Actinobacteria from Extreme Niches in Morocco and Their Plant Growth-Promoting Potentials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites, Sample Collection, and Isolation of Actinobacteria
2.2. Molecular Identification of Actinobacterial Isolates
2.3. Evaluation of Plant Growth-Promoting Potentials
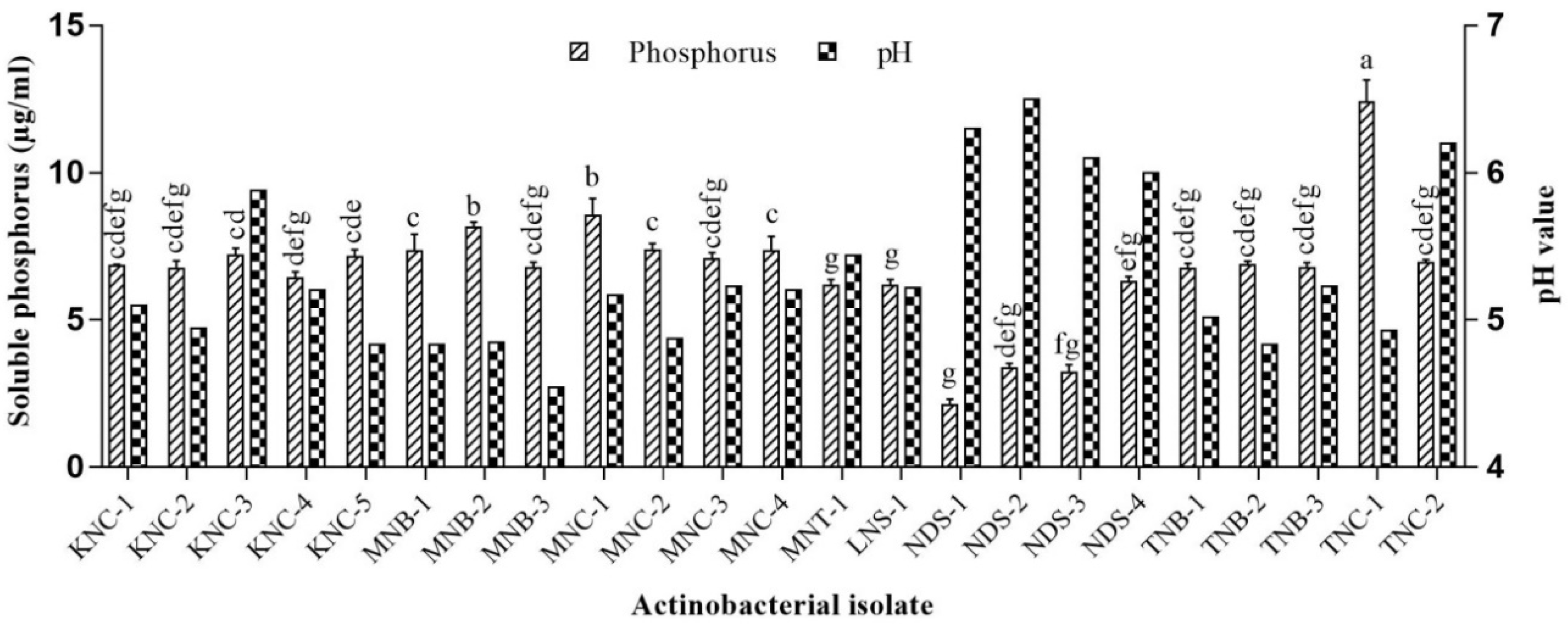
2.3.1. P Solubilization
2.3.2. K Solubilization
2.3.3. N Fixation
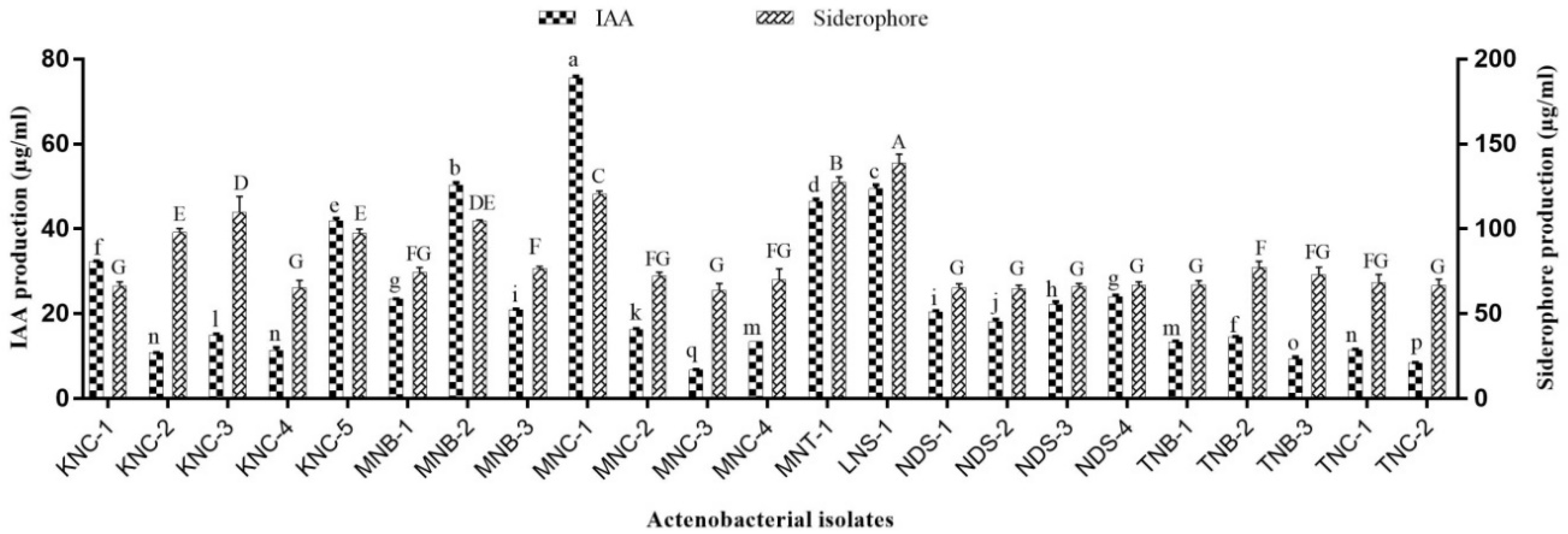
2.3.4. IAA Production
2.3.5. Siderophore Estimation
2.4. Statistical Analysis
3. Results and Discussion
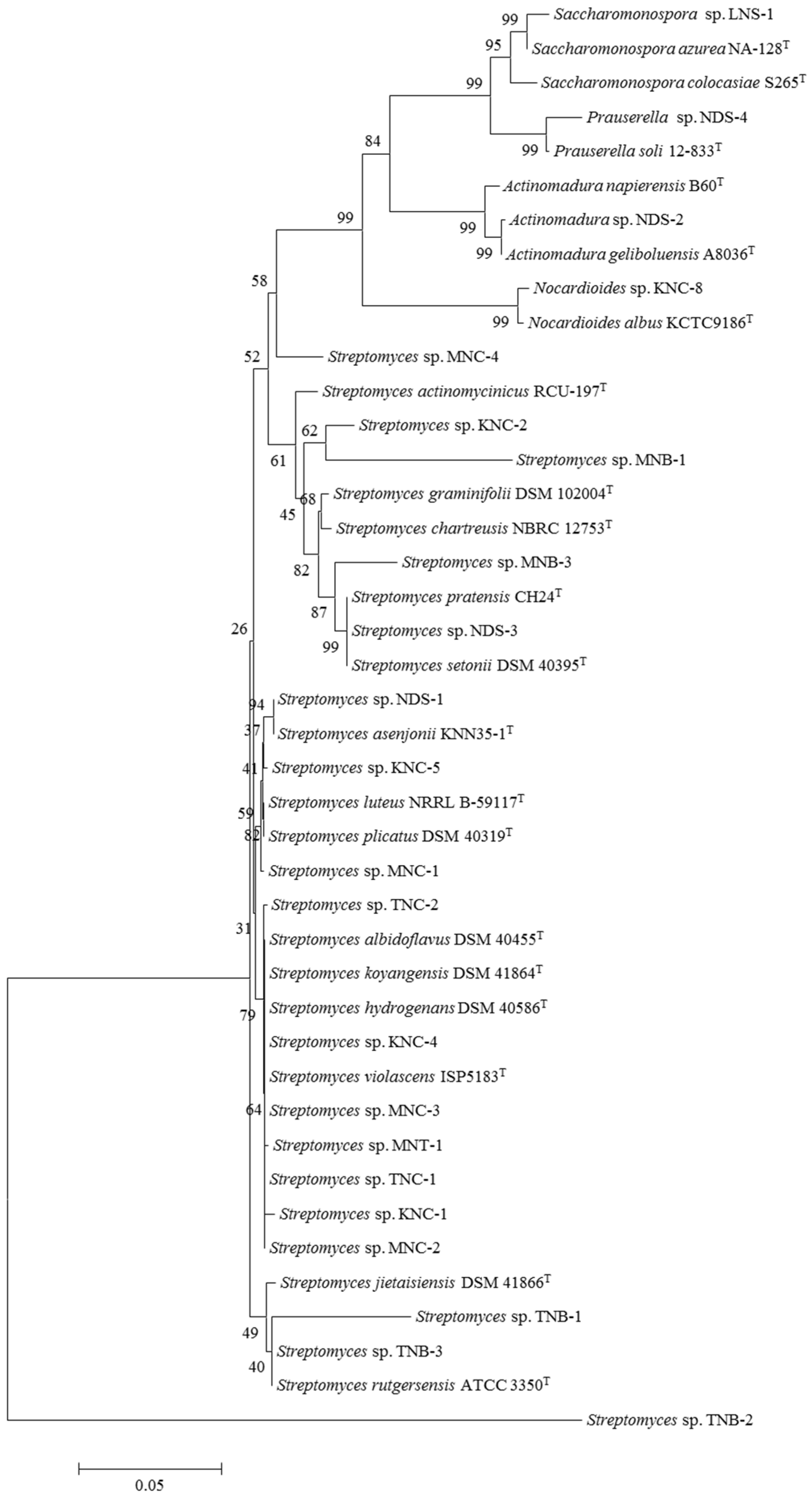
3.1. Actinobacterial Diversity in Different Moroccan Extreme Environments
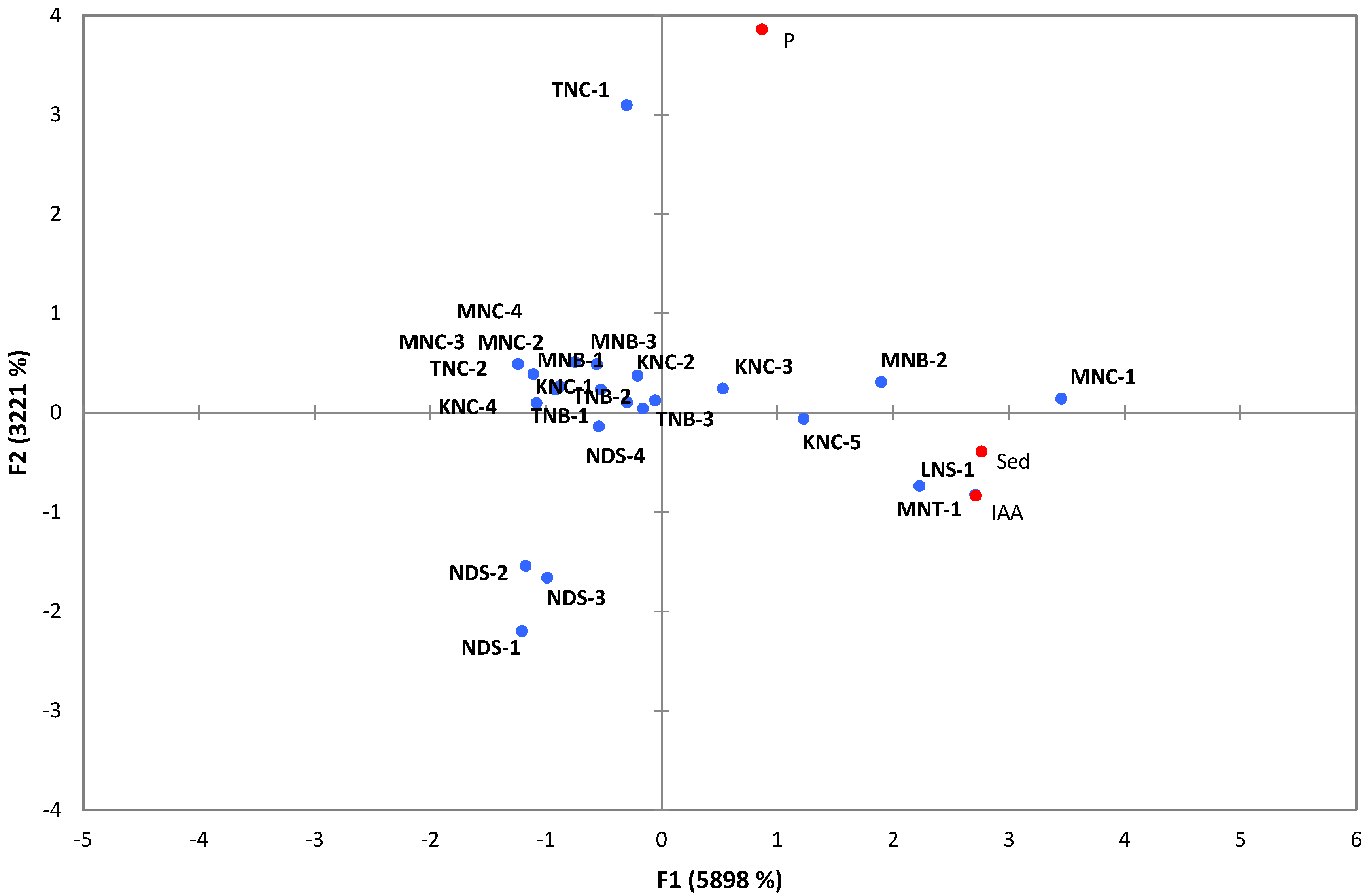
3.2. PGP Potentials of Extremophilic Actinobacteria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohamed, H.; Miloud, B.; Zohra, F.; García-Arenzana, J.M.; Veloso, A.; Rodríguez-Couto, S. Isolation and Characterization of Actinobacteria from Algerian Sahara Soils with Antimicrobial Activities. Int. J. Mol. Cell. Med. 2017, 6, 109–120. [Google Scholar] [PubMed]
- Loqman, S.; Bouizgarne, B.; Barka, E.A.; Clement, C.; Von Jan, M.; Spröer, C.; Klenk, H.-P.; Ouhdouch, Y. Streptomyces thinghirensis sp. nov., isolated from rhizosphere soil of Vitis vinifera. Int. J. Syst. Evol. Microbiol. 2009, 59, 3063–3067. [Google Scholar] [CrossRef] [PubMed]
- Hamdali, H.; Virolle, M.J.; von Jan, M.; Sproer, C.; Klenk, H.-P.; Ouhdouch, Y. Streptomyces youssoufiensis sp. nov., isolated from a Moroccan phosphate mine. Int. J. Syst. Evol. Microbiol. 2011, 61, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Steven, B.; Pollard, W.H.; Greer, C.W.; Whyte, L.G. Microbial diversity and activity through a permafrost/ground ice core profile from the Canadian high Arctic. Environ. Microbiol. 2008, 10, 3388–3403. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, V.; Øvreås, L. Microbial Diversity, Life Strategies, and Adaptation to Life in Extreme Soils. Microbiol. Extrem. Soils 2008, 13, 15–43. [Google Scholar]
- Trenozhnikova, L.; Azizan, A. Discovery of Actinomycetes from Extreme Environments with Potential to Produce Novel Antibiotics. Central Asian J. Glob. Health 2018, 7, 1–15. [Google Scholar] [CrossRef]
- Chianu, J.N.; Chianu, J.N.; Mairura, F. Mineral fertilizers in the farming systems of sub-Saharan Africa. A review. Agron. Sustain. Dev. 2012, 32, 545–566. [Google Scholar] [CrossRef]
- Jog, R.; Nareshkumar, G.; Rajkumar, S. Plant growth promoting potential and soil enzyme production of the most abundant Streptomyces spp. from wheat rhizosphere. J. Appl. Microbiol. 2012, 113, 1154–1164. [Google Scholar] [CrossRef]
- Sathya, A.; Vijayabharathi, R.; Gopalakrishnan, S. Plant growth-promoting actinobacteria: A new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotech 2017, 7, 102. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Tamreihao, K.; Ningthoujam, D.S.; Nimaichand, S.; Singh, E.S.; Reena, P.; Singh, S.H.; Nongthomba, U.; Singh, S.E. Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol. Rep. 2016, 192, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qiu, Z.; Tan, H.; Cao, L. Siderophore production by actinobacteria. BioMetals 2014, 27, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Li, W.J.; Dastager, S.G.; Hozzein, W.N. Editorial: Actinobacteria in Special and Extreme Habitats: Diversity, Function Roles, and Environmental Adaptations. Front. Microbiol. 2016, 7, 1203. [Google Scholar] [CrossRef] [PubMed]
- Meklat, A.; Sabaou, N.; Zitouni, A.; Mathieu, F.; Lebrihi, A. Isolation, Taxonomy, and Antagonistic Properties of Halophilic Actinomycetes in Saharan Soils of Algeria. Appl. Environ. Microbiol. 2011, 77, 6710–6714. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Bai, J.L.; Yang, H.T.; Zhang, W.D.; Xiong, Y.W.; Ding, P.; Qin, S. Phylogenetic diversity and investigation of plant growth-promoting traits of actinobacteria in coastal salt marsh plant rhizospheres from Jiangsu, China. Syst. Appl. Microbiol. 2018, 41, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Babavalian, H.; Amoozegar, M.A.; Pourbabaee, A.A.; Moghaddam, M.M.; Shakeri, F. Isolation and identification of moderately halophilic bacteria producing hydrolytic enzymes from the largest hypersaline playa in Iran. Microbiology 2013, 82, 466–474. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Li, W.J.; Ali, M.I.A.; Hammouda, O.; Mousa, A.S.; Xu, L.H.; Jiang, C.L. Nocardiopsis alkaliphila sp. nov., a novel alkaliphilic actinomycete isolated from desert soil in Egypt. Int. J. Syst. Evol. Microbiol. 2004, 54, 247–252. [Google Scholar] [CrossRef]
- Driche, E.H.; Sabaou, N.; Bijani, C.; Zitouni, A.; Pont, F.; Mathieu, F.; Badji, B. Streptomyces sp. AT37 isolated from a Saharan soil produces a furanone derivative active against multidrug-resistant Staphylococcus aureus. World J. Microbiol. Biotechnol. 2017, 33, 105. [Google Scholar] [CrossRef]
- Weisburg, W.; Barns, S.; Pelletier, D.; Lane, D. 16S ribosomal DNA amplification for phylogenetic studyitle. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Pawlowski, J.; Holzmann, M. Molecular phylogeny of Foraminifera a review. Eur. J. Protistol. 2002, 38, 1–10. [Google Scholar] [CrossRef]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.; Blackshields, G.; Brown, N.; Chenna, R.; Mcgettigan, P.; Mc William, H.; Valentin, F.; Wallace, I.; Wilm, A.; López, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Bechtaoui, N.; Raklami, A.; Tahiri, A.-I.; Benidire, L.; El Alaoui, A.; Meddich, A.; Göttfert, M.; Oufdou, K. Characterization of plant growth promoting rhizobacteria and their benefits on growth and phosphate nutrition of faba bean and wheat. Biol. Open 2019, 8, bio043968. [Google Scholar] [CrossRef] [PubMed]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Aeron, A.; Kumar, A.; Kim, K.; Bajpai, V.K. Potassium solubilizing rhizobacteria (KSR): Isolation, identification, and K-release dynamics from waste mica. Ecol. Eng. 2015, 81, 340–347. [Google Scholar] [CrossRef]
- Dahal, B.; NandaKafle, G.; Perkins, L.; Brözel, V.S. Diversity of free-Living nitrogen fixing Streptomyces in soils of the badlands of South Dakota. Microbiol. Rep. 2016, 195, 31–39. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Chaudhari, H.G.; Kasture, V.M.; Dhavale, D.D.; Chopade, B.A. Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumoniae strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J. Exp. Biol. 2009, 47, 993–1000. [Google Scholar]
- Rodrigues, A.A.; Araújo, M.V.F.; Soares, M.D.S.; De Oliveira, B.F.R.; Sibov, S.T.; Vieira, J.D.G. Isolation and Screening for Multi-trait Plant Growth Promoting Actinobacteria From Organic Sugarcane Rhizosphere. Int. J. Microbiol. Res. 2018, 10, 1193. [Google Scholar] [CrossRef]
- Hu, Q.-P.; Xu, J.-G. A simple double-layered chrome azurol S agar (SD-CASA) plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. African J. Microbiol. Res. 2011, 5, 4321–4327. [Google Scholar]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Baakza, A.; Dave, B.P.; Dube, H.C. Chemical nature, ligand denticity and quantification of fungal siderophores. Indian J. Exp. Biol. 2004, 42, 96–105. [Google Scholar] [PubMed]
- Marcoux, E.; Belkabir, A.; Gibson, H.L.; Lentz, D.; Ruffet, G. Draa Sfar, Morocco: A Visean (331 Ma) pyrrhotite-rich, polymetallic volcanogenic massive sulphide deposit in a Hercynian sediment-dominant terrane. Ore Geol. Rev. 2008, 33, 307–328. [Google Scholar] [CrossRef]
- Albarracin, V.H.; Alonso-Vega, P.; Trujillo, M.E.; Amoroso, M.J.; Abate, C.M. Amycolatopsis tucumanensis sp. nov., a copper-resistant actinobacterium isolated from polluted sediments. Int. J. Syst. Evol. Microbiol. 2010, 60, 397–401. [Google Scholar] [CrossRef]
- Ali, N.; Dashti, N.; Al-Mailem, D.; Eliyas, M.; Radwan, S. Indigenous soil bacteria with the combined potential for hydrocarbon consumption and heavy metal resistance. Environ. Sci. Pollut. Res. 2012, 19, 812–820. [Google Scholar] [CrossRef]
- Richards, J.W.; Krumholz, G.D.; Chval, M.S.; Tisa, L.S. Heavy Metal Resistance Patterns of Frankia Strains. Appl. Environ. Microbiol. 2002, 68, 923–927. [Google Scholar] [CrossRef]
- Köberl, M.; Müller, H.; Ramadan, E.M.; Berg, G. Desert Farming Benefits from Microbial Potential in Arid Soils and Promotes Diversity and Plant Health. PLoS ONE 2011, 6, e24452. [Google Scholar] [CrossRef]
- Harwani, D. Biodiversity of Rare Thermophilic Actinomycetes in the Great Indian Thar Desert: An Overview. Indo Am. J. Pharm. Res. 2013, 3. [Google Scholar]
- Santhanam, R.; Rong, X.; Huang, Y.; Andrews, B.A.; Asenjo, J.A.; Goodfellow, M. Streptomyces bullii sp. nov., isolated from a hyper-arid Atacama Desert soil. Antonie Van Leeuwenhoek 2013, 103, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Kurapova, A.I.; Zenova, G.M.; Sudnitsyn, I.I.; Kizilova, A.K.; Manucharova, N.A.; Norovsuren, Z.; Zvyagintsev, D.G. Thermotolerant and thermophilic actinomycetes from soils of Mongolia desert steppe zone. Microbiology 2012, 81, 98–108. [Google Scholar] [CrossRef]
- Lahoum, A.; Aouiche, A.; Bouras, N.; Verheecke, C.; Klenk, H.-P.; Sabaou, N.; Mathieu, F. Antifungal activity of a Saharan strain of Actinomadura sp. ACD1 against toxigenic fungi and other pathogenic microorganisms. J. Mycol. Médicale 2016, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Badji, B.; Mostefaoui, A.; Sabaou, N.; Mathieu, F.; Lebrihi, A. Identification of a new strain of Actinomadura isolated from Saharan soil and partial characterization of its antifungal compounds. Afr. J. Biotechnol. 2011, 10, 13878–13886. [Google Scholar]
- Zitouni, A.; Boudjella, H.; Lamari, L.; Badji, B.; Mathieu, F.; Lebrihi, A.; Sabaou, N. Nocardiopsis and Saccharothrix genera in Saharan soils in Algeria: Isolation, biological activities and partial characterization of antibiotics. Res. Microbiol. 2005, 156, 984–993. [Google Scholar] [CrossRef]
- Khamna, S.; Yokota, A.; Lumyong, S. Actinomycetes isolated from medicinal plant rhizosphere soils: Diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J. Microbiol. Biotechnol. 2009, 25, 649–655. [Google Scholar] [CrossRef]
- Aouar, L.; Lerat, S.; Ouffroukh, A.; Boulahrouf, A.; Beaulieu, C. Taxonomic identification of rhizospheric actinobacteria isolated from Algerian semi-arid soil exhibiting antagonistic activities against plant fungal pathogens. Can. J. Plant Pathol. 2012, 34, 165–176. [Google Scholar] [CrossRef]
- Solans, M.; Scervino, J.M.; Messuti, M.I.; Vobis, G.; Wall, L.G. Potential biocontrol actinobacteria: Rhizospheric isolates from the Argentine Pampas lowlands legumes. J. Basic Microbiol. 2016, 56, 1289–1298. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef]
- Lindh, M.V.; Riemann, L.; Baltar, F.; Romero-Oliva, C.; Salomon, P.S.; Granéli, E.; Pinhassi, J. Consequences of increased temperature and acidification on bacterioplankton community composition during a mesocosm spring bloom in the Baltic Sea. Environ. Microbiol. Rep. 2013, 5, 252–262. [Google Scholar] [CrossRef]
- Gong, J.; Shi, F.; Ma, B.; Dong, J.; Pachiadaki, M.; Zhang, X.; Edgcomb, V.P. Depth shapes α- and β-diversities of microbial eukaryotes in surficial sediments of coastal ecosystems. Environ. Microbiol. 2015, 17, 3722–3737. [Google Scholar] [CrossRef] [PubMed]
- Ouchari, L.; Boukeskasse, A.; Bouizgarne, B.; Ouhdouch, Y. Antimicrobial potential of actinomycetes isolated from the unexplored hot Merzouga desert and their taxonomic diversity. Biol. Open 2019, 8, bio035410. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Aalbersberg, W. Marine actinomycetes: An ongoing source of novel bioactive metabolites. Microbiol. Res. 2012, 167, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Pimentel-Elardo, S.M.; Hanora, A.; Radwan, M.; Abou-El-Ela, S.H.; Ahmed, S.; Hentschel, U. Isolation, Phylogenetic Analysis and Anti-infective Activity Screening of Marine Sponge-Associated Actinomycetes. Mar. Drugs 2010, 8, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, L.A.; Stach, J.E.M.; Pathom-aree, W.; Ward, A.C.; Bull, A.T.; Goodfellow, M. Diversity of cultivable actinobacteria in geographically widespread marine sediments. Antonie Van Leeuwenhoek 2005, 87, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Hewavitharana, A.K.; Shaw, P.N.; Fuerst, J.A. Discovery of a New Source of Rifamycin Antibiotics in Marine Sponge Actinobacteria by Phylogenetic Prediction. Appl. Environ. Microbiol. 2006, 72, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, D.; Bharathi, S.; Radhakrishnan, M.; Balagurunathan, R. Bioprospecting of actinobacteria from Yelagiri hills with special reference to antibacterial activity. J. Chem. Pharm. Res 2011, 3, 439–446. [Google Scholar]
- Dalisay, D.S.; Williams, D.E.; Wang, X.L.; Centko, R.; Chen, J.; Andersen, R.J. Marine Sediment-Derived Streptomyces Bacteria from British Columbia, Canada Are a Promising Microbiota Resource for the Discovery of Antimicrobial Natural Products. PLoS ONE 2013, 8, e77078. [Google Scholar] [CrossRef]
- Dharmaraj, S. Marine Streptomyces as a novel source of bioactive substances. World J. Microbiol. Biotechnol. 2010, 26, 2123–2139. [Google Scholar] [CrossRef]
- Ramesh, S.; Mathivanan, N. Screening of marine actinomycetes isolated from the Bay of Bengal, India for antimicrobial activity and industrial enzymes. World J. Microbiol. Biotechnol. 2009, 25, 2103–2111. [Google Scholar] [CrossRef]
- Matos, A.D.M.; Gomez, I.C.P.; Nietsche, S.; Xavier, A.A.; Gomes, W.S.; Dos Santos Neto, J.A.; Pereira, M.C.T. Phosphate solubilization by endophytic bacteria isolated from banana trees. An. Acad. Bras. Cienc. 2017, 89, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Bhadauria, S.; Kumar, P.; Lal, H.; Mondal, R.; Verma, D. Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol. Lett. 2000, 182, 291–296. [Google Scholar] [CrossRef]
- De Oliveira Mendes, G.; Moreira de Freitas, A.L.; Liparini Pereira, O.; Ribeiro da Silva, I.; Bojkov Vassilev, N.; Dutra Costa, M. Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Ann. Microbiol. 2014, 64, 239–249. [Google Scholar] [CrossRef]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.-A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Hamdali, H.; Bouizgarne, B.; Hafidi, M.; Lebrihi, A.; Virolle, M.J.; Ouhdouch, Y. Screening for rock phosphate solubilizing Actinomycetes from Moroccan phosphate mines. Appl. Soil Ecol. 2008, 38, 12–19. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Nassar, A.H.; Hardy, G.E.S.J.; Sivasithamparam, K. Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J. Appl. Microbiol. 2009, 106, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Dastager, S.G.; Damare, S. Marine Actinobacteria Showing Phosphate-Solubilizing Efficiency in Chorao Island, Goa, India. Curr. Microbiol. 2013, 66, 421–427. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Sheng, X.F.; He, L.Y. Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006, 52, 66–72. [Google Scholar] [CrossRef]
- Zarjani, K.J.; Aliasgharzad, N.; Oustan, S.; Emadi, M.; Ahmadi, A. Isolation and characterization of potassium solubilizing bacteria in some Iranian soils. Arch. Agron. Soil Sci. 2013, 59, 1713–1723. [Google Scholar] [CrossRef]
- Prajapati, K.; Sharma, M.C.; Modi, H.A. Growth Promoting Effect of Potassium Solubilizing Microorganisms on Okra (Abelmoscus Esculantus). Int. J. Agric. Sci. 2013, 31, 181–186. [Google Scholar]
- Liu, D.; Lian, B.; Dong, H. Isolation of Paenibacillus sp. and Assessment of its Potential for Enhancing Mineral Weathering. Geomicrobiol. J. 2012, 29, 413–421. [Google Scholar] [CrossRef]
- Suseela, R.B.; Srinivasan, V.; Sangeeth, K.P. Paenibacillus glucanolyticus, a promising potassium solubilizing bacterium isolated from black pepper (Piper nigrum L.) rhizosphere. J. Spices Aromat. Crop. 2012, 21, 118–124. [Google Scholar]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Pierrat, J.C.; Mustin, C.; Frey-Klett, P. Effect of the Mycorrhizosphere on the Genotypic and Metabolic Diversity of the Bacterial Communities Involved in Mineral Weathering in a Forest Soil. Appl. Environ. Microbiol. 2007, 73, 3019–3027. [Google Scholar] [CrossRef]
- Ribbe, M.; Gadkari, D.; Meyer, O. N 2 Fixation by Streptomyces thermoautotrophicus Involves a Molybdenum-Dinitrogenase and a Manganese-Superoxide Oxidoreductase That Couple N 2 Reduction to the Oxidation of Superoxide Produced from O 2 by a Molybdenum-CO Dehydrogenase. J. Biol. Chem. 1997, 272, 26627–26633. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.R.; Silvester, W.B. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 1993, 57, 293–319. [Google Scholar]
- Sellstedt, A.; Richau, K.H. Aspects of nitrogen-fixing Actinobacteria, in particular free-living and symbiotic Frankia. FEMS Microbiol. Lett. 2013, 342, 179–186. [Google Scholar] [CrossRef]
- Gtari, M.; Ghodhbane-Gtari, F.; Nouioui, I.; Beauchemin, N.; Tisa, L.S. Phylogenetic perspectives of nitrogen-fixing actinobacteria. Arch. Microbiol. 2012, 194, 3–11. [Google Scholar] [CrossRef]
- Anwar, S.; Ali, B.; Sajid, I. Screening of Rhizospheric Actinomycetes for Various In-vitro and In-vivo Plant Growth Promoting (PGP) Traits and for Agroactive Compounds. Front. Microbiol. 2016, 7, 1334–1345. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Microbial producers of plant growth stimulators and their practical use: A review. Appl. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Nassar, A.H.; Sivasithamparam, K. Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Appl. Soil Ecol. 2008, 39, 161–171. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil 2008, 308, 161–174. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.K.; Tewari, S.; Singh, R. Multifaceted Plant-Associated Microbes and Their Mechanisms Diminish the Concept of Direct and Indirect PGPRs. In Plant Microbe Symbiosis: Fundamentals and Advances; Springer: New Delhi, India, 2013; pp. 411–449. [Google Scholar]
- Singh, R.; Pandey, K.D.; Monika, S.; Kumar, A. PGPR Isolates from the Rhizosphere of Vegetable Crop Momordica charantia: Characterization and Application as Biofertilizer. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1789–1802. [Google Scholar]
- Oliveira, P.H.; Batagov, A.; Ward, J.; Baganz, F.; Krabben, P. Identification of erythrobactin, a hydroxamate-type siderophore produced by Saccharopolyspora erythraea. Lett. Appl. Microbiol. 2006, 42, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Kalinovskaya, N.I.; Romanenko, L.A.; Irisawa, T.; Ermakova, S.P.; Kalinovsky, A.I. Marine isolate Citricoccus sp. KMM 3890 as a source of a cyclic siderophore nocardamine with antitumor activity. Microbiol. Res. 2011, 166, 654–661. [Google Scholar] [CrossRef]
- Mazzei, E.; Iorio, M.; Maffioli, S.I.; Sosio, M.; Donadio, S. Characterization of madurastatin C1, a novel siderophore from Actinomadura sp. J. Antibiot. 2012, 65, 267–269. [Google Scholar] [CrossRef][Green Version]
- Mukai, A.; Komaki, H.; Takagi, M.; Shin-ya, K. Novel siderophore, JBIR-16, isolated from Nocardia tenerifensis NBRC 101015. J. Antibiot. 2009, 62, 601–603. [Google Scholar] [CrossRef]
- Kodani, S.; Kobayakawa, F.; Hidaki, M. Isolation and structure determination of new siderophore tsukubachelin B from Streptomyces sp. TM-74. Nat. Prod. Res. 2013, 27, 775–781. [Google Scholar] [CrossRef] [PubMed]
| Sample | Origin | Site Characteristic | Geographic Coordinate | Physicochemical Property | ||
|---|---|---|---|---|---|---|
| pH | EC (mS/ms) | TOC (%) | ||||
| Mountain soil | Toubkal | Highest mountain peak in Morocco and the Arab world | 4167 m; 31.05917″ N −7.91583″ W | 7.9 | 2.14 | 1.02 |
| Desert soil | Merzouga | Highest dunes in Morocco | 150 m; 31.147643″ N −3.974280″ O | 8.04 | 0.95 | 0.96 |
| Rhizospheric soil | Rif | Rhizosphere of Cannabis sativa grown in Rif plains | 34.920059″ N −4.561078″ O | 8.62 | 4.99 | 1.58 |
| Marine soil | Marchica | Largest lagoon in Morocco | 35.156468″ N −2.904342″ W | 8.31 | 20.41 | 0.34 |
| Mining soil | Draa Sfar | Significant potential for polymetallic ore | 31.704270″ N −8.135748″ W | 5.4 | 6.5 | 1.13 |
| Molecular Characteristics | PGP Characteristics | |||||
|---|---|---|---|---|---|---|
| Site of Isolation | Isolate Code | 16 rRNA Gene Accession Number | Similarity % | Closest Type Strain | N2 Fixation | K Solubilization |
| Rhizospheric soil | KNC-1 | MN161857 | 98.03 | Streptomyces koyangensis DSM 41864T | ++ | + |
| KNC-2 | MN161858 | 96.06 | Streptomyces actinomycinicus RCU-197T | +++ | +++ | |
| KNC-3 | MN161861 | 98.23 | Nocardioides albus KCTC9186T | ++ | ++ | |
| KNC-4 | MN161862 | 99.38 | Streptomyces chartreusis NBRC 12753T | +++ | ++ | |
| KNC-5 | MN161863 | 99.79 | Streptomyces setonii DSM 40395T | ++ | ++ | |
| Desert soil | MNB-1 | MN164446 | 96.20 | Streptomyces graminifolii DSM 102004T | +++ | + |
| MNB-2 | MN164450 | 99.31 | Streptomyces luteus NRRL B-59117T | +++ | +++ | |
| MNB-3 | MN164447 | 94.12 | Streptomyces pratensis CH24T | + | ++ | |
| MNC-1 | MN161860 | 97.76 | Streptomyces asenjonii KMN35-1T | ++ | ++ | |
| MNC-2 | MN161865 | 99.17 | Streptomyces plicatus DSM 40319T | +++ | ++ | |
| MNC-3 | MN161850 | 98.65 | Streptomyces violascens ISP5183T | +++ | +++ | |
| MNC-4 | MN161866 | 98.45 | Streptomyces albidoflavus DSM 40455T | +++ | +++ | |
| MNT-1 | MN161867 | 99.30 | Streptomyces hydrogenans DSM 40586T | ++ | ++ | |
| Marine soil | LNS-1 | MN161851 | 95.39 | Saccharomonospora azurea NA-128T | + | + |
| Mining soil | NDS-1 | MN161854 | 97.80 | Streptomyces asenjonii KMN35-1T | + | + |
| NDS-2 | MN161853 | 98.03 | Actinomadura napierensis B60T | +++ | ++ | |
| NDS-3 | MN161864 | 99.68 | Streptomyces albidoflavus DSM 40455T | - | - | |
| NDS-4 | MN161855 | 98.42 | Prauserella soli 12-833T | - | + | |
| Mountain soil | TNB-1 | MN164448 | 96.05 | Streptomyces jietaisiensis DSM 41866T | +++ | + |
| TNB-2 | MN164449 | 98.85 | Streptomyces albidoflavus DSM 40455T | +++ | + | |
| TNB-3 | MN161859 | 97.11 | Streptomyces rutgersensis ATCC 3350T | ++ | + | |
| TNC-1 | MN161852 | 98.56 | Streptomyces violascens ISP5183T | +++ | +++ | |
| TNC-2 | MN161856 | 98.38 | Streptomyces hydrogenans DSM 40586T | +++ | +++ | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafis, A.; Raklami, A.; Bechtaoui, N.; El Khalloufi, F.; El Alaoui, A.; Glick, B.R.; Hafidi, M.; Kouisni, L.; Ouhdouch, Y.; Hassani, L. Actinobacteria from Extreme Niches in Morocco and Their Plant Growth-Promoting Potentials. Diversity 2019, 11, 139. https://doi.org/10.3390/d11080139
Nafis A, Raklami A, Bechtaoui N, El Khalloufi F, El Alaoui A, Glick BR, Hafidi M, Kouisni L, Ouhdouch Y, Hassani L. Actinobacteria from Extreme Niches in Morocco and Their Plant Growth-Promoting Potentials. Diversity. 2019; 11(8):139. https://doi.org/10.3390/d11080139
Chicago/Turabian StyleNafis, Ahmed, Anas Raklami, Noura Bechtaoui, Fatima El Khalloufi, Abdelkhalek El Alaoui, Bernard R. Glick, Mohamed Hafidi, Lamfeddal Kouisni, Yedir Ouhdouch, and Lahcen Hassani. 2019. "Actinobacteria from Extreme Niches in Morocco and Their Plant Growth-Promoting Potentials" Diversity 11, no. 8: 139. https://doi.org/10.3390/d11080139
APA StyleNafis, A., Raklami, A., Bechtaoui, N., El Khalloufi, F., El Alaoui, A., Glick, B. R., Hafidi, M., Kouisni, L., Ouhdouch, Y., & Hassani, L. (2019). Actinobacteria from Extreme Niches in Morocco and Their Plant Growth-Promoting Potentials. Diversity, 11(8), 139. https://doi.org/10.3390/d11080139






