Biogeography, Systematics, and Ecomorphology of Pacific Island Anoles
Abstract
1. Introduction
1.1. Isla Malpelo
1.2. Isla Cocos
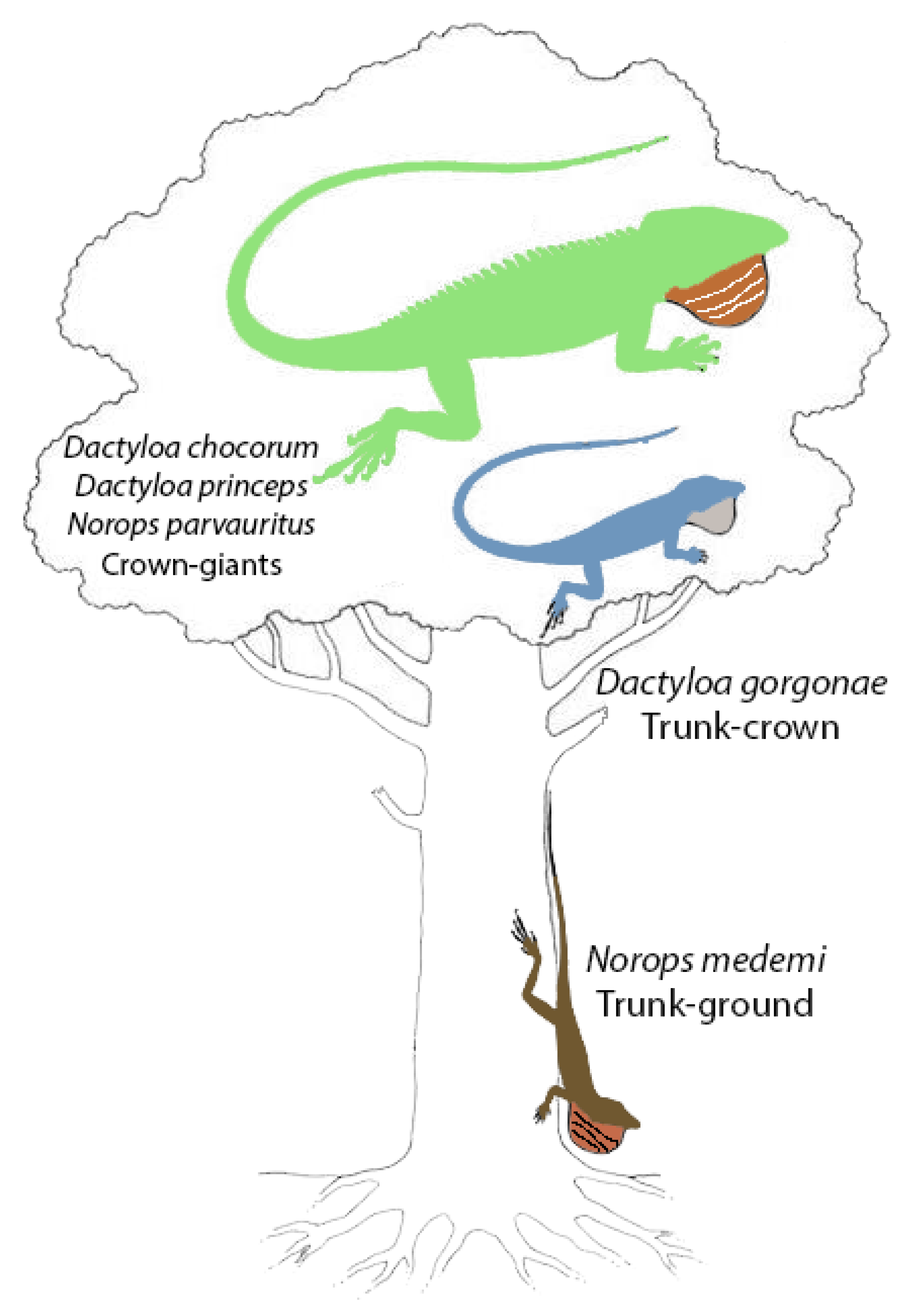
1.3. Isla Gorgona
2. Methods
2.1. DNA Sequencing and Phylogenetic Reconstruction
2.2. Morphology and Ecology
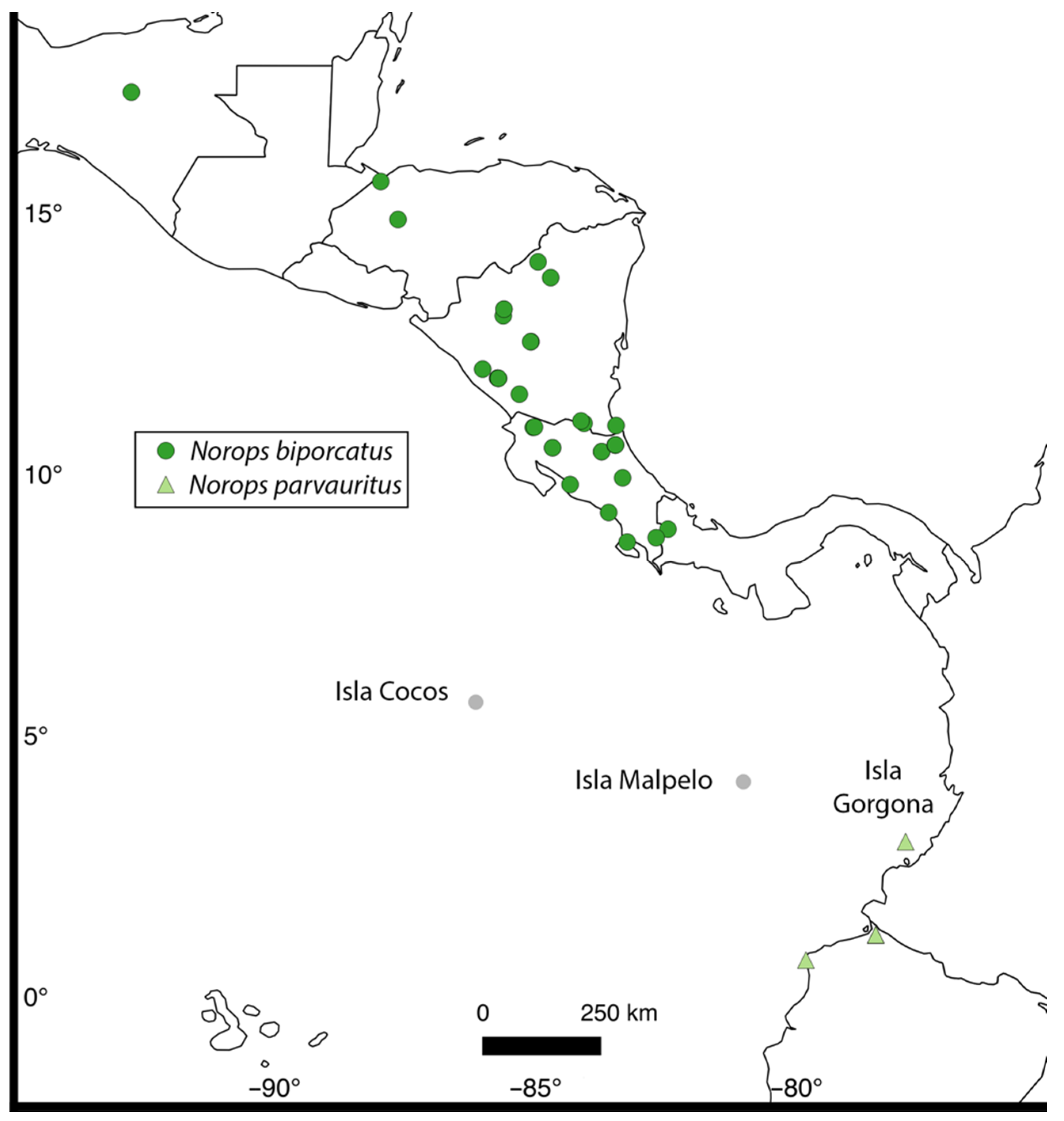
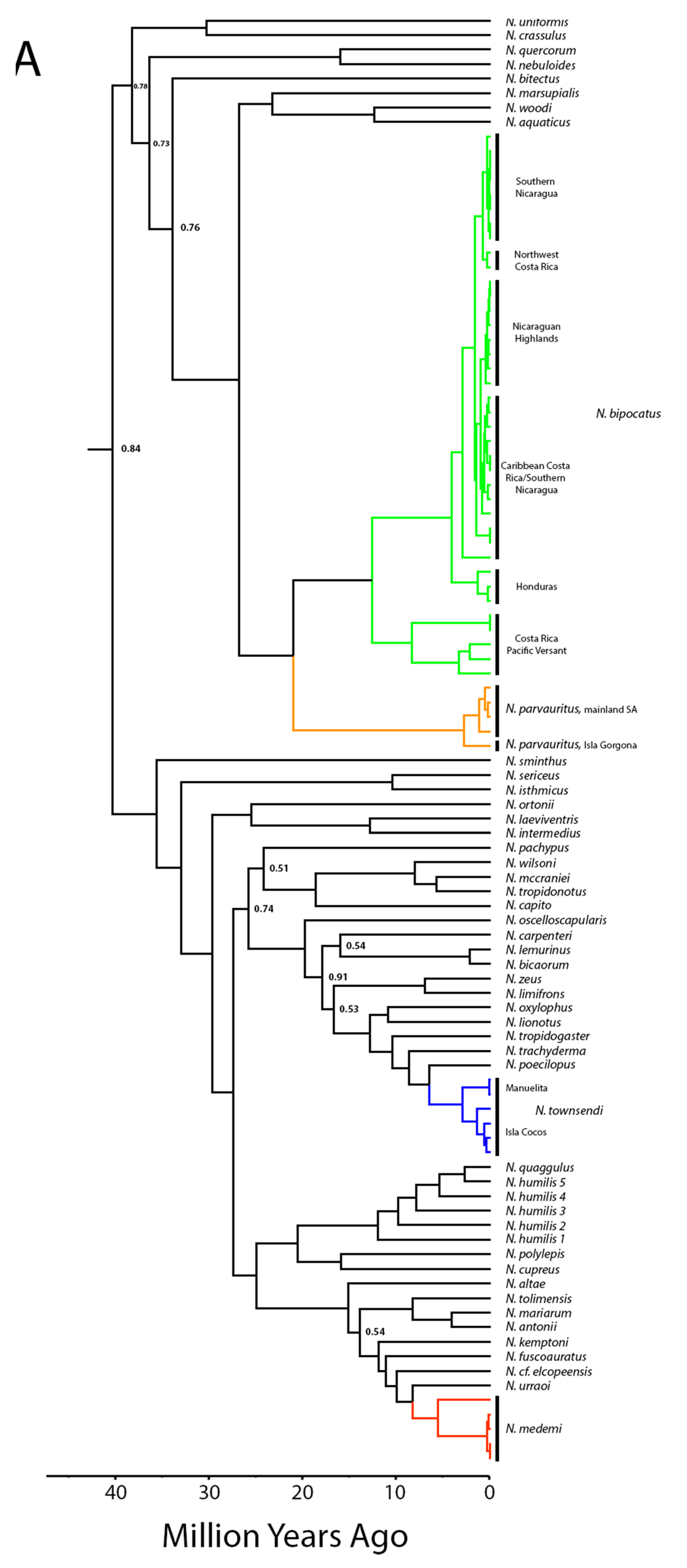
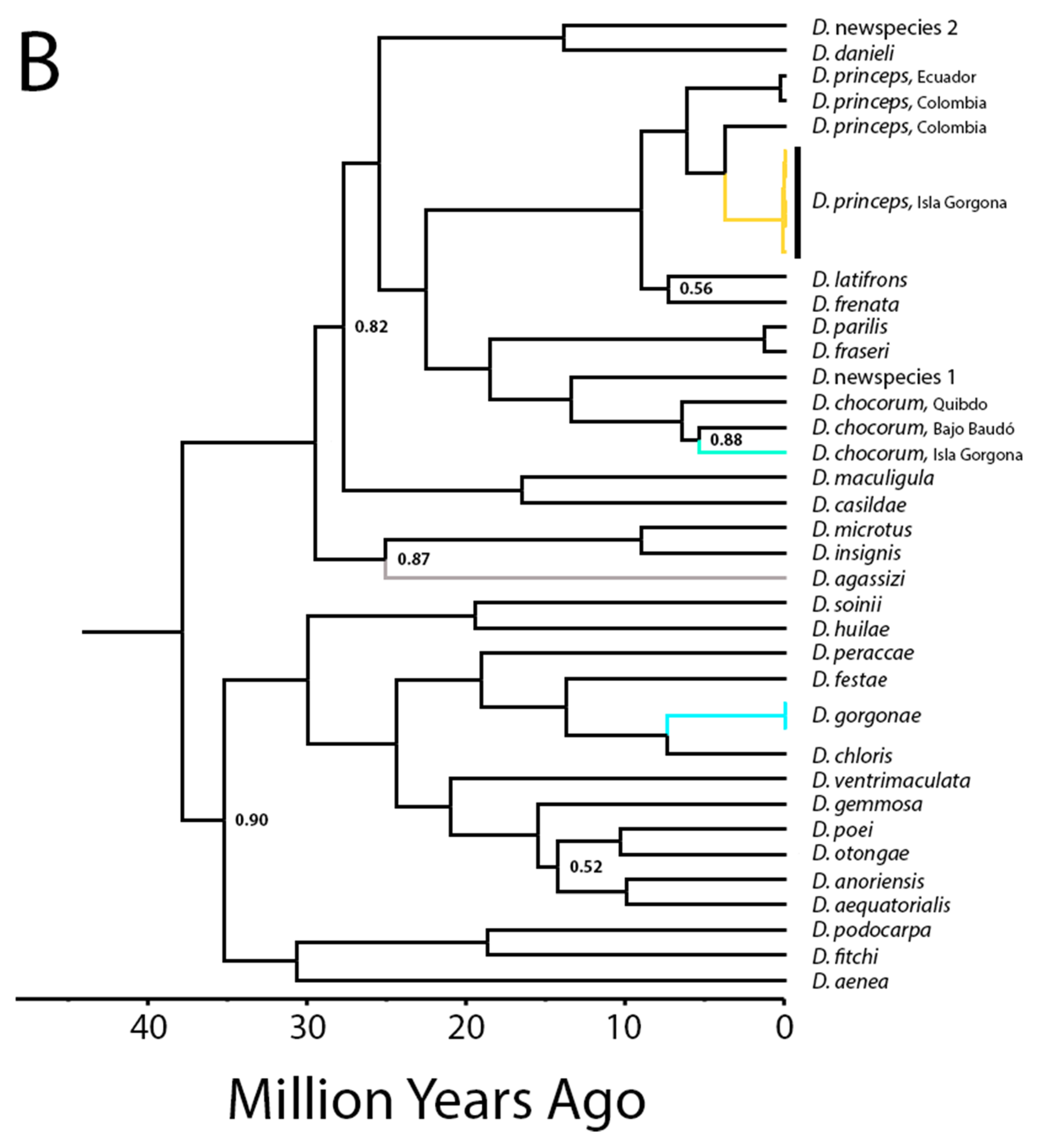
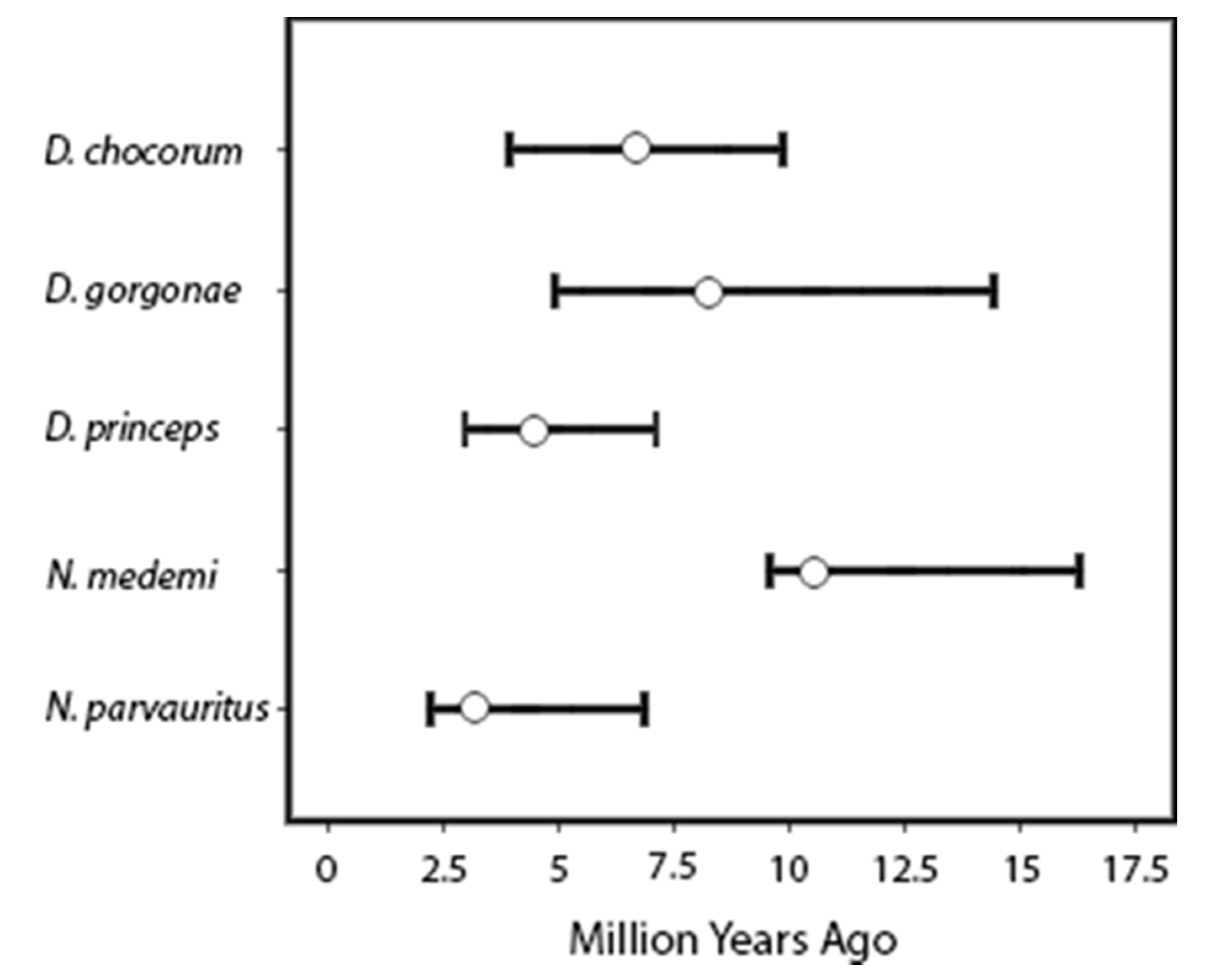
3. Results
Morphological Data
4. Discussion
4.1. Biogeographic and Phylogenetic Interpretations
4.2. Taxonomic Implications
4.3. Natural History Observations
4.4. Conservation of Island Fauna
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiba, S. Ecological and morphological patterns in communities of land snails of the genus Mandarina from the Bonin Islands. J. Evol. Biol. 2004, 17, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R. Community assembly through adaptive radiation in Hawaiian spiders. Science 2004, 303, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Parent, C.E.; Caccone, A.; Petren, K. Colonization and diversification of Galápagos terrestrial fauna: A phylogenetic and biogeographical synthesis. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3347–3361. [Google Scholar] [CrossRef] [PubMed]
- Carlquist, S.J. Island Biology; Columbia University Press: New York, NY, USA, 1974. [Google Scholar]
- Hendriks, K.P.; Alciatore, G.; Schilthuizen, M.; Etienne, R.S. Phylogeography of Bornean land snails suggests long-distance dispersal as a cause of endemism. J. Biogeogr. 2019, 46, 932–944. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Ali, J.R.; Aitchison, J.C. Exploring the combined role of eustasy and oceanic island thermal subsidence in shaping biodiversity on the Galápagos. J. Biogeogr. 2014, 41, 1227–1241. [Google Scholar] [CrossRef]
- Williams, E.E. A critique of Guyer and Savage (1986): Cladistic relationships among anoles (Sauria: Iguanidae): Are the data available to reclassify the anoles? In Biogeography of the West Indies: Past, Present, and Future; Sandhill Crane Press: Gainesville, FL, USA, 1989; pp. 286–341. [Google Scholar]
- Crother, B.I.; Parenti, L.R. Assumptions Inhibiting Progress in Comparative Biology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Guyer, C.; Savage, J.M. Cladistic relationships among anoles (Sauria: Iguanidae). Syst. Zool. 1986, 35, 509–531. [Google Scholar] [CrossRef]
- Nicholson, K.E.; Crother, B.I.; Guyer, C.; Savage, J.M. Translating a clade-based classification into one that is valid under the international code of zoological nomenclature: The case of the lizards of the family Dactyloidae (Order Squamata). Zootaxa 2018, 4461, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 29 June 2019).
- Losos, J.B. Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles; University of California Press: Berkeley, CA, USA, 2009. [Google Scholar]
- Irschick, D.L.; Vitt, L.J.; Zani, P.A.; Losos, J.B. A comparison of evolutionary radiations in mainland and West Indian Anolis lizards. Ecology 1997, 78, 2191–2203. [Google Scholar] [CrossRef]
- Irschick, D.L.; Losos, J.B. A comparative analysis of the ecological significance of maximal locomotor performance in Caribbean Anolis lizards. Evolution 1998, 52, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Losos, J.B. The evolution of convergent community structure in Caribbean Anolis communities. Syst. Biol. 1992, 41, 403–420. [Google Scholar] [CrossRef]
- Losos, J.B.; Warheit, K.I.; Schoener, T.W. Adaptive differentiation following experimental island colonization in Anolis lizards. Nature 1997, 387, 70–73. [Google Scholar] [CrossRef]
- Williams, E.E. The ecology of colonization as seen in the zoogeography of Anolis lizards on small islands. Q. Rev. Biol. 1969, 44, 345–389. [Google Scholar] [CrossRef]
- Hedges, S.B.; Hass, C.A.; Maxson, L.R. Caribbean biogeography: Molecular evidence for dispersal in West Indian terrestrial vertebrates. Proc. Natl. Acad. Sci. USA 1992, 89, 1909–1913. [Google Scholar] [CrossRef]
- Poe, S.; Nieto-Montes de Oca, A.; Torres-Carvajal, O.; de Queiroz, K.; Velasco, J.A.; Truett, B.; Gray, L.N.; Ryan, M.J.; Köhler, G.; Ayala-Varela, F.; et al. A phylogenetic, biogeographic, and taxonomic study of all extant species of Anolis (Squamata; Iguanidae). Syst. Biol. 2017, 66, 663–697. [Google Scholar] [CrossRef]
- Nicholson, K.E.; Crother, B.I.; Guyer, C.; Savage, J.M. It is time for a new classification of anoles. Zootaxa 2012, 3477, 1–108. [Google Scholar] [CrossRef]
- Schoener, T.W. Size patterns in West Indian Anolis lizards: I. size and species diversity. Syst. Biol. 1969, 18, 386–401. [Google Scholar] [CrossRef]
- Blankers, T.; Townsend, T.M.; Pepe, K.; Reeder, T.W.; Wiens, J.J. Contrasting global-scale evolutionary radiations: Phylogeny, diversification, and morphological evolution in the major clades of iguanian lizards. Biol. J. Linn. Soc. 2013, 108, 127–143. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Tavera, J.; Castañeda, D.R. When did anoles diverge? An analysis of multiple dating strategies. Mol. Phylogenet. Evol. 2018, 127, 655–668. [Google Scholar] [CrossRef]
- Poe, S.; Goheen, J.R.; Hulebak, E.P. Convergent exaptation and adaptation in solitary island lizards. Proc. R. Soc. B Biol. Sci. 2007, 274, 2231–2237. [Google Scholar] [CrossRef]
- Hoernle, K.; van den Bogaard, P.; Werner, R.; Lissinna, B.; Hauff, F.; Alvarado, G.; Garbe-Schönberg, D. Missing history (16–17 Ma) of the Galápagos hotspot: Implications for the tectonic and biological evolution of the Americas. Geology 2002, 30, 795–798. [Google Scholar] [CrossRef]
- Stead, J.A. Field observations of the geology of Malpelo Island. Smithson. Contrib. Zool. 1975, 176, 17–26. [Google Scholar]
- Gorman, G.C.; Chorba, T.L. Terrestrial biology of Malpelo Island: A historical review. Smithson. Contrib. Zool. 1975, 176, 9–12. [Google Scholar]
- Hertlein, L.G. Contribution to the biogeography of Cocos Island, including a bibliography. Proc. Calif. Acad. Sci. USA 1963, 32, 219–289. [Google Scholar]
- Kiester, A.R.; Hoffman, J.A. Reconnaissance and mapping of Malpelo Island. Smithson. Contrib. Zool. 1975, 176, 13–20. [Google Scholar]
- Wolda, H. The ecosystem on Malpelo Island. Smithson. Contrib. Zool. 1975, 176, 21–26. [Google Scholar]
- López-Victoria, M.; Wolters, V.; Werding, B. Nazca Booby (Sula granti) inputs maintain the terrestrial food web of Malpelo Island. J. Ornithol. 2009, 150, 865–870. [Google Scholar] [CrossRef]
- López-Victoria, M. The lizards of Malpelo (Colombia): Some topics on their ecology and threats. Caldesia 2006, 28, 129–134. [Google Scholar]
- Rand, A.S.; Gorman, G.C.; Rand, W.M. Natural history, behavior, and ecology and Anolis agassizi. Smithson. Contrib. Zool. 1975, 176, 27–38. [Google Scholar]
- Castañeda, M.D.R.; de Queiroz, K. Phylogenetic relationships of the Dactyloa clade of Anolis lizards based on nuclear and mitochondrial DNA sequence data. Mol. Phylogenetics Evol. 2011, 61, 784–800. [Google Scholar] [CrossRef]
- Castañeda, M.D.R.; de Queiroz, K. Phylogeny of the Dactyloa clade of Anolis lizards: New insights from combining morphological and molecular data. Bull. Mus. Comp. Zool. 2013, 160, 345–398. [Google Scholar] [CrossRef]
- Prates, I.; Rodrigues, M.T.; Melo-Sampaio, P.R.; Carnaval, A.C. Phylogenetic relationships of Amazonian anole lizards (Dactyloa): Taxonomic implications, new insights about phenotypic evolution and the timing of diversification. Mol. Phylogenetics Evol. 2015, 82, 258–268. [Google Scholar] [CrossRef]
- Carpenter, C.C. The display of the Cocos Island anole. Herpetologica 1965, 21, 256–260. [Google Scholar]
- Castillo, P.; Batiza, R.; Vanko, D.; Malavassi, E.; Barquero, J.; Fernandez, E. Anomalously young volcanoes on old hot-spot traces. I. Geology and petrology of Cocos Island. Geology and petrology of Cocos Island. Geol. Soc. Am. Bull. 1998, 100, 1400–1414. [Google Scholar] [CrossRef]
- Poe, S. Phylogeny of anoles. Herpetol. Monogr. 2004, 18, 37–89. [Google Scholar] [CrossRef]
- Grisales-Martínez, F.A.; Velasco, J.A.; Bolívar, W.; Williams, E.E.; Daza, J.M. The taxonomic and phylogenetic status of some poorly known Anolis species from the Andes of Colombia with the description of a nomen nudum taxon. Zootaxa 2017, 4303, 213–230. [Google Scholar] [CrossRef]
- Nicholson, K.E.; Guyer, C.; Phillips, J.G. Biogeographic origin of mainland Norops (Squamata: Dactyloidae). In Assumptions Inhibiting Progress in Comparative Biology; CRC Press: Boca Raton, FL, USA, 2017; pp. 169–184. [Google Scholar]
- Phillips, J.G.; Deitloff, J.; Guyer, C.; Hutteman, S.; Nicholson, K.E. Biogeography and evolution of a widespread Central American lizard species complex: Norops humilis, (Squamata: Dactyloidae). BMC Evol. Biol. 2015, 15, 143. [Google Scholar] [CrossRef]
- Castro, F.; Ayala, S.; Carvajal, H. Los saurios de las islas Gorgona y Gorgonilla. In Gorgona; Universidad de los Andes: Cundinamarca, Colombia, 1979; pp. 189–218. [Google Scholar]
- UASPNN. El Sistema de Parques Nacionales Naturales de Colombia; Unidad Administrativa Especial del Sistema de Parques Nacionales Naturales (UASPNN), Ministerio del Medio Ambiente. Editorial Nomos: Bogotá, Colombia, 1998. [Google Scholar]
- Echeverría, L.M.; Aitken, B. Pyroclastic rocks: Another manifestation of ultramafic volcanism of Gorgona Island. Colombia. Contrib. Mineral. Petrol. 1986, 92, 428–436. [Google Scholar] [CrossRef]
- Gomez, H. Algunos aspectos neotectónicos hacia el suroeste del Litoral Pacífico colombiano. Rev. CIAF 1986, 11, 281–296. [Google Scholar]
- Kerr, A.C. La Isla de Gorgona, Colombia: A petrological enigma? Lithos 2005, 84, 77–101. [Google Scholar] [CrossRef]
- Serrano, L.; Ferrari, L.; Martínez, M.L.; Petrone, C.M.; Jaramillo, C. An integrative geologic, geochronologic and geochemical study of Gorgona Island, Colombia: Implications for the formation of the Caribbean Large Igneous Province. Earth Planet. Sci. Lett. 2011, 309, 324–336. [Google Scholar] [CrossRef]
- Von Prahl, H.; Guhl, F.; Grögl, M. Gorgona; Futura Groupo Editorial Ltd.: Bogota, Colombia, 1979. [Google Scholar]
- Aguirre, J.; Rangel, O. La isla Gorgona y sus ecosistemas. In Colombia Pacífico; Fondo para la Protección del Medio Ambiente (FEN): Bogotá, Colombia, 1993; Volume I, pp. 106–170. [Google Scholar]
- Alberico, M. Biogeografía terrestre. Capitulo XI. In Isla de Gorgona; Banco Popular: Bogotá, Colombia, 1986; pp. 223–244. [Google Scholar]
- Murillo, M.T.; Lozano, G. Hacia la realización de una flórula del Parque Nacional Natural Islas de Gorgona y Gorgonilla, Cauca, Colombia. Rev. Acad. Col. Cie. Exactas Físicas y Naturales 1989, 12, 277–304. [Google Scholar]
- Yockteng, R.; Cavelier, J. Diversidad y mecanismos de dispersión de árboles de la Isla Gorgona y de los bosques húmedos tropicales del Pacífico colombo-ecuatoriano. Rev. Biol. Trop. 1998, 46, 45–53. [Google Scholar]
- Armstead, J.V.; Ayala-Varela, F.; Torres-Carvajal, O.; Ryan, M.J.; Poe, S. Systematics and ecology of Anolis biporcatus (Squamata: Iguanidae). Salamandra 2017, 53, 285–293. [Google Scholar]
- Köhler, G.; McCranie, J.R.; Nicholson, K.E.; Kreutz, J. Geographic variation in hemipenial morphology in Norops humilis (Peters 1863), and the systematic status of Norops quaggulus (Cope 1885) (Reptilia, Squamata, Polychrotidae). Senckenbergiana Biol. 2003, 82, 213–222. [Google Scholar]
- Selander, R.K. Sexual dimorphism and differential niche utilization in birds. Condor 1966, 68, 113–151. [Google Scholar] [CrossRef]
- Clegg, S.M.; Owens, P.F. The ‘island rule’ in birds: Medium body size and its ecological explanation. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 1359–1365. [Google Scholar] [CrossRef]
- Dayan, T.; Simberloff, D. Size patterns among competitors: Ecological character displacement and character release in mammals, with special reference to island populations. Mammal Rev. 1998, 28, 99–124. [Google Scholar] [CrossRef]
- Schoener, T.W. The ecological significance of sexual dimorphism in size in the lizard Anolis conspersus. Science 1967, 155, 474–477. [Google Scholar] [CrossRef]
- Schoener, T.W. Size patterns in West Indian Anolis lizards. II. Correlations with the sizes of particular sympatric species-displacement and convergence. Am. Nat. 1970, 104, 155–174. [Google Scholar] [CrossRef]
- Velasco, J.A.; Poe, S.; González-Salazar, C.; Flores-Villela, O. Solitary ecology as a phenomenon extending beyond insular systems: Exaptive evolution in Anolis lizards. Biol. Lett. 2019, 15, 20190056. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v.1.6. 2014. Available online: http://tree.bio.ed.ac.uk/software/tracer/ (accessed on 9 August 2018).
- Macey, J.R.; Schulte II, J.A.; Ananjeva, N.B.; Larson, A.; Rastegar-Pouyani, N.; Shammakov, S.M.; Papenfuss, T.J. Phylogenetic relationships among Agamid Lizards of the Laudakia caucasia species group: Testing hypotheses of biogeographic fragmentation and an area cladogram for the Iranian Plateau. Mol. Phylogenetics Evol. 1998, 10, 118–131. [Google Scholar] [CrossRef]
- Glor, R.E.; Kolbe, J.J.; Powell, R.; Larson, A.; Losos, J.B. Phylogenetic analysis of ecological and morphological diversification in Hispaniolan trunk-ground anoles (Anolis cybotes group). Evolution 2003, 57, 2383–2397. [Google Scholar] [CrossRef]
- Glor, R.E.; Losos, J.B.; Larson, A. Out of Cuba: Overwater dispersal and speciation among lizards in the Anolis carolinensis subgroup. Mol. Ecol. 2005, 14, 2419–2432. [Google Scholar] [CrossRef]
- Guarnizo, C.E.; Werneck, F.P.; Giugliano, L.G.; Santos, M.G.; Fenker, J.; Sousa, L.; D’Angiolella, A.B.; dos Santos, A.R.; Strüssmann, C.; Rodrigues, M.T.; et al. Cryptic lineages and diversification of an endemic lizard (Squamata, Dactyloidae) of the Cerrado hotspot. Mol. Phylogenetics Evol. 2016, 94, 279–289. [Google Scholar] [CrossRef]
- Ng, J.; Glor, R.E. Genetic differentiation among populations of a Hispaniolan trunk anole that exhibit geographical variation in dewlap color. Mol. Ecol. 2011, 20, 4302–4317. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.G.; Strickland, T.R.; Kolbe, J.J.; Falk, B.G.; Perry, G.; Revell, L.J.; Losos, J.B. Archipelagic genetics in a widespread Caribbean anole. J. Biogeogr. 2017, 44, 2631–2647. [Google Scholar] [CrossRef]
- Torres-Carvajal, O.; de Queiroz, K. Phylogeny of hoplocercine lizards (Squamata: Iguania) with estimates of relative divergence times. Mol. Phylogenetics Evol. 2009, 50, 31–43. [Google Scholar] [CrossRef]
- Zheng, Y.; Peng, R.; Kuro-o, M.; Zeng, X. Exploring patterns and extent of bias in estimating divergence time from mitochondrial DNA sequence data in a particular lineage: A case study of salamanders (Order Caudata). Mol. Biol. Evol. 2011, 28, 2521–2535. [Google Scholar] [CrossRef]
- Poe, S.; Scarpetta, S.; Schaad, E.W. A new species of Anolis (Squamata: Iguanidae) from Panama. Amphib. Reptile Conserv. 2015, 9, 1–13. [Google Scholar]
- Macedonia, J.M.; Clark, D.L. Headbob display structure in the naturalized Anolis lizards of Bermuda: Sex, context, and population effects. J. Herpetol. 2003, 37, 266–277. [Google Scholar] [CrossRef]
- Thorpe, R.S.; Barlow, A.; Malhotra, A.; Surget-Groba, Y. Widespread parallel population adaptation to climate variation across a radiation: Implications for adaptation to climate change. Mol Ecol. 2015, 24, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Espinosa, M.L.; Forero, A.B. Morphological diversification in solitary endemic anoles: Anolis concolor and Anolis pinchoti from San Andrés and Providence Islands, Colombia. South Am. J. Herpetol. 2011, 6, 205–210. [Google Scholar] [CrossRef]
- Gartner, G.E.A.; Gamble, T.; Jaffe, A.L.; Harrison, A.; Losos, J.B. Left-right dewlap asymmetry and phylogeography of Anolis lineatus on Aruba and Curaçao. Biol. J. Linn. Soc. 2013, 110, 409–426. [Google Scholar] [CrossRef][Green Version]
- Williams, E.E. Ecomorphs, faunas, island size, and diverse end points in island radiations of Anolis. In Lizard Ecology: Studies of a Model Organism; Harvard University Press: Cambridge, MA, USA, 1983; pp. 326–370. [Google Scholar]
- Savage, J.M.; Talbot, J.J. The giant anoline lizards of Costa Rica and western Panama. Copeia 1978, 1978, 480–492. [Google Scholar] [CrossRef]
- Williams, E.E. New or problematic Anolis from Colombia. V. Anolis danieli, a new species of the latifrons species group and a reassessment of Anolis apollinaris Boulenger, 1919. Breviora 1988, 489, 1–25. [Google Scholar]
- Farris, D.W.; Jaramillo, C.; Bayona, G.; Restrepo-Moreno, S.A.; Montes, C.; Cardona, A.; Mora, A.; Speakman, R.J.; Glascock, M.D.; Valencia, V. Fracturing of the Panamanian Isthmus during initial collision with South America. Geology 2011, 39, 1007–1010. [Google Scholar] [CrossRef]
- Montes, C.; Cardona, A.; Jaramillo, C.; Pardo, A.; Silva, J.C.; Valencia, V.; Ayala, C.; Pérez-Angel, L.C.; Rodriguez-Parra, L.A.; Ramirez, V.; et al. Middle Miocene closure of the Central American seaway. Science 2012, 348, 226–229. [Google Scholar] [CrossRef]
- Elmer, K.R.; Bonett, R.M.; Wake, D.B.; Lougheed, S.C. Early Miocene origin and cryptic diversification of South American salamanders. BMC Evol. Biol. 2013, 13, 59. [Google Scholar] [CrossRef]
- Montes, C.; Cardona, A.; McFadden, R.; Morón, S.E.; Silva, C.A.; Restrepo-Moreno, S.; Bayona, G.A. Evidence for middle Eocene and younger land emergence in central Panama: Implications for Isthmus closure. Geol. Soc. Am. Bull. 2012, 124, 780–799. [Google Scholar] [CrossRef]
- Coates, A.G.; Obando, J.A. The geologic evolution of the Central American isthmus. In Evolution and Environment in Tropical America; University of Chicago Press: Chicago, IL, USA, 1996; pp. 21–56. [Google Scholar]
- MacFadden, B.J. Extinct mammalian biodiversity of the ancient New World tropics. Trends Ecol. Evol. 2006, 21, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Censky, E.J.; Hodge, K.; Dudley, J. Over-water dispersal of lizards due to hurricanes. Nature 1988, 395, 556. [Google Scholar] [CrossRef]
- Etheridge, R.E. The Relationships of the Anoles (Reptilia: Sauria: Iguanidae): An Interpretation Based on Skeletal Morphology. Ph.D. Thesis, University Microfilms, Ann Arbor, MI, USA, 1959. [Google Scholar]
- Savage, J.M.; Guyer, C. Infrageneric classification and species composition of the anole genera, Anolis, Ctenonotus, Dactyloa, Norops, and Semiurus (Sauria: Iguanidae). Amphib. Reptil. 1989, 10, 105–116. [Google Scholar] [CrossRef]
- Caccone, A.; Gibbs, J.P.; Ketmaier, V.; Suatoni, E.; Powell, J.R. Origin and evolutionary relationships of giant Galapagos tortoises. Proc. Natl. Acad. Sci. USA 1999, 96, 13223–13228. [Google Scholar] [CrossRef] [PubMed]
- Caccone, A.; Gentile, G.; Gibbs, J.P.; Fritts, T.H.; Snell, H.L.; Betts, J.; Powell, J.R. Phylogeography and history of giant Galapagos tortoises. Evolution 2002, 56, 2052–2066. [Google Scholar]
- Huber, M.; Caballero, R. Eocene El Nino: Evidence for robust tropical dynamics in the “hothouse”. Science 2003, 299, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.V.; Dekens, P.S.; McCarthy, M.; Ravelo, A.C.; Barreiro, M.; Pacanowski, R.C.; Philander, S.G. The Pliocene paradox (mechanisms for a permanent El Niño). Science 2006, 312, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.J.; Chong, G.; Houston, J.; Mather, A.E. 150 million years of climate stability: Evidence from the Atacama Desert, northern Chile. J. Geol. Soc. 2005, 162, 421–444. [Google Scholar] [CrossRef]
- Bessudo, S.; Soler, G.A.; Klimley, A.P.; Ketchum, J.T.; Hearn, A.; Arauz, R. Residency of the scalloped hammerhead shark (Sphyrna lewini) at Malpelo Island and evidence of migration to other islands in the Eastern Tropical Pacific. Environ. Biol. Fishes 2011, 91, 165–176. [Google Scholar] [CrossRef]
- Jiménez, L.; Acosta, A.; Chong, N. Population structure of Megabalanus peninsularis in Malpelo Island, Colombia. Rev. Biol. Trop. 2016, 51, 461–468. [Google Scholar]
- O’Connor, M.; Bruno, J.; Gaines, S. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl. Acad. Sci. USA 2007, 104, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Rovirosa, F.; Richards, M. Emergence/subsidence histories along the Carnegie and Cocos Ridges and their bearing upon biological speciation in the Galápagos. Geochem. Geophys. Geosystems 2018, 19, 4099–4129. [Google Scholar] [CrossRef]
- Savage, J.M. The Amphibians and Reptiles of Costa Rica: A Herpetofauna Between Two Continents, Between Two Seas; University of Chicago Press: Chicago, IL, USA, 2002; p. 954. [Google Scholar]
- Losos, J.B.; de Queiroz, K. Evolutionary consequences of ecological release in Caribbean Anolis lizards. Biol. J. Linn. Soc. 1997, 61, 459–483. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Cubas, J.; Irl, S.D.; Villafuerte, R.; Bello-Rodríguez, V.; Rodríguez-Luengo, J.L.; del Arco, M.; Martín-Esquivel, J.L.; González-Mancebo, J.M. Endemic plant species are more palatable to introduced herbivores than non-endemics. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2019, 286, 20190136. [Google Scholar] [CrossRef]
- Regnier, C.; Achaz, G.; Lambert, A.; Cowie, R.H.; Bouchet, P.; Fontaine, B. Mass extinction in poorly known taxa. Proc. Natl. Acad. Sci. USA 2015, 112, 7761–7766. [Google Scholar] [CrossRef]
- Solem, A. How many Hawaiian land snail species are left and what can we do for them? Bish. Mus. Occas. Pap. 1990, 30, 27–40. [Google Scholar]


| Node | Fossil | 95% | Rate | 95% |
|---|---|---|---|---|
| Dactyloa agassizi + Dactyloa insignis/Dactyloa microtus | 29.2 | 21.6–37.2 | 33.4 | 22.4–36.4 |
| Dactyloa chocorum from Isla Gorgona vs. mainland | 6.3 | 3.8–9.9 | 6.7 | 4.1–9.1 |
| All Dactyloa chocorum coalescence | 7.6 | 4.8–11.2 | 7.3 | 5.3–10.5 |
| Dactyloa princeps from Isla Gorgona vs. mainland | 4.5 | 2.7–7.1 | 3.0 | 2.8–6.5 |
| All Dactyloa princeps coalescence | 7.3 | 5.1–10.5 | 7.4 | 5.2–9.4 |
| Dactyloa chloris + Dactyloa gorgonae | 8.6 | 4.9–13.2 | 12.8 | 5.3–12.2 |
| Norops medemi coalescence (within Isla Gorgona) | 6.6 | 5.2–9.6 | 6.1 | 5.0–8.0 |
| Norops medemi + Norops urraoi | 9.6 | 10.2–16.3 | 8.1 | 7.7–11.8 |
| Norops townsendi coalescence (Isla Cocos vs. Manuelita) | 3.5 | 2.2–4.7 | 3.1 | 2.4–4.9 |
| Norops poecilopus + Norops townsendi | 7.6 | 5.1–10.1 | 6.7 | 5.6–10.0 |
| Norops parvauritus coalescence | 3.2 | 2.4–6.6 | 3.8 | 2.0–4.8 |
| Norops biporcatus coalescence | 14.7 | 11.6–20.7 | 12.5 | 11.4–18.3 |
| Norops biporcatus + Norops parvauritus | 24.4 | 20.2–32.7 | 21.3 | 19.4–29.7 |
| Sex/Species | N | SVL (mm) | Mass (g) | # Lamelle | PH (m) | PD (m) | # Mites |
|---|---|---|---|---|---|---|---|
| Isla Malpelo | |||||||
| Dactyloa agassizi, female | 18 | 67.3 (± 5.9) | 7.6 (± 2.2) | 36.8 (± 1.7) | 1.4 (± 1.2) | 0.3 (± 0.3) | |
| Dactyloa agassizi, male | 29 | 92.9 (± 9.0) | 24.1 (± 6.9) | 37.1 (± 1.7) | 1.5 (± 1.1) | 0.3 (± 0.4) | |
| p | <0.0001 | <0.0001 | 0.53 | 0.76 | 0.88 | ||
| Isla Cocos | |||||||
| Norops townsendi, female | 16 | 46.6 (± 4.2) | 2.1 (± 0.7) | 18.5 (± 1.6) | 0.9 (± 0.6) | 0.07 (± 0.06) | 5.2 (± 6.4) |
| Norops townsendi, male | 61 | 50.5 (± 4.0) | 2.6 (± 0.8) | 18.5 (± 1.2) | 1.4 (± 1.2) | 0.09 (± 1.0) | 7.9 (± 13.3) |
| p | 0.002 | 0.05 | 0.83 | 0.12 | 0.53 | 0.52 | |
| Isla Gorgona | |||||||
| Dactyloa princeps, female | 10 | 102.1 (± 14.0) | 21.1 (± 8.4) | 22.9 (± 0.9) | 2.4 (± 1.3) | 17.3 (± 9.6) | |
| Dactyloa princeps, male | 9 | 139.8 (± 7.9) | 49.1 (± 7.3) | 24.0 (± 1.1) | 3.6 (± 1.3) | 11.4 (± 4.9) | |
| p | <0.0001 | <0.0001 | 0.04 | 0.07 | 0.12 | ||
| Norops medemi, female | 5 | 51.0 (± 2.0) | 2.3 (± 0.2) | 15.0 (± 0.0) | 3.1 (± 2.3) | 5.2 (± 4.5) | |
| Norops medemi, male | 5 | 48.8 (± 3.9) | 1.9 (± 0.3) | 15.8 (± 0.5) | 2.0 (± 0.2) | 5.9 (± 7.7) | |
| p | 0.30 | 0.02 | 0.01 | 0.37 | 0.87 |
| Species | Male SVL (mm) | Female SVL (mm) | F/M Ratio | Reference |
|---|---|---|---|---|
| Dactyloa agassizi | 92.9 (± 9.0) | 67.3 (± 5.9) | 0.72 | This study |
| Dactyloa extrema | 65.9 | 52 | 0.79 | [74] |
| Dactyloa luciae | 72.6 | 52.1 | 0.72 | [75] |
| Dactyloa roquet | 67.3 (± 7.5) | 52.1 (± 6.3) | 0.77 | [22] |
| Norops concolor | 69.3 (± 0.7) | 50.1 (± 0.3) | 0.72 | [76] |
| Norops lineatus | 69.1 | 58.1 | 0.84 | [77] |
| Norops pinchoti | 47.7 (± 0.1) | 40.2 (± 0.2) | 0.84 | [76] |
| Norops townsendi | 50.5 (± 4.0) | 46.6 (± 4.2) | 0.92 | This study |
| Population | SVL (mm) | mass (g) | Fore (mm) | Hind (mm) | HW (mm) | HH (mm) | HL (mm) | # lamellae | PH (m) | PD (cm) | # mites |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerro Yglesias | 45.9 (± 3.0) | 1.6 (± 0.4) | 21.5 (± 1.4) | 40.4 (± 3.5) | 7.0 (± 0.5) | 5.4 (± 0.6) | 12.4 (± 1.2) | 17.7 (± 1.2) | 0.8 (± 0.1) | 11.2 (± 9.9) | 7.7 (± 8.9) |
| Playa by Station | 48 (± 2.4) | 2.4 (± 0.4) | 22.9 (± 1.8) | 40.1 (± 2.8) | 7.6 (± 0.4) | 5.7 (± 0.4) | 13.3 (± 0.8) | 18.4 (± 1.2) | 1.8 (± 0.9) | 8.3 (± 12.1) | N/A |
| Chatham Trail | 48.9 (± 3.3) | 2.1 (± 0.3) | 22.8 (± 1.3) | 40.8 (± 1.6) | 7.5 (± 0.4) | 5.6 (± 0.3) | 13.2 (± 0.5) | 18.2 (± 1.3) | 1.7 (± 1.5) | 8.2 (± 6.8) | 10.4 (± 15.8) |
| Playa + Trail | 48.3 (± 2.8) | 2.3 (± 0.4) | 22.8 (± 1.6) | 40.3 (± 2.4) | 7.5 (± 0.4) | 5.7 (± 0.4) | 13.3 (± 0.7) | 18.3 (± 1.2) | 1.8 (± 1.2) | 8.3 (± 9.9) | 10.4 (± 15.8) |
| Manuelita Islet | 55.2 (± 3.2) | 3.5 (± 0.7) | 26.4 (± 2.3) | 45.9 (± 3.9) | 8.3 (± 0.6) | 6.3 (± 0.4) | 14.5 (± 1.0) | 19.5 (± 0.9) | 0.4 (± 0.1) | 6.4 (± 6.6) | 3.4 (± 6.5) |
| Population | SVL | mass | Fore | Hind | HW | HH | HL | lamellae | PH | PD | mites |
| Playa v Trail | 0.32 | 0.004 | 0.10 | 0.002 | 0.23 | 0.61 | 0.05 | 0.69 | 0.34 | 0.17 | N/A |
| Playa v Cerro | 0.003 | 0.0003 | 0.002 | 0.30 | 0.001 | 0.06 | 0.001 | 0.21 | 0.03 | 0.69 | N/A |
| Cerro v Trail | 0.003 | 0.00001 | 0.02 | 0.31 | 0.01 | 0.19 | 0.04 | 0.15 | 0.0002 | 0.14 | 0.61 |
| Playa v Islet | < 0.00001 | < 0.00001 | < 0.00001 | 0.00001 | 0.0002 | < 0.00001 | 0.0003 | 0.0006 | 0.0001 | 0.20 | N/A |
| Trail v Islet | < 0.00001 | < 0.00001 | < 0.00001 | < 0.00001 | 0.00001 | < 0.00001 | 0.00002 | 0.004 | < 0.00001 | 0.77 | 0.09 |
| Cerro v Islet | < 0.00001 | < 0.00001 | < 0.00001 | 0.001 | 0.00001 | 0.00005 | 0.00003 | 0.0001 | < 0.00001 | 0.12 | 0.15 |
| Population | SVL | mass | Fore | Hind | HW | HH | HL | lamellae | PH | PD | mites |
| Main v Cerro | 0.02 | 0.00001 | 0.02 | 0.97 | 0.0004 | 0.07 | 0.001 | 0.14 | 0.01 | 0.36 | 0.60 |
| Main v Islet | < 0.00001 | < 0.00001 | < 0.00001 | < 0.00001 | < 0.00001 | < 0.00001 | < 0.00001 | 0.0004 | < 0.00001 | 0.46 | 0.08 |
| Cerro v Islet | < 0.00001 | < 0.00001 | < 0.00001 | 0.001 | 0.00001 | 0.00005 | 0.00003 | 0.0001 | < 0.00001 | 0.12 | 0.15 |
| Species | N | Perch Height (m) | Perch Diameter (cm) |
|---|---|---|---|
| Dactyloa chocorum | 1 * | 2.2 | 12.0 |
| Dactyloa gorgonae | 8 | 15.6 (± 7.3) | 18.2 (± 7.1) |
| Dactyloa princeps | 21 | 3.1 (± 1.5) | 14.5 (± 7.9) |
| Norops medemi | 30 | 3.4 (± 2.6) | 7.9 (± 5.3) |
| Norops parvauritus | 1 * | 4.4 | 93.0 |
| D. gorgonae vs. D. princeps | < 0.00001 | 0.26 | |
| D. gorgonae vs. N. medemi | < 0.00001 | 0.10 | |
| D. princeps vs. N. medemi | 0.66 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, J.G.; Burton, S.E.; Womack, M.M.; Pulver, E.; Nicholson, K.E. Biogeography, Systematics, and Ecomorphology of Pacific Island Anoles. Diversity 2019, 11, 141. https://doi.org/10.3390/d11090141
Phillips JG, Burton SE, Womack MM, Pulver E, Nicholson KE. Biogeography, Systematics, and Ecomorphology of Pacific Island Anoles. Diversity. 2019; 11(9):141. https://doi.org/10.3390/d11090141
Chicago/Turabian StylePhillips, John G., Sarah E. Burton, Margarita M. Womack, Evan Pulver, and Kirsten E. Nicholson. 2019. "Biogeography, Systematics, and Ecomorphology of Pacific Island Anoles" Diversity 11, no. 9: 141. https://doi.org/10.3390/d11090141
APA StylePhillips, J. G., Burton, S. E., Womack, M. M., Pulver, E., & Nicholson, K. E. (2019). Biogeography, Systematics, and Ecomorphology of Pacific Island Anoles. Diversity, 11(9), 141. https://doi.org/10.3390/d11090141





