Endemic Infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for Amphibian Conservation at Regional and Species Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Assessment
2.2. Field Dataset
2.3. Combined Dataset
2.3.1. Herpetological Provinces
2.3.2. Altitudinal Belt
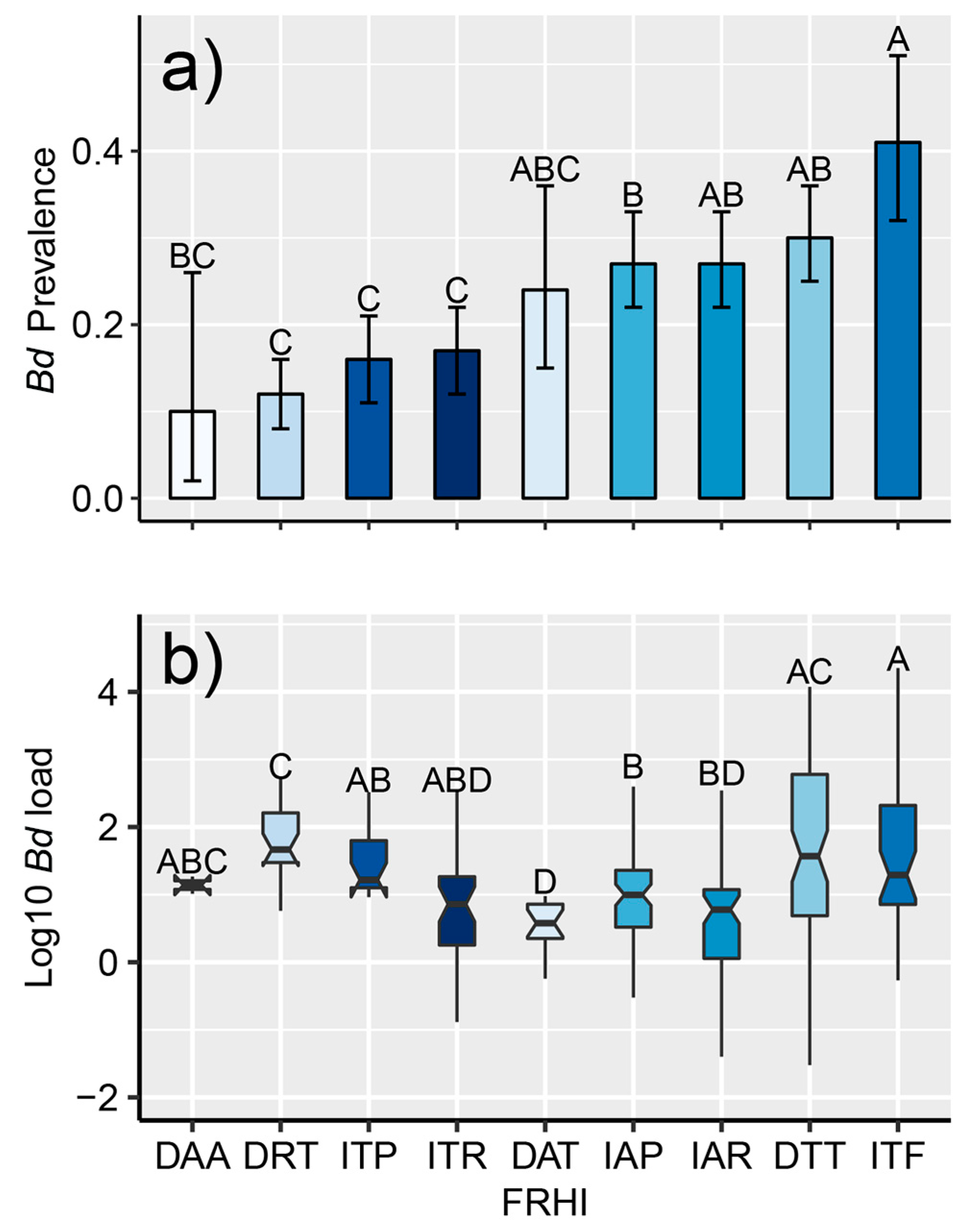
2.3.3. Foraging-Reproduction Habitat Index
2.4. Statistical Analysis
3. Results
3.1. Species Assessment
3.2. Endemic Dynamics
4. Discussion
4.1. Species Assessment
4.2. Post-Decline Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novacek, M.J.; Cleland, E.E. The current biodiversity extinction event: Scenarios for mitigation and recovery. Proc. Natl. Acad. Sci. USA 2001, 98, 5466–5470. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef] [PubMed]
- Monastersky, R. Life—A status report. Nature 2014, 516, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.; Waller, R. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Catenazzi, A. State of the World’s Amphibians. Annu. Rev. Environ. Resour. 2015, 40, 91–119. [Google Scholar] [CrossRef]
- Daszak, P.; Berger, L.; Cunningham, A.A.; Hyatt, A.D.; Green, D.E.; Speare, R. Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 1999, 5, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.P. Amphibian decline and extinction: What we know and what we need to learn. Dis. Aquat. Organ. 2010, 92, 93–99. [Google Scholar] [CrossRef] [PubMed]
- La Marca, E.; Lips, K.R.; Lotters, S.; Puschendorf, R.; Ibanez, R.; Rueda-Almonacid, J.V.; Schulte, R.; Marty, C.; Castro, F.; Manzanilla-Puppo, J.; et al. Catastrophic population declines and extinctions in Neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 2005, 37, 190–201. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Gerber, B.D.; Converse, S.J.; Muths, E.; Crockett, H.J.; Mosher, B.A.; Bailey, L.L. Identifying species conservation strategies to reduce disease-associated declines. Conserv. Lett. 2018, 11, e12393. [Google Scholar] [CrossRef]
- Meredith, H.; Van Buren, C.; Antwis, R.E. Making amphibian conservation more effective. Conserv. Evid. 2016, 13, 1–6. [Google Scholar]
- Savage, J.M. The Amphibians and Reptiles of Costa Rica: A Herpetofauna between Two Continents, between Two Deas; University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.0. Available online: http://research.amnh.org/vz/herpetology/amphibia/ (accessed on 27 June 2019).
- Bagley, J.C.; Johnson, J.B. Phylogeography and biogeography of the lower Central American Neotropics: Diversification between two continents and between two seas. Biol. Rev. 2014, 89, 767–790. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, F. Situación de los anfibios de Costa Rica. Biocenosis 2009, 22, 95–108. [Google Scholar]
- Pounds, J.A.; Crump, M.L. Amphibian declines and climate disturbance: The case of the golden toad and the harlequin frog. Conserv. Biol. 1994, 8, 72–85. [Google Scholar] [CrossRef]
- González-Maya, J.F.; Belant, J.L.; Wyatt, S.A.; Schipper, J.; Cardenal, J.; Corrales, D.; Cruz-Lizano, I.; Hoepker, A.; Escobedo-Galván, A.H.; Castañeda, F.; et al. Renewing hope: The rediscovery of Atelopus varius in Costa Rica. Amphib. Reptil. 2013, 34, 573–578. [Google Scholar] [CrossRef]
- Chaves, G.; Zumbado-Ulate, H.; García-Rodríguez, A.; Gómez, E.; Vredenburg, V.T.; Ryan, M.J. Rediscovery of the critically endangered streamside frog, Craugastor taurus (Craugastoridae), in Costa Rica. Trop. Conserv. Sci. 2014, 7, 628–638. [Google Scholar] [CrossRef]
- Abarca, J.; Chaves, G.; García-Rodríguez, A.; Vargas, R. Reconsidering extinction: Rediscovery of Incilius holdridgei (Anura: Bufonidae) in Costa Rica after 25 years. Herpetol. Rev. 2010, 41, 150. [Google Scholar]
- Hero, J.-M.; Williams, S.E.; Magnusson, W.E. Ecological traits of declining amphibians in upland areas of eastern Australia. J. Zool. 2005, 267, 221–232. [Google Scholar] [CrossRef]
- Mendelson, J.R.; Whitfield, S.M.; Sredl, M.J. A recovery engine strategy for amphibian conservation in the context of disease. Biol. Conserv. 2019, 236, 188–191. [Google Scholar] [CrossRef]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.R.; Green, D.E.; Papendick, R. Chytridiomycosis in wild frogs from southern Costa Rica. J. Herpetol. 2003, 37, 215–218. [Google Scholar] [CrossRef]
- Puschendorf, R.; Bolaños, F.; Chaves, G. The amphibian chytrid fungus along an altitudinal transect before the first reported declines in Costa Rica. Biol. Conserv. 2006, 132, 136–142. [Google Scholar] [CrossRef]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pounds, A.J.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zumbado-Ulate, H.; Bolaños, F.; Willink, B.; Soley-Guardia, F. Population status and natural history notes on the critically endangered stream dwelling frog Craugastor ranoides (Craugastoridae) in a Costa Rican tropical dry forest. Herpetol. Conserv. Biol. 2011, 6, 455–464. [Google Scholar]
- Puschendorf, R.; Carnaval, A.C.; VanDerWal, J.; Zumbado-Ulate, H.; Chaves, G.; Bolaños, F.; Alford, R.A. Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: Proposing climatic refuges as a conservation tool. Divers. Distrib. 2009, 15, 401–408. [Google Scholar] [CrossRef]
- Whitfield, S.M.; Bell, K.E.; Philippi, T.; Sasa, M.; Bolaños, F.; Chaves, G.; Savage, J.M.; Donnelly, M.A. Amphibian and reptile declines over 35 years at La Selva, Costa Rica. Proc. Natl. Acad. Sci. USA 2007, 104, 8352–8356. [Google Scholar] [CrossRef]
- Christie, M.R.; Searle, C.L. Evolutionary rescue in a host–pathogen system results in coexistence not clearance. Evol. Appl. 2018, 11, 681–693. [Google Scholar] [CrossRef]
- Retallick, R.W.R.; Miera, V. Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis. Aquat. Organ. 2007, 75, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.J.; Vredenburg, V.T.; Knapp, R.A.; Rachowicz, L.J. Investigating the population-level effects of chytridiomycosis: An emerging infectious disease of amphibians. Ecology 2005, 86, 3149–3159. [Google Scholar] [CrossRef]
- Rachowicz, L.J.; Knapp, R.A.; Morgan, J.A.T.; Stice, M.J.; Vredenburg, V.T.; Parker, J.M.; Briggs, C.J. Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 2006, 87, 1671–1683. [Google Scholar] [CrossRef]
- Retallick, R.W.R.; McCallum, H.; Speare, R. Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biol. 2004, 2, e351. [Google Scholar] [CrossRef] [PubMed]
- Searle, C.L.; Biga, L.M.; Spatafora, J.W.; Blaustein, A.R. A dilution effect in the emerging amphibian pathogen Batrachochytrium dendrobatidis. Proc. Natl. Acad. Sci. USA 2011, 108, 16322–16326. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, V.A.; Moore, A.T.; Young, G.R.; Komar, N.; Reisen, W.K.; Brown, C.R. An enzootic vector-borne virus is amplified at epizootic levels by an invasive avian host. Proc. R. Soc. B Biol. Sci. 2011, 278, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.J.; Knapp, R.A.; Vredenburg, V.T. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc. Natl. Acad. Sci. USA 2010, 107, 9695–9700. [Google Scholar] [CrossRef]
- Miller, C.A.; Tasse Taboue, G.C.; Ekane, M.M.P.; Robak, M.; Sesink Clee, P.R.; Richards-Zawacki, C.; Fokam, E.B.; Fuashi, N.A.; Anthony, N.M. Distribution modeling and lineage diversity of the chytrid fungus Batrachochytrium dendrobatidis (Bd) in a central African amphibian hotspot. PLoS ONE 2018, 13, e0199288. [Google Scholar] [CrossRef]
- Hitchman, S.M.; Mather, M.E.; Smith, J.M.; Fencl, J.S. Identifying keystone habitats with a mosaic approach can improve biodiversity conservation in disturbed ecosystems. Glob. Chang. Biol. 2018, 24, 308–321. [Google Scholar] [CrossRef]
- Vredenburg, V.T.; Knapp, R.A.; Tunstall, T.S.; Briggs, C.J. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl. Acad. Sci. USA 2010, 107, 9689–9694. [Google Scholar] [CrossRef]
- Brem, F.; Lips, K. Batrachochytrium dendrobatidis infection patterns among Panamanian amphibian species, habitats and elevations during epizootic and enzootic stages. Dis. Aquat. Organ. 2008, 81, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Kriger, K.M.; Hero, J.-M. The chytrid fungus Batrachochytrium dendrobatidis is non-randomly distributed across amphibian breeding habitats. Divers. Distrib. 2007, 13, 781–788. [Google Scholar] [CrossRef]
- Heard, G.W.; Scroggie, M.P.; Ramsey, D.S.L.; Clemann, N.; Hodgson, J.A.; Thomas, C.D. Can habitat management mitigate disease impacts on threatened amphibians? Conserv. Lett. 2018, 11, e12375. [Google Scholar] [CrossRef]
- Scheele, B.C.; Hunter, D.A.; Grogan, L.F.; Berger, L.; Kolby, J.E.; Mcfadden, M.S.; Marantelli, G.; Skerratt, L.F.; Driscoll, D.A. Interventions for reducing extinction risk in chytridiomycosis-threatened amphibians. Conserv. Biol. 2014, 28, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, F.; Savage, J.M.; Chaves, G. Anfibios y Reptiles de Costa Rica. Listas Zoológicas Actualizadas UCR. Available online: http://museo.biologia.ucr.ac.cr/Listas/Anteriores/HerpCREsp.htm (accessed on 27 June 2019).
- Savage, J.M.; Bolaños, F. A checklist of the amphibians and reptiles of Costa Rica: Additions and nomenclatural revisions. Zootaxa 2009, 2005, 1–23. [Google Scholar] [CrossRef]
- Sasa, M.; Chaves, G.; Porras, L.W. The Costa Rican herpetofauna: Conservation status and future perspectives. In Conservation of Mesoamerican Amphibians and Reptiles; Townsend, J.H., Johnson, J.D., Eds.; Eagle Mountain Press: Salt Lake City, UT, USA, 2010; pp. 510–603. [Google Scholar]
- Chaves, G.; Bolaños, F.; Rodríguez, J.E.; Matamoros, Y. Actualización de las Listas Rojas Nacionales de Costa Rica. Anfibios y Reptiles; Conservation Breeding Specialist Group (SSC/IUCN)/CBSG Mesoamerica): San José, Costa Rica, 2014. [Google Scholar]
- IUCN The IUCN Red List of Threatened Species. Version 2019-1. Available online: https://www.iucnredlist.org/en (accessed on 27 June 2019).
- Wilson, L.D.; McCranie, J.R. The conservation status of the herpetofauna of Honduras. Amphib. Reptile Conserv. 2004, 3, 6–33. [Google Scholar] [PubMed]
- Voyles, J.; Young, S.; Berger, L.; Campbell, C.; Voyles, W.F.; Dinudom, A.; Cook, D.; Webb, R.; Alford, R.A.; Skerratt, L.F.; et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 2009, 326, 582–585. [Google Scholar] [CrossRef]
- Kriger, K.M.; Hines, H.B.; Hyatt, A.D.; Boyle, D.G.; Hero, J.-M. Techniques for detecting chytridiomycosis in wild frogs: Comparing histology with real-time Taqman PCR. Dis. Aquat. Organ. 2006, 71, 141–148. [Google Scholar] [CrossRef]
- Skerratt, L.; Berger, L.; Hines, H.; McDonald, K.; Mendez, D.; Speare, R. Survey protocol for detecting chytridiomycosis in all Australian frog populations. Dis. Aquat. Organ. 2008, 80, 85–94. [Google Scholar] [CrossRef]
- Boyle, D.G.; Boyle, D.B.; Olsen, V.; Morgan, J.A.T.; Hyatt, A.D. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Organ. 2004, 60, 141–148. [Google Scholar] [CrossRef]
- Hyatt, A.D.; Boyle, D.G.; Olsen, V.; Boyle, D.B.; Berger, L.; Obendorf, D.; Dalton, A.; Kriger, K.; Hero, M.; Hines, H.; et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2007, 73, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Kriger, K.M.; Hero, J.-M.; Ashton, K.J. Cost efficiency in the detection of chytridiomycosis using PCR assay. Dis. Aquat. Organ. 2006, 71, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Picco, A.M.; Collins, J.P. Fungal and viral pathogen occurrence in Costa Rican amphibians. J. Herpetol. 2007, 41, 746–749. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Hawley, T.J.; Waits, L.P. Local and regional patterns of amphibian chytrid prevalence on the Osa Peninsula, Costa Rica. Herpetol. Rev. 2009, 40, 309–311. [Google Scholar]
- Whitfield, S.M.; Geerdes, E.; Chacon, I.; Ballestero Rodriguez, E.; Jimenez, R.; Donnelly, M.; Kerby, J. Infection and co-infection by the amphibian chytrid fungus and ranavirus in wild Costa Rican frogs. Dis. Aquat. Organ. 2013, 104, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zumbado-Ulate, H.; Bolaños, F.; Gutiérrez-Espeleta, G.; Puschendorf, R. Extremely low prevalence of Batrachochytrium dendrobatidis in frog populations from Neotropical dry forest of Costa Rica supports the existence of a climatic refuge from disease. EcoHealth 2014, 11, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Zumbado-Ulate, H.; García-Rodríguez, A.; Vredenburg, V.T.; Searle, C.L. Infection with Batrachochytrium dendrobatidis is common in tropical lowland habitats: Implications for amphibian conservation. Ecol. Evol. 2019, 9, 4917–4930. [Google Scholar] [CrossRef] [PubMed]
- Saenz, D.; Adams, C.K.; Pierce, J.B.; Laurencio, D. Occurrence of Batrachochytrium dendrobatidis in an anuran community in the southeastern Talamanca region of Costa Rica. Herpetol. Rev. 2009, 40, 311–313. [Google Scholar]
- Abarca, J.G. Quitridiomicosis en Costa Rica: Aislamiento y Descripción de Cepas Circulantes del Patógeno y Análisis de la Microbiota del Hospedero como Posible Factor en la Incidencia de la Enfermedad. Master’s Thesis, Universidad de Costa Rica, Heredia, Costa Rica, 2018. [Google Scholar]
- Lips, K.R.; Diffendorfer, J.; Mendelson, J.R.; Sears, M.W. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008, 6, e72. [Google Scholar] [CrossRef]
- Lips, K.R.; Burrowes, P.A.; Mendelson, J.R.; Parra-Olea, G. Amphibian declines in Latin America: Widespread population declines, extinctions, and impacts. Biotropica 2005, 37, 163–165. [Google Scholar] [CrossRef]
- Whitfield, S.M.; Lips, K.R.; Donnelly, M.A. Amphibian decline and conservation in Central America. Copeia 2016, 104, 351–379. [Google Scholar] [CrossRef]
- Arias, E.; Chaves, G. 140 years after William M. Gabb’s climb to Cerro Pico Blanco. Mesoam. Herpetol. 2014, 1, 176–180. [Google Scholar]
- Holdridge, L.R. Life Zone Ecology; Tropical Science Center: San Jose, Costa Rica, 1967. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 22 February 2019).
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Ryan, M.; Lips, K.; Giermakowski, J.T. New species of Pristimantis (Anura: Terrarana: Strabomantinae) from lower Central America. J. Herpetol. 2010, 44, 193–200. [Google Scholar] [CrossRef]
- Batista, A.; Hertz, A.; Köhler, G.; Mebert, K.; Vesely, M. Morphological variation and phylogeography of frogs related to Pristimantis caryophyllaceus (Anura: Terrarana: Craugastoridae) in Panama. Salamandra 2014, 50, 155–171. [Google Scholar]
- Kubicki, B.; Salazar, S.; Puschendorf, R. A new species of glassfrog, genus Hyalinobatrachium (Anura: Centrolenidae), from the Caribbean foothills of Costa Rica. Zootaxa 2015, 3920, 069–084. [Google Scholar] [CrossRef]
- Arias, E.; Chaves, G.; Parra-Olea, G. A new species of Craugastor (Anura: Craugastoridae) from the montane rainforest of the Cordillera de Talamanca, Costa Rica. Phyllomedusa J. Herpetol. 2018, 17, 211–232. [Google Scholar] [CrossRef]
- Arias, E.; Chaves, G.; Crawford, A.J.; Parra-Olea, G. A new species of the Craugastor podiciferus species group (Anura: Craugastoridae) from the premontane forest of southwestern Costa Rica. Zootaxa 2016, 4132, 347–363. [Google Scholar] [CrossRef]
- Arias, E.; Hertz, A.; Parra-Olea, G. Taxonomic assessment of Craugastor podiciferus (Anura: Craugastoridae) in lower Central America with the description of two new species. Amphib. Reptile Conserv. 2019, 13, 173–197. [Google Scholar]
- Arias, E.; Chaves, G.; Salazar, S.; Salazar-Zúñiga, J.A.; García-Rodríguez, A. A new species of dink frog, genus Diasporus (Anura: Eleutherodactylidae), from the Caribbean foothills of the Cordillera de Talamanca, Costa Rica. Zootaxa 2019, 4609, 269–288. [Google Scholar] [CrossRef]
- Barquero, M.D.; Araya, M.F. First record of the Greenhouse frog, Eleutherodactylus planirostris (Anura: Eleutherodactylidae), in Costa Rica. Herpetol. Notes 2016, 9, 145–147. [Google Scholar]
- Kubicki, B.; Salazar, S. Discovery of the golden-eyed fringe-limbed treefrog, Ecnomiohyla bailarina (Anura: Hylidae), in the Caribbean foothills of southeastern Costa Rica. Mesoam. Herpetol. 2015, 2, 76–86. [Google Scholar]
- McCranie, J.R. Morphological and systematic comments on the Caribbean lowland population of Smilisca baudinii (Anura: Hylidae: Hylinae) in northeastern Honduras, with the resurrection of Hyla manisorum. Mesoam. Herpetol. 2017, 4, 513–526. [Google Scholar]
- Gray, A.R. Review of the genus Cruziohyla (Anura: Phyllomedusidae), with description of a new species. Zootaxa 2018, 4450, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, B.; Arias, E. A beautiful new yellow salamander, genus Bolitoglossa (Caudata: Plethodontidae), from the northeastern slopes of the Cordillera de Talamanca, Costa Rica. Zootaxa 2016, 4184, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Boza-Oviedo, E.; Rovito, S.M.; Chaves, G.; Garcia-Rodriguez, A.; Artavia, L.G.; Bolaños, F.; Wake, D.B. Salamanders from the eastern Cordillera de Talamanca, Costa Rica, with descriptions of five new species (Plethodontidae: Bolitoglossa, Nototriton, and Oedipina) and natural history notes from recent expeditions. Zootaxa 2012, 3309, 36–61. [Google Scholar] [CrossRef]
- Arias, E.; Kubicki, B. A new moss salamander, genus Nototriton (Caudata: Plethodontidae), from the Cordillera de Talamanca, in the Costa Rica-Panama border region. Zootaxa 2018, 4369, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, B. A new species of salamander (Caudata: Plethodontidae: Oedipina) from the central Caribbean foothills of Costa Rica. Mesoam. Herpetol. 2016, 3, 819–840. [Google Scholar]
- Kubicki, B.; Arias, E. Vulcan’s Slender Caecilian, Caecilia volcani, in Costa Rica. Mesoam. Herpetol. 2017, 4, 488–492. [Google Scholar]
- Jiménez, R.; Alvarado, G. Craugastor escoces (Anura: Craugastoridae) reappears after 30 years: Rediscovery of an “extinct” Neotropical frog. Amphib. Reptil. 2017, 38, 257–259. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Chaves, G.; Benavides-Varela, C.; Puschendorf, R. Where are the survivors? Tracking relictual populations of endangered frogs in Costa Rica. Divers. Distrib. 2012, 18, 204–212. [Google Scholar] [CrossRef]
- Lewis, C.H.R.; Richards-Zawacki, C.L.; Ibáñez, R.; Luedtke, J.; Voyles, J.; Houser, P.; Gratwicke, B. Conserving Panamanian harlequin frogs by integrating captive-breeding and research programs. Biol. Conserv. 2019, 236, 180–187. [Google Scholar] [CrossRef]
- Searle, C.L.; Gervasi, S.S.; Hua, J.; Hammond, J.I.; Relyea, R.A.; Olson, D.H.; Blaustein, A.R. Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conserv. Biol. 2011, 25, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Young, B.E.; Lips, K.R.; Reaser, J.K.; Ibáñez, R.; Salas, A.W.; Cedeño, J.R.; Coloma, L.A.; Ron, S.; La Marca, E.; Meyer, J.R.; et al. Population declines and priorities for amphibian conservation in Latin America. Conserv. Biol. 2001, 15, 1213–1223. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, S.M.; Kerby, J.; Gentry, L.R.; Donnelly, M.A. Temporal variation in infection prevalence by the amphibian chytrid fungus in three species of frogs at La Selva, Costa Rica. Biotropica 2012, 44, 779–784. [Google Scholar] [CrossRef]
- Whitfield, S.M. (Zoo Miami, FL, USA). Personal Communication, 2019.
- Whitfield, S.M.; Alvarado, G.; Abarca, J.; Zumbado-Ulate, H.; Zuñiga, I.; Wainwright, M.; Kerby, J. Differential patterns of Batrachochytrium dendrobatidis infection in relict amphibian populations following severe disease-associated declines. Dis. Aquat. Organ. 2017, 126, 33–41. [Google Scholar] [CrossRef]
- Puschendorf, R.; Hoskin, C.J.; Cashins, S.D.; McDonald, K.; Skerratt, L.F.; Vanderwal, J.; Alford, R.A. Environmental refuge from disease-driven amphibian extinction. Conserv. Biol. 2011, 25, 956–964. [Google Scholar] [CrossRef]
- Perez, R.; Richards-Zawacki, C.L.; Krohn, A.R.; Robak, M.; Griffith, E.J.; Ross, H.; Gratwicke, B.; Ibanez, R.; Voyles, J. Field surveys in Western Panama indicate populations of Atelopus varius frogs are persisting in regions where Batrachochytrium dendrobatidis is now enzootic. Amphib. Reptile Conserv. 2014, 8, 30–35. [Google Scholar]
- Woodhams, D.C.; Kilburn, V.L.; Reinert, L.K.; Voyles, J.; Medina, D.; Ibáñez, R.; Hyatt, A.D.; Boyle, D.G.; Pask, J.D.; Green, D.M.; et al. Chytridiomycosis and amphibian population declines continue to spread eastward in Panama. EcoHealth 2008, 5, 268–274. [Google Scholar] [CrossRef]
- Kilburn, V.L.; Ibáñez, R.; Sanjur, O.; Bermingham, E.; Suraci, J.P.; Green, D.M. Ubiquity of the pathogenic chytrid fungus, Batrachochytrium dendrobatidis, in anuran communities in Panamá. EcoHealth 2010, 7, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Chaves, V.J.; Madrigal-Elizondo, V.; Chaves, G.; Morera-Chacón, B.; Garcia-Rodriguez, A.; Bolaños, F. View of shifts in the diversity of an amphibian community from a premontane forest of San Ramón, Costa Rica. Rev. Biol. Trop. 2019, 67, 259–273. [Google Scholar]
- Garner, T.W.J.; Walker, S.; Bosch, J.; Leech, S.; Marcus Rowcliffe, J.; Cunningham, A.A.; Fisher, M.C. Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos 2009, 118, 783–791. [Google Scholar] [CrossRef]
- Kolby, J.E.; Ramirez, S.D.; Berger, L.; Richards-Hrdlicka, K.L.; Jocque, M.; Skerratt, L.F. Terrestrial dispersal and potential environmental transmission of the amphibian chytrid fungus (Batrachochytrium dendrobatidis). PLoS ONE 2015, 10, e0125386. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Lips, K.R.; Eichholz, M.W. Decline and extirpation of an endangered Panamanian stream frog population (Craugastor punctariolus) due to an outbreak of chytridiomycosis. Biol. Conserv. 2008, 141, 1636–1647. [Google Scholar] [CrossRef]
- Campbell, J.A.; Savage, J.M. Taxonomic reconsideration of Middle American frogs of the Eleutherodactylus rugulosus group (Anura: Leptodactylidae): A reconnaissance of subtle nuances among frogs. Herpetol. Monogr. 2000, 14, 186–292. [Google Scholar] [CrossRef]
- Lips, K.R.; Reeve, J.D.; Witters, L.R. Ecological traits predicting amphibian population declines in Central America. Conserv. Biol. 2003, 17, 1078–1088. [Google Scholar] [CrossRef]
- Köhler, G.; Batista, A.; Carrizo, A.; Hertz, A. Field notes on Craugastor azueroensis (Savage, 1975) (Amphibia: Anura: Craugastoridae). Herpetol Notes 2012, 5, 157. [Google Scholar]
- Hanken, J.; Wake, D.B.; Savage, J.M. A solution to the large black salamander problem (genus Bolitoglossa) in Costa Rica and Panamá. Copeia 2005, 2005, 227–245. [Google Scholar] [CrossRef]
- Sunyer, J.; Wake, D.B.; Obando, L. Distributional data for Bolitoglossa (Amphibia, Caudata, Plethodontidae) from Nicaragua and Costa Rica. Herpetol. Rev. 2012, 43, 564–568. [Google Scholar]
- AlMutairi, B.S.; Grossmann, I.; Small, M.J. Climate model projections for future seasonal rainfall cycle statistics in Northwest Costa Rica. Int. J. Climatol. 2019, 39, 2933–2946. [Google Scholar] [CrossRef]
- Grenyer, R.; Orme, C.D.L.; Jackson, S.F.; Thomas, G.H.; Davies, R.G.; Davies, T.J.; Jones, K.E.; Olson, V.A.; Ridgely, R.S.; Rasmussen, P.C.; et al. Global distribution and conservation of rare and threatened vertebrates. Nature 2006, 444, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.C.; Bosch, J.; Briggs, C.J.; Cashins, S.; Davis, L.R.; Lauer, A.; Muths, E.; Puschendorf, R.; Schmidt, B.R.; Sheafor, B.; et al. Mitigating amphibian disease: Strategies to maintain wild populations and control chytridiomycosis. Front. Zool. 2011, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Garner, T.W.J.; Schmidt, B.R.; Martel, A.; Pasmans, F.; Muths, E.; Cunningham, A.A.; Weldon, C.; Fisher, M.C.; Bosch, J. Mitigating amphibian chytridiomycosis in nature. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160207. [Google Scholar] [CrossRef] [PubMed]
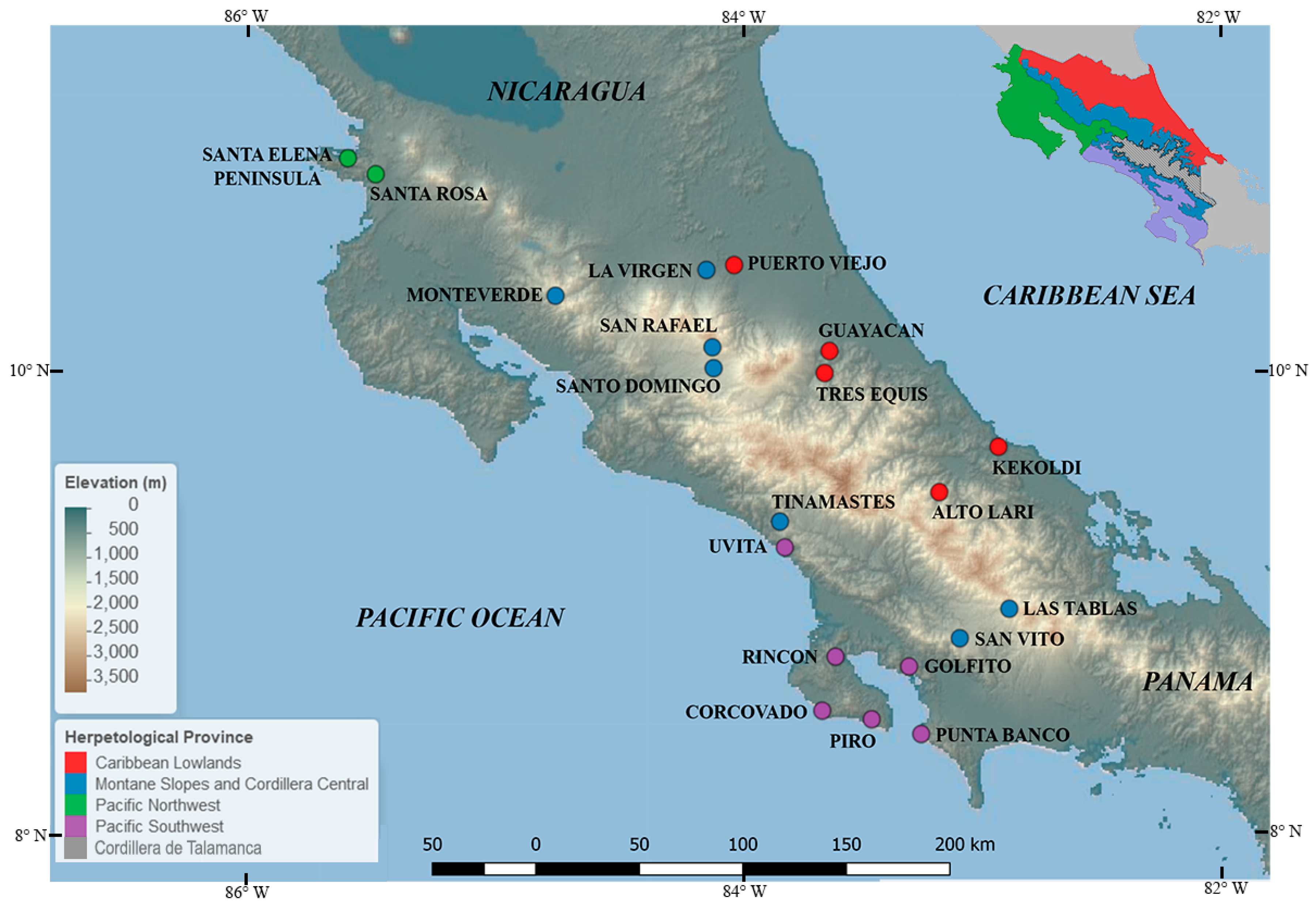


| Study Site (Elevation m) and Herpetological Provinces | Sampling Period | % of Infection (n Sampled) | Altitudinal Belt | Reference |
|---|---|---|---|---|
| Monteverde (1400–2000), MSCC | July 2005 | 12.2 (41) | Lower montane | [59] |
| San Vito de Coto Brus (1120–1385), MSCC | 9.3 (43) | Premontane | ||
| Rincón de Osa (0–100), PS | May–June 2006 | 0.1 (91) | Lowland | [60] |
| Piro (0–100), PS | 0.0 (62) | Lowland | ||
| Corcovado (0–100), PS | 0.1 (25) | Lowland | ||
| Kekoldi (0–100), CL | January 2008 | 7.9 (126) | Lowland | [64] |
| La Virgen de Sarapiquí (0–200), CL | January–March 2011 | 21.3 (253) | Lowland | [61] |
| Santa Elena Peninsula (0–200), PN | January–March 2007–2008 | 0.0 (310) | Lowland | [62] |
| Santa Rosa (0–200), PN | 9.0 (100) | Lowland | ||
| Punta Banco–Burica (0–100), PS | November–December 2011 | 68.6 (35) | Lowland | [63] |
| Rincón de Osa (0–100), PS | 0.0 (25) | Lowland | ||
| Puerto Viejo de Sarapiquí (0–200), CL | 67.4 (144) | Lowland | ||
| Guayacán de Siquirres (400–600), CL | 47.9 (144) | Lowland | ||
| San Vito de Coto Brus (1120–1385), MSCC | Unknown/not indicated | 10.5 (19) | Lowland | [65] |
| Punta Banco–Burica (0–100), PS | 0.0 (20) | Lowland | ||
| Guayacán de Siquirres (400–600), CL | 5.3 (19) | Lowland | ||
| San Rafael de Heredia (1800), MSCC | 66.7 (15) | Lower montane | ||
| Santo Domingo de Heredia (1000–1200), MSCC | 45.5 (11) | Premontane | ||
| Las Tablas (1350), MSCC | 28.6 (14) | Lower montane |
| FRHI | Species | Taxonomic Group (Examples) |
|---|---|---|
| DAA | 2 | Diasporus spp. (dink frogs, e.g., Diasporus diastema) |
| DAT | 5 | Pristimantis spp. (rain frogs, e.g., Pristimantis cerasinus) |
| DRT | 3 | Craugastor punctariolus clade (robber frogs, e.g., Craugastor taurus) |
| C. fitzingeri (Pacific side) | ||
| DTT | 13 | Craugastor spp. (leaf-litter frogs. e.g., Craugastor bransfordi) |
| C. fitzingeri (Caribbean side) | ||
| Plethodontidae (e.g., Oedipina gracilis) | ||
| IAP | 17 | Hylidae (pond-breeding treefrogs, e.g., Boana rufitela) |
| IAR | 15 | Centrolenidae (glass frogs, e.g., Teratohyla pulverata) |
| Hylidae (stream-breeding treefrogs, e.g., Duellmanohyla rufioculis) | ||
| ITF | 4 | Dendrobatidae (Poison-dart frogs, e.g., Oophaga pumilio) |
| ITP | 12 | Leptodactylidae (Leptodactylid frogs, e.g., Leptodactylus melanonotus) |
| Microhylidae (sheep frogs, e.g., Hypopachus variolosus) | ||
| Ranidae (Ranid frogs, e.g., Lithobates forreri) | ||
| Bufonidae (toads, e.g., Incilius coccifer) | ||
| ITR | 7 | Bufonidae (river toads, e.g., Rhaebo haematiticus) |
| Order | Family | Species | Source |
|---|---|---|---|
| Anura | Centrolenidae | Hyalinobatrachium dianae | [75] |
| Craugastoridae | Craugastor aenigmaticus | [76] | |
| Craugastor gabbi | [77] | ||
| Craugastor zunigai | [78] | ||
| Eleutherodactylidae | Diasporus amirae | [79] | |
| Eleutherodactylus planirostris * | [80] | ||
| Hylidae | Ecnomiohyla bailarina | [81] | |
| Ecnomiohyla veraguensis | Unpublished | ||
| Smilisca manisorum | [82] | ||
| Phyllomedusidae | Cruziohyla sylviae | [83] | |
| Caudata | Plethodontidae | Bolitoglossa aurae | [84] |
| Bolitoglossa aureogularis | [85] | ||
| Bolitoglossa kamuk | [85] | ||
| Bolitoglossa pygmaea | Unpublished | ||
| Bolitoglossa splendida | [85] | ||
| Nototriton costaricense | [86] | ||
| Nototriton matama | [85] | ||
| Oedipina berlini | [87] | ||
| Oedipina nimaso | [85] | ||
| Gymnophiona | Caeciliidae | Caecilia volcani | [88] |
| Species (FRHI) | Bd + Swabs | Bd Load Average (SE) | Log10 Bd Load Average (SE) |
|---|---|---|---|
| Agalychnis callidryas (IAP) | 4 | 8.19 (3.81) | 0.77 (0.21) |
| Agalychnis spurrelli (IAP) | 5 | 39.83 (32.47) | 1.10 (0.30) |
| Boana rufitela (IAP) | 8 | 8.41 (4.12) | 0.53 (0.23) |
| Bolitoglossa colonnea (DTT) | 1 | 1.83 (0.00) | 0.26 (0.00) |
| Cochranella granulosa (IAR) | 1 | 3.95 (0.00) | 0.60 (0.00) |
| Craugastor bransfordi (DTT) | 23 | 1007.06 (483.50) | 1.78 (0.25) |
| Craugastor crassidigitus (DTT) | 5 | 1636.74 (1583.43) | 1.69 (0.64) |
| Craugastor fitzingeri (DTT, DRT) | 44 | 951.48 (310.27) | 1.97 (0.17) |
| Craugastor megacephalus (DTT) | 1 | 0.62 (0.00) | −0.21 (0.00) |
| Craugastor mimus (DTT) | 9 | 125.48 (74.69) | 1.01 (0.46) |
| Craugastor ranoides (DRT) | 3 | 187.40 (174.13) | 1.65 (0.55) |
| Craugastor stejnegerianus (DTT) | 2 | 2.18 (0.95) | 0.29 (0.20) |
| Craugastor taurus (DRT) | 12 | 11,632.50 (6564.67) | 2.51 (0.41) |
| Dendropsophus ebraccatus (IAP) | 34 | 315.85 (194.09) | 1.00 (0.18) |
| Diasporus diastema (DAA) | 2 | 14.44 (4.12) | 1.14 (0.13) |
| Duellmanohyla rufioculis (IAR) | 1 | 3.65 (0.00) | 0.56 (0.00) |
| Engystomops pustulosus (ITP) | 11 | 34.83 (13.26) | 1.11 (0.3) |
| Espadarana prosoblepon (IAR) | 3 | 3691.59 (3684.73) | 1.06 (1.75) |
| Hyalinobatrachium colymbiphyllum (IAR) | 1 | 0.01 (0.00) | −2.00 (0.00) |
| Hyalinobatrachium valerioi (IAR) | 2 | 8.38 (2.04) | 0.91 (0.11) |
| Incilius melanochlorus (ITR) | 2 | 23.27 (19.93) | 1.08 (0.56) |
| Leptodactylus melanonotus (ITP) | 4 | 11.86 (0.66) | 1.07 (0.02) |
| Leptodactylus poecilochilus (ITP) | 1 | 1073.45 (0.00) | 3.03 (0.00) |
| Leptodactylus savagei (ITP) | 1 | 33.49 (0.00) | 1.52 (0.00) |
| Lithobates forreri (ITP) | 2 | 569.24 (241.10) | 2.71 (0.20) |
| Lithobates warszewitschii (ITR) | 14 | 978.92 (801.60) | 1.47 (0.31) |
| Oophaga granulifera (ITF) | 9 | 23.92 (11.31) | 1.20 (0.11) |
| Oophaga pumilio (ITF) | 34 | 1765.81 (778.67) | 1.71 (0.25) |
| Pristimantis cerasinus (DAT) | 9 | 14.82 (10.97) | 0.47 (0.32) |
| Pristimantis ridens (DAT) | 7 | 48.37 (32.34) | 0.69 (0.50) |
| Rhaebo haematiticus (ITR) | 22 | 239.20 (178.56) | 0.70 (0.26) |
| Scinax boulengeri (IAP) | 1 | 195.20 (0.00) | 2.29 (0.00) |
| Scinax elaeochroa (IAP) | 5 | 1384.15 (1350.51) | 1.78 (0.58) |
| Smilisca phaeota (IAP) | 4 | 37.25 (19.15) | 1.44 (0.18) |
| Smilisca sordida (IAP) | 46 | 14.96 (9.27) | 0.24 (0.16) |
| Teratohyla pulverata (IAR) | 2 | 34.53 (22.92) | 1.41 (0.35) |
| Teratohyla spinosa (IAR) | 5 | 937.99 (825.54) | 1.90 (0.57) |
| Tlalocohyla loquax (IAP) | 11 | 144.66 (107.28) | 1.22 (0.30) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zumbado-Ulate, H.; Nelson, K.N.; García-Rodríguez, A.; Chaves, G.; Arias, E.; Bolaños, F.; Whitfield, S.M.; Searle, C.L. Endemic Infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for Amphibian Conservation at Regional and Species Level. Diversity 2019, 11, 129. https://doi.org/10.3390/d11080129
Zumbado-Ulate H, Nelson KN, García-Rodríguez A, Chaves G, Arias E, Bolaños F, Whitfield SM, Searle CL. Endemic Infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for Amphibian Conservation at Regional and Species Level. Diversity. 2019; 11(8):129. https://doi.org/10.3390/d11080129
Chicago/Turabian StyleZumbado-Ulate, Héctor, Kiersten N. Nelson, Adrián García-Rodríguez, Gerardo Chaves, Erick Arias, Federico Bolaños, Steven M. Whitfield, and Catherine L. Searle. 2019. "Endemic Infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for Amphibian Conservation at Regional and Species Level" Diversity 11, no. 8: 129. https://doi.org/10.3390/d11080129
APA StyleZumbado-Ulate, H., Nelson, K. N., García-Rodríguez, A., Chaves, G., Arias, E., Bolaños, F., Whitfield, S. M., & Searle, C. L. (2019). Endemic Infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for Amphibian Conservation at Regional and Species Level. Diversity, 11(8), 129. https://doi.org/10.3390/d11080129





