossa Crater | Plant Roots |
| 3 | CPRS | La Fossa Crater | Plant Rhizospheric Soil |
| 4 | CPBS | La Fossa Crater | Plant Bulk Soil |
| 5 | LPA | Levante Bay | Plant Aerial Part |
| 6 | LPR | Levante Bay | Plant Roots |
| 7 | LPRS | Levante Bay | Plant Rhizospheric Soil |
| 8 | LPBS | Levante Bay | Plant Bulk Soil |
| 9 | CSF94 | La Fossa Crater | Soil, 94 °C |
| 10 | CSF56 | La Fossa Crater | Soil, 56 °C |
| 11 | CSCS | La Fossa Crater | Soil from Cap Surface |
| 12 | LSV11 | Levante Bay | Soil, site V11 |
| 13 | LSV13 | Levante Bay | Soil, site V13 |
| 14 | LSV14 | Levante Bay | Soil, site V14 |
| No. | Code | Site | Sample | Bacterial Plate Count (CFU/g) | Number of Isolated Strains |
|---|---|---|---|---|---|
| 1 | CPA | La Fossa Crater | Plant Aerial Part | 1.5 ± 0.7 × 104 | 14 |
| 2 | CPR | La Fossa Crater | Plant Roots | 3.8 ± 0.6 × 104 | 5 |
| 3 | CPRS | La Fossa Crater | Plant Rhizospheric Soil | 2.7 ± 2.5 × 104 | 11 |
| 4 | CPBS | La Fossa Crater | Plant Bulk Soil | 9.5 ± 0.7 × 103 | 14 |
| 5 | LPA | Levante Bay | Plant Aerial Part | 1.03 ± 0.09 × 103 | 16 |
| 6 | LPR | Levante Bay | Plant Roots | 4.9 ± 0.9 × 103 | 16 |
| 7 | LPRS | Levante Bay | Plant Rhizospheric Soil | 1.1 × 104 | 9 |
| 8 | LPBS | Levante Bay | Plant Bulk Soil | 6.1 ± 1.4 × 104 | 14 |
| 9 | CSF94 | La Fossa Crater | Soil, 94 °C | 0 | 0 |
| 10 | CSF56 | La Fossa Crater | Soil, 56 °C | 0 | 0 |
| 11 | CSCS | La Fossa Crater | Soil | 5 ± 0.007 × 102 | 2 |
| 12 | LSV11 | Levante Bay | Soil | 1 ± 0.7 × 104 | 15 |
| 13 | LSV13 | Levante Bay | Soil | 1.2 ± 0.2 × 105 | 46 |
| 14 | LSV14 | Levante Bay | Soil | 1.4 ± 1.6 × 105 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagorzi, C.; Del Duca, S.; Venturi, S.; Chiellini, C.; Bacci, G.; Fani, R.; Tassi, F. Bacterial Communities from Extreme Environments: Vulcano Island. Diversity 2019, 11, 140. https://doi.org/10.3390/d11080140
Fagorzi C, Del Duca S, Venturi S, Chiellini C, Bacci G, Fani R, Tassi F. Bacterial Communities from Extreme Environments: Vulcano Island. Diversity. 2019; 11(8):140. https://doi.org/10.3390/d11080140
Chicago/Turabian StyleFagorzi, Camilla, Sara Del Duca, Stefania Venturi, Carolina Chiellini, Giovanni Bacci, Renato Fani, and Franco Tassi. 2019. "Bacterial Communities from Extreme Environments: Vulcano Island" Diversity 11, no. 8: 140. https://doi.org/10.3390/d11080140
APA StyleFagorzi, C., Del Duca, S., Venturi, S., Chiellini, C., Bacci, G., Fani, R., & Tassi, F. (2019). Bacterial Communities from Extreme Environments: Vulcano Island. Diversity, 11(8), 140. https://doi.org/10.3390/d11080140







