Abstract
Gastrotricha is a group of meiofaunal-sized, free-living invertebrates present in all aquatic ecosystems. The phylum includes over 860 species globally, of which 505 nominal species have been recorded in marine sandy sediments; another 355 taxa inhabit the freshwater environments, where they are recurrent members of the periphyton and epibenthos, and, to a lesser degree, of the plankton and interstitial fauna. Gastrotrichs are part of the permanent meiofauna and, in general, they rank among the top five groups for abundance within meiobenthic assemblages. The diversity, abundance, and ubiquity of Gastrotricha allow us to suppose an important role for these animals in aquatic ecosystems; however, ecological studies to prove this idea have been comparatively very few. This is mainly because the small size and transparency of their bodies make gastrotrichs difficult to discover in benthic samples; moreover, their contractility and fragility make their handling and morphological survey of the specimens rather difficult. Here we offer an overview, describe the basic techniques used to study these animals, and provide a key to known genera in an attempt to promote easy identification and to increase the number of researchers who may be interested in conducting studies on this understudied ecological group of microscopic organisms.
1. Introduction
Gastrotrichs are minute (from 60 μm to 3.5 mm in total length) vermiform, acoelomate invertebrates; they inhabit the aquatic ecosystems of the world as part of the meiofaunal communities. In freshwater habitats, gastrotrichs are members of the benthos and periphyton and, to a limited degree, also of the plankton and psammon. In marine settings, these tiny animals inhabit (mostly) the interstice of the sandy habitats and are usually the third group in density among the interstitial meiofaunal taxa, behind the nematodes and the harpacticoid copepods (e.g., abundance up to 364 ind./10 cm2) [1]; however, several studies have found them to be the second or the first most abundant meiobenthic group [2,3,4,5]. In inland waters, the group usually figures among the top five most abundant taxa, and populations may attain a density of 2600 ind./10 cm2 [6]. In marine as well as in freshwater systems, the ecological role of Gastrotricha is accomplished within the detritivorous, microphagous benthic assemblage. Gastrotrichs feed on bacteria, microscopic algae, and small protists; food is ingested by aspiration thanks to the powerful, triradiated, myoepithelial pharynx. In turn, they represent prey for small macrofauna, carnivorous ciliates, and free-living flat worms. The gastrotrichs’ ecological disparity is coupled with an ample morphological diversity which may seem amazing when comparing the large and vermiform marine representatives with the tiny and tenpin-shaped freshwater forms. Despite their variety, gastrotrichs are considered to constitute a monophyletic group (phylum) based on the following synapomorphies: (1) cuticle made up of two layers, with the external layer (epicuticle) consisting of one or more plasma-membrane-like sheets (lamellar layer); (2) epicuticle covering the entire body, including the locomotor and sensorial cilia; (3) a “duo-gland system” adhesive apparatus lacking an anchor cell; and 4) peculiar helicoidal muscles enwrapping the anterior portion of the alimentary canal [7,8]. The phylum has a worldwide distribution with some 860 nominal species (as of July 2019) distributed into two orders: Chaetonotida, including 483 tenpin- or bottle-shaped species, two-thirds of which are found in inland ecosystems, and Macrodasyida, grouping 377 vermiform species, the vast majority of which are marine or, more rarely, estuarine. Only four macrodasyidan species, belonging to the genera Marinellina (1 sp.) and Redudasys (3 spp.), have been reported from freshwater habitats to date. The current classification sees the order Chaetonotida divided into 8 families and 32 genera, whereas the order Macrodasyida counts 10 families and 36 genera. The continuous description of new taxa (species, genera) and the ongoing process of re-systematization suggest that we should consider the statistics reported above as being highly conservative.
Phylogenetic relationships of the Gastrotricha have been questioned for a long time. By virtue of their morphological traits, many researchers have considered Gastrotricha to be close relatives of Nematoda, Rotifera, Gnathostomulida, or Kinorhyncha, within large assemblages such as the Aschelminthes, Pseudocoelomates, etc. However, phylogenetic analyses of the “Aschelminthes”, grounded on genetic traits (e.g., 18S rRNA gene) showed such groupings to be polyphyletic and Gastrotricha as part of the Lophotrochozoa but with unstable alliances within the clade [9]. Recent phylogenomic studies have also dismissed the Platyzoa clade, within which Gastrotricha has been allocated for some time, and have convincingly shown Gastrotricha together with the Platyhelminthes allied in a clade named Rouphozoa as a subset within the protostomian Spiralia [10,11]. Parts of the in-group phylogenetic relationships remain unclear, e.g., the evolutionary relationships between the representatives of the two orders or within the clearly paraphyletic family Chaetonotidae. Fortunately, relationships among taxa belonging to several families, especially of the Macrodasyida, are becoming less obscure [9,12,13,14,15,16,17,18,19].
Recent overviews of the gastrotrichs’ biology and morphology have been offered by several authors [20,21,22]. Updated information regarding, e.g., classification, distribution, literature, etc., can be found at the dedicated Gastrotricha World Portal [23] and through the World Register of Marine Species (WoRMS) [24].
2. Materials and Methods
2.1. Sampling
Sampling procedures in freshwater environments and marine ecosystems are usually analogous; qualitative studies implicate the gathering of sediment by mean of a scoop, spoon, plastic jar, or a hand-held planktonic net, while quantitative research typically uses corers of clear plastic or Plexiglas (2–5 cm inner diameter, 10–20 cm long). The sea-dweller taxa are typically interstitial, inhabiting preferentially clean, fine to medium sands, with some occurring in muddy substrata (e.g., Musellifer spp.) and a few that are tolerant of high sulphide or organic loads [25,26,27,28,29,30].
Qualitative intertidal sampling is typically carried out at low tide; pits are dug in the beach, and the sand from the bottom and the wall of the pits is then removed with a spoon or scoop and transferred to plastic jars (Supplementary Material Figure S1); subtidal material for qualitative studies can be taken directly by scooping up the upper 10 cm sediment surface with a plastic container (e.g., a 500 mL jar), which is immediately closed off underwater (Supplementary Material Figure S2). Jars filled with sand are then transported to the laboratory and allowed to rest for some time (1 h to overnight) at a suitable temperature. Over the hours, the fauna move upward and will enrich the top layers of the sand, facilitating the following extraction process (see below). The horizontal distribution of Gastrotricha is patchy; consequently, the collection of several small samples is more illustrative of the taxonomic assemblage of a location than a sole big sample. Interstitial forms of freshwater habitats may be collected using similar techniques. Freshwater gastrotrichs that live on the surfaces of rooted aquatic plants, along with benthic, periphytic, and semiplanktonic taxa, are qualitatively sampled by gathering bunches of vegetation together with the bottom deposits and filtering the water through a net or a sieve with mesh of appropriate size (e.g., 25–30 μm) (Supplementary Material Figure S3). The gastrotrich-enriched sample is placed in buckets and rapidly transported to the laboratory where it is subsequently moved to small aquaria, kept at a suitable temperature, and moderately oxygenated with an air stone (Supplementary Material Figure S4).
2.2. Extraction
Freshwater and marine samples should both be processed within 5–6 days to obtain the living specimens, which are normally better suited than preserved animals for taxonomic purposes (e.g., identification) since fixation generally causes artifacts that alter and/or obscure the diagnostic characteristics. For freshwater samples only, additional checks 2–4 weeks after sampling are advisable, since taxa initially absent may be found later due to the hatching of resting eggs.
Interstitial fauna can efficiently be separated (extracted) from the sand by narcotization and decantation, using a solution of MgCl2 (7% marine sample or 1% freshwater sample) as a narcotic. For this purpose, 1–2 spoons of the fauna-enriched top layer of sand (see above) are placed into a small vessel with a sufficient amount of added narcotic solution to cover the sand. The material is then swirled and allowed to sit for 5 minutes, after which it is gently swirled again and the liquid decanted into small Petri dishes (5.5 cm). At this stage, a small amount of either seawater (marine samples) or freshwater (freshwater sample) is added to each Petri dish, which is then scanned for gastrotrichs using a binocular microscope at 40–50× magnification, preferably in transmitted light (Supplementary Material Figures S5,S6).
The freshwater, non-sandy samples placed in the small aquaria, as reported above, may be processed for gastrotrichs by sucking up with a large pipet a small amount of the detritus and the overlying water and by transferring the sucked material to a large Petri dish (9.5–12 cm); the dish is then scanned for active (motile) gastrotrichs under a dissecting microscope as described above (Supplementary Material Figure S4). Alternatively, material collected with the large pipet may be transferred to a glass flask, with an equal quantity of 2% MgCl2 solution added, aliquoted into Petri dishes of suitable diameter, and thence analyzed for narcotized gastrotrichs under a dissecting microscope.
For obvious reasons, quantitative studies should be based on fixed material. To reduce artifacts that may hamper species identification, treatment of the freshly collected material (samples) with a solution of magnesium chloride (7% for marine or 1% for freshwater samples) for 5–10 min is very much suggested before the material is fixed. Fixation may be done using a solution of 10% borax-buffered formalin; later, some rose bengal (1%) may be added to ease sorting. Gastrotrich specimens in the quantitative samples may be extracted from the sandy substrata using the same techniques used for other meiofaunal taxa, e.g., by elutriation and multiple decantations. Extraction from samples containing fine sediment and rich in detritus can be carried out by centrifugation using the silica gel LUDOX AM (d = 1.210) to create a gradient [31]. The supernatant should be filtered using a 20–30 μm mesh sieve to concentrate the gastrotrichs.
2.3. Morphological Analysis
Morphometric data should be acquired on living, relaxed specimens mounted on a microscope slide and covered with a square coverslip (15–18 mm). As the mounting of a gastrotrich may be tricky, the following practice carried out routinely at the first author laboratory may facilitate the task. To mount the specimen of interest, a drop of the same medium the specimen is extracted from is put on a clean microscope slide and a single gastrotrich is transferred to it by using a micropipette (mouth or hand held). In the case of freshwater medium, to relax/anesthetize the specimen, a small amount of 1% magnesium chloride solution can be added to the liquid containing the gastrotrich; alternatively, small crystals of alkaloids such as novocaine or procaine are put at the edge of the water so they dissolve gradually in the water, anesthetizing the animal. Thereafter, a clean coverslip (cover glass) is carefully put on the water. To avoid excessive animal compression, the coverslip should not be used as it is; instead, small modelling clay posts are attached beneath its corners before it is put in place (Supplementary Material Figures S7,S8). As deep morphological survey requires the use of oil immersion optics (e.g., 60×, 100×) it is important the specimen be positioned far from the sides of the coverslip. Proper positioning of the specimen at (or near) the center of the slide and its dorso-ventral orientation may be attained by adding a tiny amount of the liquid medium to the cover glass sides or by absorbing the liquid with a piece of blotting paper. Animals gently compressed between the slide and the coverslip are then observed under an upright biological microscope, preferably using DIC (differential interference contrast) lenses (Supplementary Material Figure S9). Fine anatomical traits may necessitate SEM observation; for this purpose, specimens are opportunely prepared (e.g., by hesamethildysilazane or the CPD (critical point drying) technique) [32,33].
Identification of formalin-fixed gastrotrichs from a location may be facilitated by a preliminary identification of the local fauna based on living specimens. Regardless, identification of preserved material can be executed on animals included in watery solutions, or better, established on (semi)permanent mounts. The latter can be set by including the gastrotrichs in a solution of glycerol–formalin (1:3), and the coverslip is then sealed with nail polish or Glyceel. Alternatively, specimens may be mounted in absolute glycerol on an H-S slide after immersion in a 10% glycerol–ethanol solution, which is allowed to evaporate in an oven at 40 °C for 2–4 days [34]. However, in many cases, permanent mounts—even in the case of uncontracted, well-oriented specimens—do not permit a full identification as many of the diagnostic traits deteriorate over time. Consequently, for taxonomic purposes, photos or high-resolution video sequences of living, relaxed specimens may deliver superior long-lasting records of the anatomical characteristics of a species compared with specimens mounted on microscope slides.
2.4. Taxonomic Key
The following key, modified from [35], encompasses the valid families and genera of Gastrotricha (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19 and Figure 20) described to date [24]. Two families (Redudasyidae and Hummondasyidae, belonging to Macrodasyida) and five genera (Bifidochaetus and Cephalionotus belonging to Chaetonotida and Anandrodasys, Hummondasys, Thaidasys, and Kryptodasys belonging to Macrodasyida) included herein have been established since the publication of the previous keys [12,13,14,15,36,37]. For the inclusion of Megadasys among the Planodasyidae (order Macrodasyida), see [18].
The key is designed to be used by researchers and students who have a general knowledge on how to identify animals but may not have much expertise on Gastrotricha; it is practical in style and is grounded on relevant discriminatory traits as they appear in relaxed mature animals. In most cases, anatomical traits are those which are easily visible using differential interference contrast optics and which are countable. However, to facilitate the assignment, it is imperative that the mounted specimen to be identified is oriented in a dorso-ventral fashion. The following abbreviations are used in the key: PhIJ, pharyngo-intestinal junction; TbA, adhesive tubes of the anterior series; TbD, adhesive tubes of the dorsal series; TbP, adhesive tubes of the posterior series; TbV, adhesive tubes of the ventral series.
3. Results
Key to Families and Genera of Gastrotricha
- 1a
- Body flask-, bottle- or tenpin-shaped; posterior body region usually furcate (furca), less often rounded off or bifurcate; cuticle usually forming ornamentations such as scales and spines; TbA, TbD, and TbL absent; TbP present, numbering 2 (rarely 4 or 0) at the distal end of the furcal rami; mouth narrow; pharynx lacking pores. Freshwater, marine, and brackish: periphytic, epibenthic, and interstitial, occasionally semiplanktonic. Order CHAETONOTIDA, Suborder PAUCITUBULATINA (Figure 1A–C) ……………….…….... 38
- 1b
- Different from the above. …………….………..…………………………………..……....…….... 2
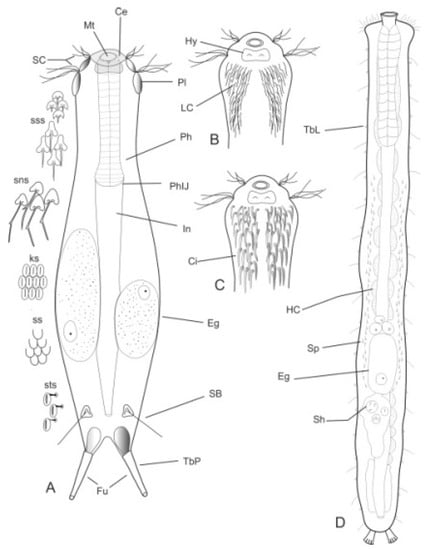
Figure 1.
Drawings of hypothetical Chaetonotida: (A–C) Paucitubulatina and (D) Multitubulatina (Neodasys). (A) Habitus, dorsal view; (B,C) anterior region, ventral view, showing different arrangement of the locomotor ciliations. Abbreviations: Ce, cephalion; Ci, locomotor cirri; eg, egg; Fu, furca; HC, hemoglobin-containing cell; Hy, hypostomion; In, intestine; LC, locomotor cilia; Mt, mouth; Ph, pharynx; PhIJ, pharyngo-intestinal junction; Pl, pleuria; SB, sensorial bristle; SC, sensorial cilia; sk, scales with a keel; sns, scales with notched spines; Sh, spermatophores; Sp, spermatozoa; ss, scales smooth; sss, scales with simple spines; sts, scale with a stalk; TbL, lateral adhesive tubes; TbP, posterior adhesive tubes. (A–C), original; scales redrawn and modified from [38]; (D), redrawn and modified from [39].
- 2a (1b)
- Body vermiform; cuticle naked, not forming scales and/or spines; TbA and TbD absent; TbL present in the form of numerous papillae along each side; TbP, some per side, fused at their bases forming two adhesive structures; mouth narrow, pharynx lacking pores. Uncommon; marine: interstitial. Order CHAETONOTIDA, Suborder MULTITUBULATINA, NEODASYIDAE …………………………………………………….……... Neodasys (Figure 1D)
- 2b (1b)
- Body usually vermiform, occasionally tenpin-shaped; cuticle naked or forming ornamentations such as plates and multi-pointed hooks; TbA, TbL, and TbP present, usually numerous; TbD present in several taxa; mouth opening narrow to broad; pharyngeal pores usually present. Marine and brackish, rarely fresh water: interstitial. Order MACRODASYIDA (Figure 2) ........... 3
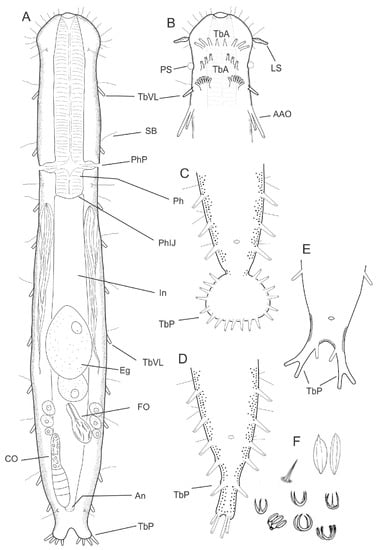
Figure 2.
Drawing of a hypothetical Macrodasyida. (A) Habitus showing the internal organs, dorsal view; (B) anterior region, ventral view, showing some of the possible arrangements of adhesive tubes of the anterior series, i.e., borne, singly, directly from the body surface (the two most anterior ones), and borne in a group on a fleshy extensible base (the posterior one); (C–E), some of the possible configurations of the posterior region, ventral view; (F) some of the possible spines and scales found among Macrodasyida. Abbreviations: AAO, accessory adhesive organs; An, anus; CO, caudal organ; Eg, egg; Fo, frontal organ; In, intestine; LS, leaf-like sensorial organ; Ph, pharynx; PhIJ, pharyngo-intestinal junction; PhP, pharyngeal pores; PS, piston pit sensorial organ; TbA, anterior adhesive tubes; borne singly, directly from the body; TbP, posterior adhesive tubes; TbVL, ventrolateral adhesive tubes; Te, testicle. (A–C) original; (D) modified from [13]; (E) modified [15].
- 3a (2b)
- Marine or brackish. ...……………………...……….……………………………………………… 4
- 3b (2b)
- Freshwater. ...…………...…………………....……………….......…………………....……..……37
- 4a (3a)
- Body tenpin-shaped; head well discernible, including most of pharynx; TbD absent; posterior body region lobed, furcate, or bifurcate. Cuticle bare or developing thickenings and ridges. ..……………………………………………………………………………...……...… 5
- 4b (3a)
- Body vermiform, head usually indistinct or, when distinct, includes only part of pharynx; cuticle naked or developing spines and/or scales. …...……….………………….…….....…... 9
- 5a (4a)
- Cuticle naked; dorsal side of the trunk naked; chordoid organ not present. Common to rare; marine and brackish: interstitial. DACTYLOPODOLIDAE (Figure 3) ….………..…...……. 6
- 5b (4a)
- Cuticle often developing thickenings and ridges; if naked, the trunk bears, on the dorsal side, long rod-like structures; chordoid organ present. Rare; marine: interstitial. XENODASYIDAE (Figure 4) ……………………………………..………………….…....…..… 8
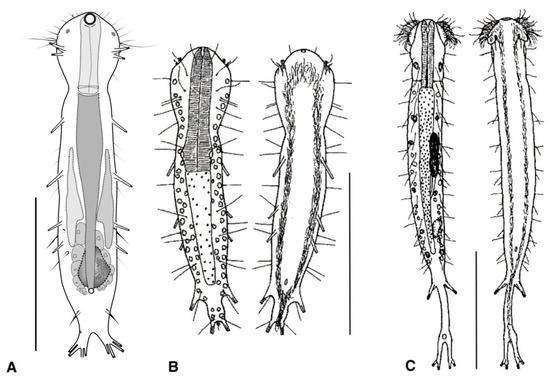
Figure 3.
Macrodasyida, Dactylopodolidae: models of (A) Dactylopodola, (B) Dendropodola, and (C) Dendrodasys. Scale bars = 100 μm. (A) from [40] with modifications, (B) from [41] with modifications, and (C) from [42] with modifications.
- 6a (5a)
- Head simple or bearing two sensorial tentacles; cuticular covering bare; posterior body region bilobed; TbL present. Regionally common; marine: interstitial. .......... Dactylopodola (Figure 3A)
- 6b (5a)
- Head simple or with crenulated lateral lobes; cuticular covering bare; posterior body region bifurcate; TbL absent. ………………………….………………………………………………….. 7
- 7a (6b)
- Head simple, cuticle naked. Rare; marine: interstitial. …………….. Dendropodola (Figure 3B)
- 7b (6b)
- Head with elongate crenulated lateral lobes. Uncommon; marine: interstitial. ………………………………………………………………………………. Dendrodasys (Figure 3C)
- 8a (5b)
- Trunk region without tentacles, but presenting dented lateral sides; posterior body region furcate; distal rami, each showing a small TbP. Rare; marine: interstitial. ......................................................................................................................... Xenodasys (Figure 4A)
- 8b (5b)
- Trunk region bearing numerous tentacles; lateral sides of the trunk region parallel, lacking indentations; posterior region furcate; each ramus showing an adhesive pad at the end. Rare; marine: interstitial. ………………………………………..………........ Chordodasiopsis (Figure 4B)
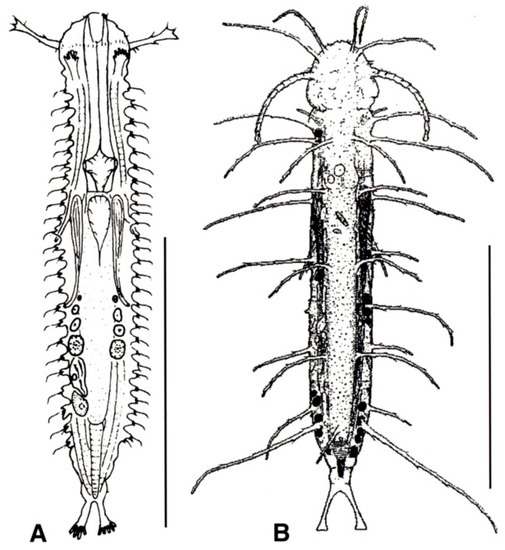
Figure 4.
Macrodasyida, Xenodasyidae: models of (A) Xenodasys and (B) Chordodasiopsis. Scale bars = 200 μm. (A) from [39] with modifications, (B) from [43] with modifications.
- 9a (4b)
- TbA, usually 4 or more per side, occasionally 2 or 3, at the end of extensible fleshy base (Figure 2B); pharynx with pores located at the base. ................................................................. 10
- 9b (4b)
- TbA, generally 1 to 3 per side, occasionally 4 or more, arising singly and directly from the body surface; pharynx with pores at the base or in the middle. .............................................. 16
- 10a (9a)
- Head generally well demarcated posteriorly by a furrow; posterior body region tapered into a medial process, truncated, rounded, or broadly expanded, but never two-lobed. CEPHALODASYIDAE (partim) (Figure 5) .................................................................................. 11
- 10b (9a)
- Head normally not clearly delimited; posterior body region two-lobed. TURBANELLIDAE (partim) (Figure 6) ........................................................................................................................... 12
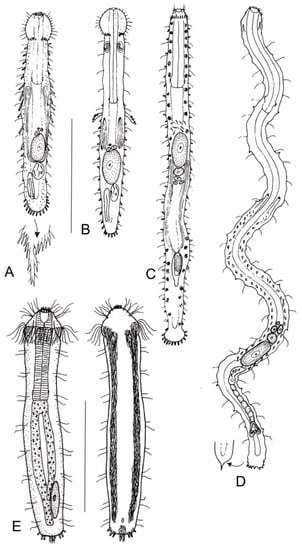
Figure 5.
Macrodasyida, Cephalodasyidae: models of (A) Cephalodasys, (B) Pleurodasys, (C) Mesodasys, (D) Dolichodasys, and (E) Paradasys. Scale bars = 200 μm. (A–D) from [39] with modifications, (E) from [35] with modifications.
- 11a (10a)
- Head surrounded by very thick and dense sensory cilia; a couple of accessory adhesive organs present near the PhIJ, laterally directed; each organ comprising 3–4 tubes of unequal length; a couple of club-shaped gravireceptor organs on the dorsal side of the posterior cephalic region may be present. Rare; marine: interstitial. ……...….. Pleurodasys (Figure 5B)
- 11b (10a)
- Cephalic sensory cilia and accessory adhesive organs described above are absent. Regionally common; marine and brackish: interstitial. ................... Cephalodasys (Figure 5A)
- 12a (10b)
- Head showing elongate lateral tentacles. …………………………………….….....………… 13
- 12b (10b)
- Head without tentacles, occasionally with conical lobes. ……………………...….......…… 14
- 13a (12a)
- TbL numerous. Uncommon; marine: interstitial. ……………..…..…. Dinodasys (Figure 6A)
- 13b (12a)
- TbL lacking, paired TbV inserted just past the PhIJ. Rare; marine: interstitial. ................. ............................................................................................................. Pseudoturbanella (Figure 6B)
- 14a (12b)
- Paired accessory adhesive organs in the anterior pharyngeal region; organs are posteriorly directed, and each is made up of two tubes of unequal lengths. Common; marine and brackish: interstitial. ........………….…..…………………..…..…… Paraturbanella (Figure 6C)
- 14b (12b)
- Accessory adhesive tubes described above are either absent or present in different body regions. ………………………………………………………………………………………...…... 15
- 15a (14b)
- Accessory adhesive tubes not present. Common; marine and brackish: interstitial. ………….……………………………………………………………….……Turbanella (Figure 6E)
- 15b (14b)
- Accessory adhesive tubes present, close to the PhIJ. Rare; marine: interstitial. …………………..…..………………...........................................…... Prostobuccantia (Figure 6D)
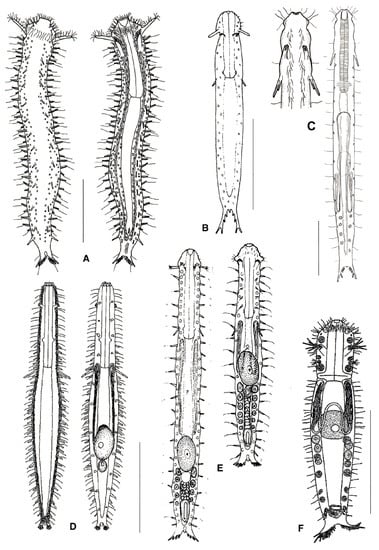
Figure 6.
Macrodasyida, Turbanellidae: models of (A) Dinodasys, (B) Pseudoturbanella, (C) Paraturbanella, (D) Prostobuccantia, (E) Turbanella, and (F) Desmodasys. Scale bars = 200 μm. (A) from [44] with modifications, (B) from [39] with modifications, (C) from [45] with modifications, (D) from [46] with modifications, and (F) from [47] with modifications.
- 16a (9b)
- Pharynx with pores far from the base; posterior body region unilobed, ovoidal in shape, or tapering off. MACRODASYIDAE (Figure 7) …......................................................................…. 17
- 16b (9b)
- Pharynx with pores at the base; posterior end of body not tapering off ……………...….… 20
- 17a (16a)
- Head bearing a lateral leaf-like sensorial organ; posterior body region unilobed, ovoidal in shape. Rare; marine: interstitial. …….…………….…………………. Thaidasys (Figure 7B,C)
- 17b (16a)
- Head bearing lateral piston pit sensorial organs; posterior body region tapering into a medial process. ...……………………………………..…………………………………………… 18
- 18a (17b)
- Posterior process in the form of a long tail. Regionally common; marine: interstitial and epibenthic. …………………………………………….……………….……. Urodasys (Figure 7E)
- 18b (17b)
- Posterior process short or in the form of a short tail. ………………….……………….…… 19
- 19a (18b)
- Frontal organ posterior to the largest egg; spermatozoa filiform. Common; marine: interstitial. …..……………….…..……..………………………..…..…... Macrodasys (Figure 7D)
- 19b (18b)
- Frontal organ anterior to the largest egg; spermatozoa stout. Uncommon; marine: interstitial. ……………………...……..…………………………….…... Kryptodasys (Figure 7A)
- 20a (16b)
- Cuticle forming ornamentations such as hooks, papillae, scales, or thickenings. ..……..... 21
- 20b (16b)
- Cuticle smooth, without ornamentation such those reported above. ...……….……..….… 27
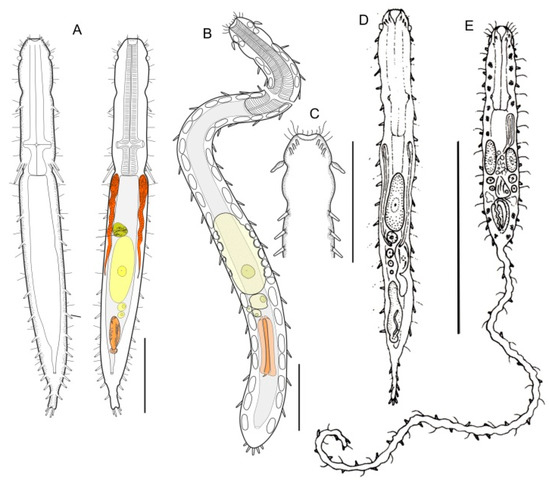
Figure 7.
Macrodasyida, Macrodasyidae: models of (A) Kryptodasys, (B, C) Thaidays, (D) Macrodasys, and (E) Urodasys. Scale bars: (A–C) = 100 μm, (D, E) = 200 μm. (A) from [15] with modifications, (B, C) from [14] with modifications, and (D, E) from [39] with modifications.
- 21a (20a)
- ) Presence of elongate scales; mouth narrow; pharynx without pores. Uncommon; marine: interstitial. LEPIDODASYIDAE ................................................…….... Lepidodasys (Figure 8)
- 21b (20a)
- Presence of variously spined hooks, large scales, or papillae; mouth opening generally broad; pharyngeal pores present. THAUMASTODERMATIDAE (partim) (Figure 9) ……………... 22
- 22a (21b)
- Presence of papillae or large scales. ...…………………..……………………….……………. 23
- 22b (21b)
- Presence of uni- or multi-spined hooks. ……………………….………………………….…. 24
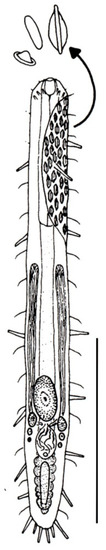
Figure 8.
Macrodasyida, Lepidodasyidae: model of Lepidodasys. Scale bar = 200 μm. From [39] with modifications.
- 23a (22a)
- Cuticle with large scales, but not papillae; on either side of the body a single row of wide spines present. Regionally common; marine: interstitial. ..........….. Diplodasys (Figure 9A)
- 23b (22a)
- Cuticle with papillae, but not scales or spines. Uncommon; marine: interstitial. ....................………………………...…………………………..……..….... Oregodasys (Figure 9B)
- 24a (22b)
- Cuticle with hooks showing a single spine; right and left testicles present; Common; marine: interstitial. .....…….……...…….…………………..……....… Acanthodasys (Figure 9C)
- 24b (22b)
- Cuticle with hooks showing more than one spine; a single testicle on the right-hand body side. ……………………………………………………………………………………………….... 25
- 25a (24b)
- Anterior body region showing conspicuous, grasping structures on either side of the mouth funnel (buccal palps); hooks bearing 5, 4, or 3 spines (penta-, tetra-, or triancres). Common; marine: interstitial. …………………………………….. Pseudostomella (Figure 9D)
- 25b (24b)
- Anterior body region without buccal palps; hooks showing 5, 4, 3, or 2 spines (penta-, tetra-, tri-, or biancres). ..…………………………………………..…………………………..…. 26
- 26a (25b)
- Head bearing two pairs of sensoria tentacles on the lateral sides; mouth narrow, hooks with four spines. Common; marine: interstitial. ………….….... Thaumastoderma (Figure 9E)
- 26b (25b)
- Head bearing no or one pair of sensorial tentacles on the lateral sides; hooks with 5, 4, 3, or 2 spines. Very common; marine: interstitial. ………............. Tetranchyroderma (Figure 9F)
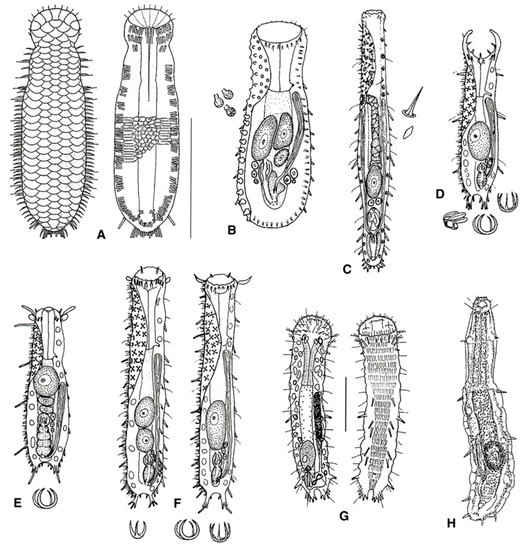
Figure 9.
Macrodasyida, Thaumastodermatidae: models of (A) Diplodasys, (B) Oregodasys, (C) Acanthodasys, (D) Pseudostomella, (E) Thaumastoderma, (F) Tetranchyroderma, (G) Ptychostomella, and (H) Hemidasys. Scale bars: (A–F, H) = 200 μm, (G) = 50 μm. (A) from [48] with modifications, (B–F) from [39] with modifications, (G) from [41] with modifications, and (H) from [49] with modifications.
- 27a (20b)
- Male apparatus absent (i.e., parthenogenetic); TbA, two groups of three tubes per side; TbL, four or five per side, TbP up to five per side. Rare; marine: interstitial. REDUDASYIDAE (partim) ……………………………………………………………….. Anandrodasys (Figure 10A)
- 27b (20b)
- These characteristics not combined. ..……………..……...………………..………………… 28
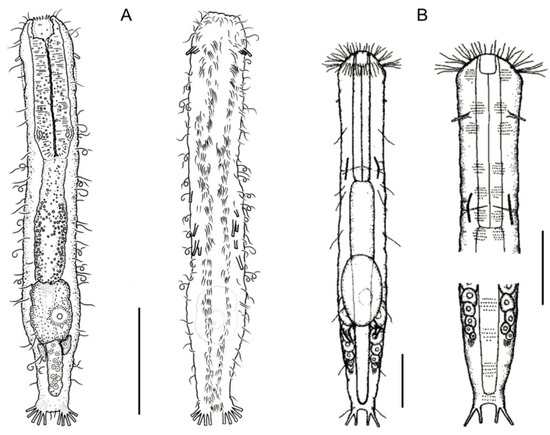
Figure 10.
Macrodasyida, Redudasyidae: models of (A) Anandrodasys and (B) Redudasys. Scale bars = 50 μm. (A) from [50] with modifications, (B) from [51] with modifications.
- 28a (27b)
- TbA, several to many, arranged in two tufts; TbL absent. Rare; marine: interstitial. TURBANELLIDAE (partim) …………………………..…………….. Desmodasys (Figure 6F)
- 28b (27b)
- TbA, few to many, but not arranged in tufts; TbL normally present or, if absent, then TbA few in number. …………....…………………………………………………..……….………….. 29
- 29a (28b)
- TbA, few; TbL few; body elongate (to about 1 mm in length) and narrow; posterior end in the form of two distinct pedicles. HUMMONDASYIDAE. …….... Hummondasys(Figure 11)
- 29b (28b)
- These characteristics not combined. ……………….…………………….…….……….…….. 30
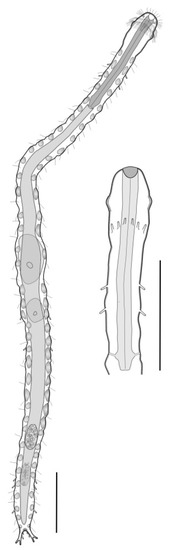
Figure 11.
Macrodasyida, Hummondasyidae: model of Hummondasys. Scale bars = 100 µm. From [13] with modifications.
- 30a (29b)
- TbA, few to many; TbL and TbP, numerous (more than 10 per side); mouth narrow (< 0.4 × head width); posterior body region in the form of a large round lobe or clearly two-lobed. PLANODASYIDAE (Figure 12) ……………………………………………..……..……...……….. 31
- 30b (29b)
- TbA or TbL numbering fewer than six tubes per side; oral opening narrow to broad, and if narrow, then posterior body region not clearly two-lobed. ...……………………………... 33
- 31a (30a)
- TbA, present in low numbers; body very long (up to 3.5 mm in length) and rather narrow; posterior body region ending as a large lobe. Uncommon; marine: interstitial. ……………………………………………………………………….…… Megadasys (Figure 12A)
- 31b (30a)
- Posterior body region distinctly two-lobed. ………………………..……..…………………. 32
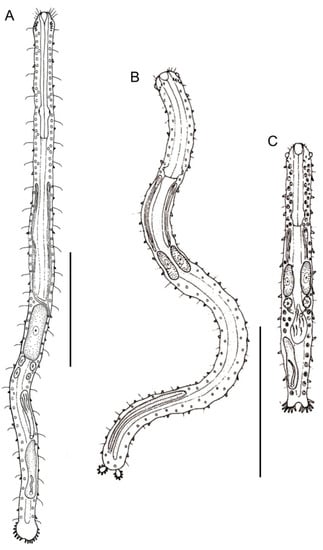
Figure 12.
Macrodasyida, Planodasyidae: models of (A) Megadasys, (B) Planodasys, and (C) Crasiella. Scale bars = 200 μm. From [39] with modifications.
- 32a (31b)
- Posterior lobes in the form of oval appendages; most anterior TbA arranged transversely; caudal organ elongate. Rare; marine: interstitial. .……………...…… Planodasys (Figure 12B)
- 32b (31b)
- Posterior lobes in the form of furcate extensions; most anterior TbA arranged longitudinally; caudal organ ovate. Uncommon; marine: interstitial. ……………………... Crasiella (Figure 12C)
- 33a (30b)
- Oral opening, narrow (< 0.4 × head width); right and left testicles present. CEPHALODASYIDAE (partim) (Figure 5) ………………………………….……………..…... 34
- 33b (30b)
- Oral opening broad (> 0.6 × head width) or, if narrow, leading to a large buccal cavity surrounded by an oral hood; a single testicle, on the right-hand side. THAUMASTODERMATIDAE (partim) (Figure 9) ….…………………………...………………. 36
- 34a (33a)
- Total body length > 1 mm; TbA, one per side; TbL in form of numerous papillae along the body sides. Uncommon; marine: interstitial. ……………………… Dolichodasys (Figure 5D)
- 34b (33a)
- Total body length < 1 mm. …………………………………..…………….…………………… 35
- 35a (34b)
- TbA, 1–4 tubes per side, arranged in two groups; TbL, 0–6 tubes per side. Uncommon; marine: interstitial. ....................................................................................... Paradasys (Figure 5E)
- 35b (34b)
- TbA, few to several per side; TbL, several to many. Common; marine: interstitial. ……………….....……………….......………………………………....…. Mesodasys (Figure 5C)
- 36a (33b)
- Oral opening, broad; locomotor cilia extending over the entire ventral surface; male genital pore not surrounded by cuticular plates. Common; marine: interstitial. ……….…………………………………………………………......… Ptychostomella(Figure 9G)
- 36b (33b)
- Oral opening, narrow, leading to a large buccal cavity covered by an oral hood; ventral locomotor cilia restricted to the pharyngeal region; male genital pore surrounded by cuticular plates. Very rare (possibly extinct); marine: interstitial …... Hemidasys (Figure 9H)
- 37a (3b)
- Total body length 300–400 μm; TbA, 1–2 per side; pharyngeal pores present. Rare; interstitial. REDUDASYIDAE (partim) .………………..…….……..… Redudasys (Figure 10B)
- 37b (3b)
- Body length up to 220 μm; TbA, one per side; pharyngeal pores absent. Rare; interstitial. INCERTAE SEDIS. …………………………..…….………………..…… Marinellina (Figure 13)
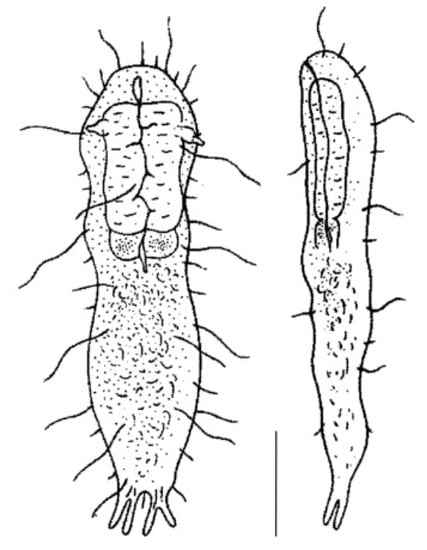
Figure 13.
Macrodasyida, INCERTAE SEDIS: model of Marinellina. Scale bar = 50 μm. From [52] with modifications.
- 38a (1a)
- 38b (1a)
- Ventral locomotor ciliation formed by single cilia, occurring in longitudinal bands or tufts, never composed of cirri (Figure 1B). Freshwater, marine, and brackish. ............................... 41
- 39a (38a)
- Cirri of the head and pharyngeal regions of two different sizes, with 1–2 transverse rows of small and short cirri anteriorly followed by transverse rows of big and longer cirri; frontal portion of pharynx with a swelling (bulb). Common; marine and brackish: interstitial. ..……………………………………….…….....….. Heteroxenotrichula (Figure 14A)
- 39b (38a)
- Cirri, all of similar size, pharynx without anterior swelling (bulb). ...………………..…… 40
- 40a (39b)
- Ovary and eggs present, testicles and spermatozoa absent; head clearly distinct; scales on the dorsal side, flat; scales of the lateral mid-trunk, pedunculated; a pair of lateral spines at the base of the furcal branches. Common; marine: interstitial. ..... Draculiciteria (Figure 14B)
- 40b (39b)
- Testicles and spermatozoa present; head in general not clearly defined; scales of the lateral mid-trunk bearing a stalk or flat; if stalked, similar to the dorsal scales. Common; marine and brackish: interstitial. ………………..……………….…. Xenotrichula (Figure 14C)
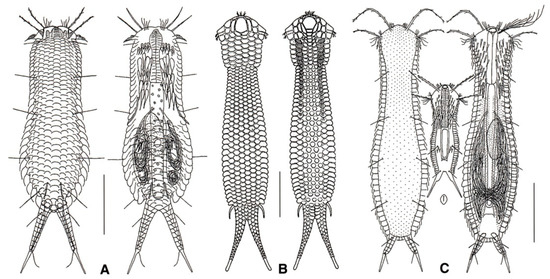
Figure 14.
Chetonotida, Xenotrichulidae: models of (A) Heteroxenotrichula, (B) Draculiciteria, and (C) Xenotrichula. Scale bars = 50 μm. (A, C) modified from [35], (B) modified from [53].
- 41a (38b)
- Posterior body region furcate or bifurcate; caudal rami with or without TbP. ……..…..... 42
- 41b (38b)
- Posterior body region rounded or truncated; perhaps showing two caudal protuberances or spines. ………………………………………………………………………………………………... 60
- 42a (41a)
- Posterior body region bifurcate, bearing four TbP or two TbP and two spiniform cuticular processes; elsewhere, cuticle smooth, not forming scales or spines. Rare; freshwater: interstitial or periphytic/epibenthic. DICHAETURIDAE …………………….….….. Dichaetura (Figure 15A)
- 42b (41a)
- Posterior body region furcate; cuticle smooth or forming spines and/or scales. ..……...... 43
- 43a (42b)
- Body cuticle smooth; caudal rami with TbP, sickle-shaped; cilia of the head not arranged into tufts. Very rare; freshwater: semiplanktonic or hyperbenthic. PROICHTHYDIIDAE (Figure 15B,C) ……………………………………..…………………………….…………..…….. 44
- 43b (42b)
- Body cuticle generally forming spines and scales; caudal rami with or without TbP; if present, caudal rami and TbP generally straight, short to very long; cilia of the head emerging as tufts or forming a continuous band around the elongate, muzzle-like frontal end. .…………………………………………………………………………………………....…… 45
- 44a (43a)
- Cilia of the head arranged as a transverse row of small elements on the dorsal side; locomotor cilia limited to head and neck, emerging as separate tufts. Freshwater: hyperbenthic …………………………………………...…….………Proichthydium (Figure 15B)
- 44b (43a)
- Cilia of the head emerging mostly from the lateral sides as single, short to very long elements; locomotor cilia distributed in two bands that run from under the head to the posterior trunk region. Freshwater: semiplanktonic. …....................................…… Proichthydioides (Figure 15C)
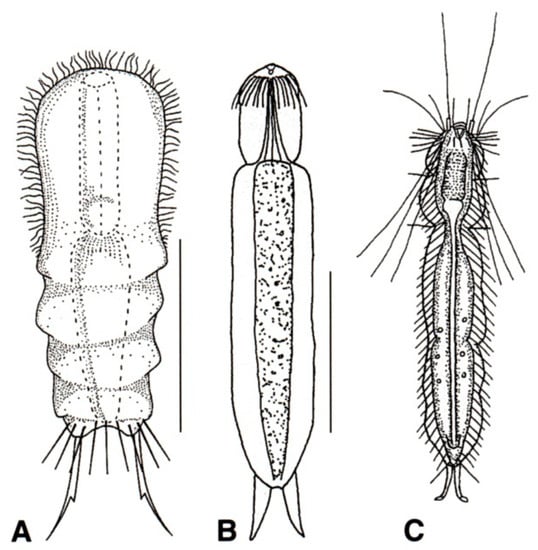
Figure 15.
Chetonotida: (A) Dichaeturidae, a model of Dichaetura. (B, C) Proichthydiidae, models of (B) Proichthydium and (C) Proichthydioides. Scale bars = 50 μm. (A) from [54] with modification, (B) from [55] with modification, and (C) re-sketched from [56].
- 45a (43b)
- 45b (43b)
- Cilia of the head organized in a band, encircling a muzzle-like frontal end; TbP numbering two or four. Uncommon to rare, marine: epibenthic or interstitial. MUSELLIFERIDAE (Figure 20) …………………………………………………………………………………………………………. 67
- 46a (45a)
- TbP at the end of the furcal rami absent. Rare; freshwater: epibenthic. …. Undula (Figure 16A)
- 46b (45a)
- TbP at the end of the furcal rami present. ...……………………………………....……...…... 47
- 47b (46b)
- Furcal base narrow (pedunculated); caudal rami segmented; cephalion and hypostomion extremely large; scales without a keel, notch, or spine. Rare; freshwater: epibenthic. …………………………………………………………...……………….. Cephalionotus (Figure 16B)
- 47b (46b)
- These characteristics not combined. ……………………………………….…..……...……… 48
- 48a (47b)
- Furcal rami very long (up to one-third of the total body length), multi-segmented, bare or with tiny spines or scales. Common, freshwater: periphytic and epibenthic. ………………………………………………………………………...… Polymerurus (Figure 16C)
- 48b (47b)
- Furcal rami from very short to mid length, not segmented, scales or spines limited to the proximal portion or lacking altogether. ………………………………………..…..………….... 49
- 49a (48b)
- Cuticular covering bare (or mostly bare) or made up of scales lacking spines; seldomly, some spines may be present at the base of the furca. .……………..…………....…………..… 50
- 49b (48b)
- Cuticular covering including scales bearing spines (spined scales) and/or a keel (spined, keeled scales and keeled scales, respectively); spines from long to very short, bearing 1–2 indentations laterally (notched spines), or simple. ………………………………………………... 57
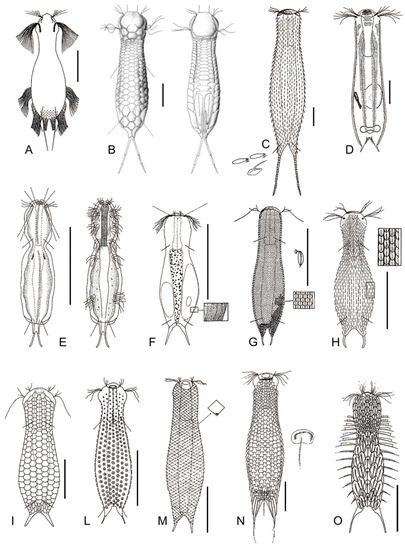
Figure 16.
Chetonotida, Chaetonotidae: models of (A) Undula, (B) Cephalionotus, (C) Polymerurus, (D) Arenotus, (E) Caudichthydium, (F) Ichthydium, (G) Aspidiophorus, (H) Heterolepidoderma, (I) Lepidodermella, (L) Fluxiderma, (M) Rhomballichthys, (N) Lepidochaetus, and (O) Halichaetonotus. Scale bars (A, C–D) = 50 μm, (B) = 25 μm. (A) from [57] with modifications, (B) from [37] with modifications, (C) from [51] with modifications, (D) from [58] with modifications, (E–H, M) from [54] with modifications, (I, L) from [59] with modifications, and (O) from [60] with modifications.
- 50a (49a)
- Cuticular covering bare, rarely a few scales and/or spines at base of the furca may be present. …………………………………………………………………………………………...... 51
- 50b (49a)
- Cuticular covering wholly or prevalently made of spineless scales. ….…….……...……... 53
- 51a (50a)
- Cuticle completely bare, very thick, obviously distinguishable from the underlying epidermal layer. Rare; freshwater: interstitial. ……...……...………..…. Arenotus (Figure 16D)
- 51b (50a)
- Cuticle thin, mostly bare, except for perhaps two terminal scales at the end ventral interciliary field; occasionally, weak striations along the body or few spines and/or scales at the furcal base may be present. Common; freshwater, rarely marine or brackish water: periphytic, epibenthic, and interstitial. …………………………………………………………………………………..…… 52
- 52a (51b)
- Furcal base pedunculated; locomotor cilia distributed in separated tufts. Uncommon; marine: interstitial. ………….……..………………..………....…. Caudichthydium (Figure 16E)
- 52b (51b)
- Furcal base not pedunculated; locomotor cilia mostly forming two longitudinal bands. Common; freshwater, rarely brackish or marine: epibenthic, periphytic, and interstitial. ………………………………………………………………….……….…Ichthydium (Figure 16F)
- 53a (50b)
- Scales, small, keeled, or stalked. ………………………………………………………………. 54
- 53b (50b)
- Scales, large and bare, round, rhomboidal, or polygonal in shape. ..………………….…... 55
- 54a (53a)
- Most scales with a stalk; occasionally, few scales may lack a stalk and bear a keel or a spine instead. Common; freshwater, brackish, and marine: epibenthic, periphytic, and interstitial. ……………………………………………………………………...…….. Aspidiophorus (Figure 16G)
- 54b (53a)
- Numerous keeled scales; occasionally, few scales may bear a spine. Common; freshwater, brackish, and marine: periphytic, epibenthic, and interstitial. ...Heterolepidoderma (Figure 16H)
- 55a (53b)
- Scales polygonal in shape. Common; freshwater, rarely brackish or marine: interstitial, epibenthic, and periphytic. ……………………………..……….....Lepidodermella (Figure 16I)
- 55b (53b)
- Scales rhomboidal or circular in shape. ………………………….………………………...… 56
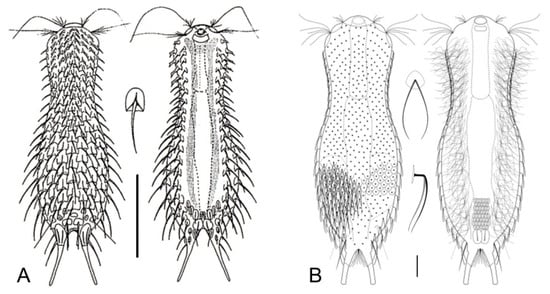
Figure 17.
Chaetonotida, Chaetonotidae: models of (A) Chaetonotus and (B) Bifidochaetus. Scale bars (A) = 50 μm, (B) = 10 μm. (A) from [61] with modifications, (B) from [62] with modifications.
- 56a (55b)
- Scales circular. Rare; freshwater: periphytic. ..................................... Fluxiderma (Figure 16L)
- 56b (55b)
- Scales rhomboidal. Rare; freshwater: periphytic. ................. Rhomballichthys (Figure 16M)
- 57a (49b)
- Scales of the ventral interciliary field similar in shape to the scales of the dorsal side; scales of the dorsal side possessing a double edge anteriorly, with or without a spine but always deprived of a keel; several pairs of thin spines of increasing length at the lateral sides of the furcal base. Rather common; freshwater: periphytic and epibenthic. ……………………………………………………………………….... Lepidochaetus (Figure 16N)
- 57b (49b)
- Scales of the ventral interciliary field dissimilar in shape from scales of the dorsal side; scales of the dorsal side with a single edge anteriorly, keeled or keeled and spined…………………... 58
- 58a (57b)
- Scales lateral to the ventral locomotor cilia with spines bearing lamellae (hydrofoil scales); scales of the dorsal side bearing a keel; seldom presence of 1–5 scales bearing spines. Common; marine and brackish: interstitial. ……………….... Halichaetonotus (Figure 16O)
- 58b (57b)
- Scales bearing spines with lamellae normally absent; if present, dorsal scales spined. .…..…. 59
- 59a (58b)
- Dorsal scales round to suboval, without keels and/or notches but carrying distally bifurcating hairlike spines. Rare; freshwater: epibenthic……...…. Bifidochaetus (Figure 17B)
- 59b (58b)
- These characteristics not combined. Very common; freshwater, marine, and brackish: epibenthic, periphytic, and interstitial. ………...……………....…… Chaetonotus (Figure 17A)
- 60a (41b)
- Posterior body region rounded-off or truncated with paired lateral projections; head bearing a pair of rod- or club-shaped tentacles; trunk bearing small, spined scales; rarely, trunk scales restricted to a small patch on the ventral side. Uncommon to rare; freshwater: hyperbenthic and semiplanktonic. NEOGOSSEIDAE (Figure 18) ………….…….…………. 61
- 60b (41b)
- Posterior body region rounded or truncated, occasionally with a very short caudal lobe or paired postero-lateral protuberance; head without tentacles; body scales reduced or absent; trunk bearing very long and movable spines arranged into groups. Uncommon to rare; freshwater: hyperbenthic, epibenthic, and semiplanktonic. DASYDYTIDAE (Figure 19) ….…. 62
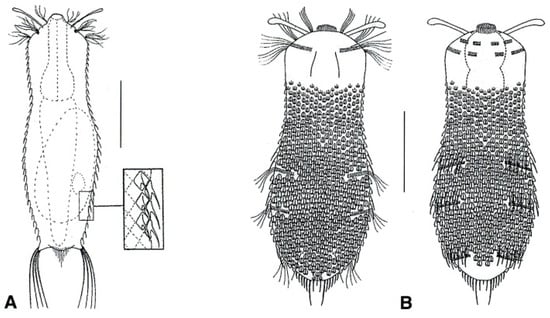
Figure 18.
Chaetonotida, Neogosseidae: models of (A) Neogossea and (B) Kijanebalola. Scale bars = 50 μm. (A) from [54] with modifications, (B) from [57] with modifications.
- 61a (60a)
- Posterior body region truncated, showing two lateral projections bearing a tuft of long spines; trunk with fine spined scales. Uncommon: epibenthic and semiplanktonic. ...................................................................................................................... Neogossea (Figure 18A)
- 61b (60a)
- Posterior body region rounded, with a central group of spines and no lateral projections; trunk with keeled scales, seldom reduced to a small group on the ventral side. Rare: epibenthic and semiplanktonic. …............................……………………..…………….…. Kijanebalola (Figure 18B)
- 62a (60b)
- Trunk region bearing long, scattered spines on the dorsal side or two caudal spines only; body scales absent; locomotor cilia arranged in two longitudinal bands; pharynx bearing two robust swellings (bulbs). Rare: epibenthic and semiplanktonic. …………………………………………...……………………….... Anacanthoderma (Figure 19A)
- 62b (60b
- ) Trunk region bearing long, lateral spines arranged into columns or groups; dorsal spines present or absent; locomotor cilia arranged in tufts; pharynx bearing a single swelling or cylindrical. ……………………………………………………………………………….…...…… 63
- 63a (62b)
- Lateral spines, simple or with notches; if present, scales large, elliptical in shape, and few in number; pharynx cylindrical (i.e., without bulbs). …………….……………...………….… 64
- 63b (62b)
- Lateral spines with a single lateral notch and bifurcate apex, or with 2–3 lateral notches and pointed apex; if present, numerous, small, keeled scales; pharynx bearing a swelling at the posterior end. ………………………………………………………………………………………… 65
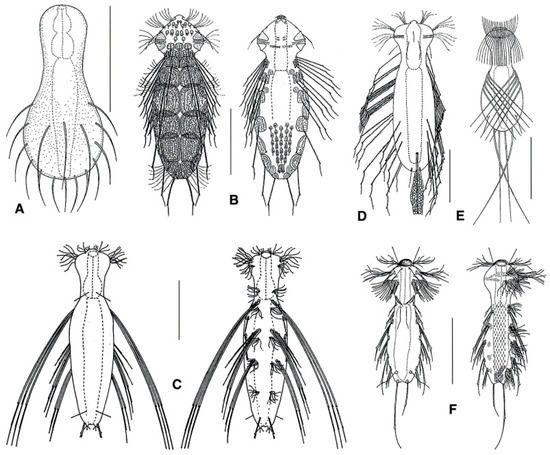
Figure 19.
Chaetonotida, Dasydytidae: models of (A) Anacanthoderma, (B) Ornamentula, (C) Stylochaeta, (D) Dasydytes, (E) Haltidytes, and (F) Setopus. Scale bars = 50 μm. (A, F) from [54] with modifications, (B–E) from [57] with modifications.
- 64a (63a)
- Trunk showing dorsal spines; two pairs of caudal spines; all spines show a noticeable lateral notch; dorsal scales, rather large and of peculiar lace-like appearance. Rare: epibenthic, periphytic, and semiplanktonic. ……………….……... Ornamentula (Figure 19B)
- 64b (63a)
- Trunk lacking dorsal spines; a single pair of caudal spines or none; if very long, the lateral spines are thick and bent basally, becoming thinner and thinner distally; lateral notch present or absent; where present, body scales are small and feebly keeled. ………...……... 66
- 65a (65b)
- Lateral spines, robust, showing pointed tip and 2–3 lateral notches; body scales lacking; posterior body region showing two bristled protuberances on the sides. Uncommon: semiplanktonic and epibenthic.……………………..…….……………Stylochaeta (Figure 19C)
- 65b (63b)
- Lateral spines, almost straight, showing a bifurcate tip and a single lateral notch; body scales present; posterior body region rounded. Uncommon: semiplanktonic, periphytic, and epibenthic ………………………….………………………………...……Dasydytes (Figure 19D)
- 66a (63b)
- Caudal spines absent or present; if present, in general of different length; lateral spines, straight, of medium length; ventral, S-shaped, jumping spines lacking. Rare: semiplanktonic and epibenthic. ......................…………………….......................................... Setopus (Figure 19F)
- 66b (64b)
- Caudal spines absent; lateral spines very long, strongly bent crossing over the dorsal side; ventral S-shaped jumping spines present. Rare: semiplanktonic and epibenthic. ……………………………………………………………….………….…. Haltidytes (Figure 19E)
- 67a (45b)
- Furcal rami each with a single TbP; body scales bearing fine spines but lacking keels. Rare; marine: interstitial or infaunal. Uncommon: epibenthic and interstitial. .……………………………………………………………………..………. Musellifer (Figure 20A)
- 67b (45b)
- Furcal rami each with two TbP; body scales, keeled. Rare: interstitial. ...................................................................................................................... Diuronotus (Figure 20B)
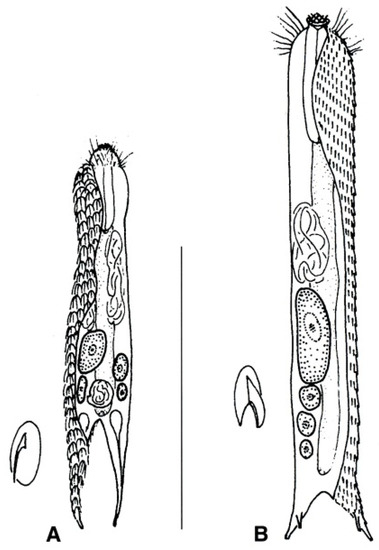
Figure 20.
Chetonotida, Muselliferidae: models of (A) Musellifer and (B) Diuronotus. Scale bar = 200 μm. From [39] with modifications.
4. Discussion
This paper was derived from a workshop on marine meiofaunal organisms of Costa Rica with a focus on Gastrotricha (and Kinorhyncha) held in January 2019 at the CIMAR (University of Costa Rica) (Supplementary Material Figure S10). During the event, intended for undergraduate and graduate students, general information on the phylum was provided in the classroom and the main techniques regarding sampling, extraction, and observation were illustrated. Techniques were put in practice by some of the students who participated in subsequent 15-day field work. The effectiveness of an early versions of the taxonomic keys was determined by the pupils based on direct observation of living, relaxed specimens (some of the students) and/or based on photographs of freshly sampled specimens (all the students). The proposed version of the keys benefited from the insightful comments which emerged during the testing. At the end of the training period, all the students were able to correctly identify at the genus level the gastrotrich involved in the testing. Based on this outcome, we are confident in the work’s usefulness to many others.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/11/7/117/s1, Figure S1: Sampling of marine gastrotrichs of the littoral zone, Figure S2: Sampling of marine gastrotrichs of the sublittoral zone, Figure S3: Sampling freshwater gastrotrichs, Figure S4: Processing of freshwater sample for in vivo studies, Figure S5: Extraction of interstitial fauna, Figure S6: Extraction of interstitial fauna, Figure S7: Mounting of the specimens, Figure S8: Mounting of the specimens, Figure S9: Morphological analysis and documentation, Figure S10: Instructors (background) and students (foreground) participating at the work-shop on marine meiofaunal organisms of Costa Rica with focus on Gastrotricha.
Author Contributions
Conceptualization, M.A.T.; Data curation, M.D.; Funding acquisition, M.A.T. and J.A.S.-C.; Investigation, J.A.S.-C., O.A.S.-B., J.D.B. and M.D.; Supervision, M.A.T.; Writing—original draft, M.A.T.; Writing—review and editing, J.A.S.-C., O.A.S.-B., G.C.-D., N.G.-O., J.D.B., M.C.-D. and M.D.Z.
Funding
The research was funded in part by a Universidad de Costa Rica (project 808-B8-184 Marine meiofauna of Costa Rica) grant to J.A.S.-C. The publishing cost was met by a MIUR grant “Finanziamento annuale individuale delle attività base di ricerca” to M.A.T.
Acknowledgments
The first draft of this paper was prepared during the workshop on marine meiofaunal organisms of Costa Rica with a focus on Gastrotricha and Kinorhyncha, held in January 2019 at the CIMAR (University of Costa Rica). We thank the director of CIMAR for supporting the event.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Todaro, M.A. Meiofauna from the Meloria Shoals: Gastrotricha, biodiversity and seasonal dynamics. Biol. Mar. Medit. 1998, 5, 587–590. [Google Scholar]
- Gray, J.S. The effects of pollution on sand meiofauna communities. Thalass. Jugosl. 1971, 7, 79–86. [Google Scholar]
- Coull, B.S. Long-term variability of estuarine meiobenthos: An 11 year study. Mar. Ecol. Prog. Ser. 1985, 24, 205–218. [Google Scholar] [CrossRef]
- Todaro, M.A.; Fleeger, J.W.; Hummon, W.D. Marine gastrotrichs from the sand beaches of the northern Gulf of Mexico: Species list and distribution. Hydrobiologia 1995, 310, 107–117. [Google Scholar] [CrossRef]
- Hochberg, R. Spatiotemporal size-class distribution of Turbanella mustela (Gastrotricha: Macrodasyida) on a northern California beach and its effect on tidal suspension. Pacific Sci. 1999, 53, 50–60. [Google Scholar]
- Nesteruk, T. Density and biomass of Gastrotricha in sediments of different types of standing waters. Hydrobiologia 1996, 24, 205–208. [Google Scholar] [CrossRef]
- Ruppert, E.E. Gastrotricha. In Microscopic Anatomy of Invertebrates, Aschelminthes; Harrison, F.W., Ruppert, E.E., Eds.; Wiley-Liss: New York, NY, USA, 1991; Volume 4, pp. 41–109. [Google Scholar]
- Hochberg, R.; Litvaitis, M.K. A muscular double helix in gastrotricha. Zool. Anz. 2001, 240, 61–68. [Google Scholar] [CrossRef]
- Todaro, M.A.; Telford, M.J.; Lockyer, A.E.; Littlewood, D.T.J. Interrelationships of the Gastrotricha and their place among the Metazoa inferred from 18S rRNA genes. Zool. Scr. 2006, 35, 251–259. [Google Scholar] [CrossRef]
- Struck, T.H.; Wey-Fabrizius, A.R.; Golombek, A.; Hering, L.; Weigert, A.; Bleidorn, C.; Klebow, S.; Iakovenko, N.; Hausdorf, B.; Petersen, M.; et al. Platyzoan paraphyly based on phylogenomic data supports a noncoelomate ancestry of Spiralia. Mol. Biol. Evol. 2014, 31, 1833–1849. [Google Scholar] [CrossRef]
- Egger, B.; Lapraz, F.; Müller, S.; Dessimoz, C.; Girstmair, J.; Skunca, N.; Rawlinson, K.A.; Cameron, C.B.; Beli, E.; Todaro, M.A.; et al. A Transcriptomic-phylogenomic analysis of the evolutionary relationships of flatworms. Curr. Biol. 2015, 25, 1–7. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Jondelius, U.; Hochberg, R.; Hummon, W.D.; Kånneby, T.; Rocha, C.E.F. Gastrotricha: a marine sister for a freshwater puzzle. PLoS ONE 2012, 7, e31740. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Leasi, F.; Hochberg, R. A new species, genus and family of marine Gastrotricha from Jamaica, with a phylogenetic analysis of Macrodasyida based on molecular data. Syst. Biodiv. 2014, 12, 473–488. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Leasi, F. An integrated morphological and molecular approach to the description and systematisation of a novel genus and species of Macrodasyida (Gastrotricha). PLoS ONE 2015, 10, e0130278. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Dal Zotto, M.; Kånneby, T.; Hochberg, R. Integrated data analysis allows the establishment of a new, cosmopolitan genus of marine Macrodasyida (Gastrotricha). Sci. Rep. 2019, 9, 7989. [Google Scholar] [CrossRef] [PubMed]
- Leasi, F.; Todaro, M.A. The muscular system of Musellifer delamarei (Renaud-Mornant, 1968) and other chaetonotidans with implications for the phylogeny and systematisation of the Paucitubulatina (Gastrotricha). Biol. J. Linn. Soc. 2008, 94, 379–398. [Google Scholar] [CrossRef]
- Kånneby, T.; Todaro, M.A.; Jondelius, U. Phylogeny of Chaetonotidae and other Paucitubulatina (Gastrotricha: Chaetonotida) and the colonization of aquatic ecosystems. Zool. Scr. 2013, 42, 88–105. [Google Scholar] [CrossRef]
- Guidi, L.; Todaro, M.A.; Ferraguti, M.; Balsamo, M. Reproductive system and spermatozoa ultrastructure support the phylogenetic proximity of Megadasys and Crasiella (Gastrotricha, Macrodasyida). Contr. Zool. 2014, 83, 119–131. [Google Scholar] [CrossRef]
- Kånneby, T.; Todaro, M.A. The phylogenetic position of Neogosseidae (Gastrotricha: Chaetonotida) and the origin of planktonic Gastrotricha. Org. Divers. Evol. 2015, 6, 1–12. [Google Scholar]
- Balsamo, M.; Grilli, P.; Guidi, L.; d’Hondt, J.L. Gastrotricha: Biology, ecology and systematics. Families Dasydytidae, Dichaeturidae, Neogosseidae, Proichthydiidae. In Identification Guides to the Plankton and Benthos of Inland Waters; Dumont, H.J.F., Ed.; Backhuys Publisher: Leiden, The Netherlands, 2014; Volume 24, pp. 1–187. [Google Scholar]
- Kånneby, T.; Hochberg, R. Phylum Gastrotricha. In Thorp and Covich’s Freshwater Invertebrates: Ecology and General Biology; Thorp, J., Rogers, D.C., Eds.; Elsevie Academic Press: Amsterdam, The Netherlands, 2015; Volume 1, pp. 211–223. [Google Scholar]
- Kieneke, A.; Schmidt-Rhaesa, A. Gastrotricha and Gnathifera. In Handbook of Zoology; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2015; Volume 3, pp. 1–134. [Google Scholar]
- Gastrotricha Web Portal. Available online: http://www.gastrotricha.unimore.it/ (accessed on 25 June 2019).
- World Register of Marine Species (WoRMS). Available online: http://www.marinespecies.org/ (accessed on 25 June 2019).
- Hummon, W.D.; Todaro, M.A.; Balsamo, M.; Tongiorgi, P. Effects of pollution on marine Gastrotricha in the northwestern Adriatic Sea. Mar. Pollut. Bull. 1990, 21, 241–243. [Google Scholar] [CrossRef]
- Todaro, M.A.; Rocha, C.E.F. Diversity and distribution of marine Gastrotricha along the northern beaches of the state of Sao Paulo (Brazil), with description of a new species of Macrodasys (Macrodasyida, Macrodasyidae). J. Nat. Hist. 2004, 38, 1605–1634. [Google Scholar] [CrossRef]
- Todaro, M.A.; Rocha, C.E.F. Further data on marine gastrotrichs from the State of São Paulo and the first records from the State of Rio de Janeiro (Brazil). Meiofauna Mar. 2005, 14, 27–31. [Google Scholar]
- Hummon, W.D. Gastrotricha. In The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon; Carlton, J.T., Ed.; University of California Press: Berkeley, CA, USA, 2007; pp. 267–268. [Google Scholar]
- Todaro, M.A.; Leasi, F.; Bizzarri, N.; Tongiorgi, P. Meiofauna densities and gastrotrich community composition in a Mediterranean sea cave. Mar. Biol. 2006, 149, 1079–1091. [Google Scholar] [CrossRef]
- Sergeeva, N.G.; Ürkmez, D.; Todaro, M.A. Significant occurrence of Musellifer profundus Vivier, 1974 (Gastrotricha, Chaetonotida) in the Black Sea. Check List 2019, 15, 219–224. [Google Scholar] [CrossRef]
- Pfannkuche, O.; Thiel, H. Sampling processing. In Introduction to the Study of Meiofauna; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 134–145. [Google Scholar]
- Todaro, M.A. Contribution to the study of the Mediterranean meiofauna: Gastrotricha from the Island of Ponza, Italy. Boll. Zool. 1992, 59, 321–333. [Google Scholar] [CrossRef]
- Hochberg, R.; Litvaitis, M.K. Hexamethyldisilazane for scanning electron microscopy of Gastrotricha. Biotech. Histochem. 2000, 75, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Chang, C.Y. Two new marine gastrotrichs of the genus Ptychostomella (Macrodasyida, Thaumastodermatidae) from South Korea. Zool. Sci. 2003, 20, 481–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Todaro, M.A.; Hummon, W.D. An overview and a dichotomous key to genera of the phylum Gastrotricha. Meiofauna Mar. 2008, 16, 3–20. [Google Scholar]
- Kolicka, M.; Dabert, M.; Dabert, J.; Kånneby, T.; Kisielewski, J. Bifidochaetus, a new Arctic genus of freshwater Chaetonotida (Gastrotricha) from Spitsbergen revealed by an integrative taxonomic approach. Invert. Syst. 2016, 30, 398–419. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Araujo, T.Q.; Lourenço, A.P.; Guidi, L.; Balsamo, M. A new genus and new species of freshwater Chaetonotidae (Gastrotricha: Chaetonotida) from Brazil with phylogenetic position inferred from nuclear and mitochondrial DNA sequences. Syst. Biodiv. 2017, 15, 49–62. [Google Scholar] [CrossRef]
- Balsamo, M.; Todaro, M.A. Gastrotricha. In Freshwater Meiofauna Biology and Ecology; Rundle, S.D., Robertson, A.I., Schmid-Araya, J.M., Eds.; Backhuys Publisher: Leiden, The Netherlands, 2002; pp. 45–61. [Google Scholar]
- Pfannkuche, O. Gastrotricha. In Introduction to the Study of Meiofauna; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 302–311. [Google Scholar]
- Todaro, M.A.; Perissinotto, R.; Bownes, S.J. Two new marine Gastrotricha from the Indian Ocean coast of South Africa. Zootaxa 2015, 3905, 193–208. [Google Scholar] [CrossRef]
- Hummon, W.D.; Todaro, M.A.; Tongiorgi, P. Italian Marine Gastrotricha: II. One new genus and ten new species of Macrodasyida. Boll. Zool. 1993, 60, 109–127. [Google Scholar] [CrossRef]
- Hummon, W.D.; Todaro, M.A.; Tongiorgi, P.; Balsamo, M. Italian marine Gastrotricha: V. Four new and one redescribed species of Macrodasyida in the Dactylopodolidae and Thaumastodermatidae. Ital. J. Zool. 1998, 65, 109–119. [Google Scholar] [CrossRef]
- Rieger, R.M.; Ruppert, E.E.; Rieger, G.E.; Schoepfer-Sterrer, C. On the fine structure of gastrotrichs, with description of Chordodasys antennatus sp. n. Zool. Scr. 1974, 3, 219–237. [Google Scholar] [CrossRef]
- Hummon, W.D. Gastrotricha of the North Atlantic Ocean: 1. Twenty four new and two redescribed species of Macrodasyida. Meiofauna Mar. 2008, 16, 117–174. [Google Scholar]
- Todaro, M.A.; Dal Zotto, M.; Bownes, S.J.; Perissinotto, R. Two new interesting species of Macrodasyida (Gastrotricha) from KwaZulu-Natal (South Africa). Proc. Biol. Soc. Wash. 2017, 130, 139–154. [Google Scholar] [CrossRef]
- Evans, W.A.; Hummon, W.D. A new genus and species of Gastrotricha from the Atlantic coast of Florida, U.S.A. Trans. Am. Microsc. Soc. 1991, 110, 321–327. [Google Scholar] [CrossRef]
- Clausen, C. Gastrotricha Macrodasyida from the Tromsø region, northern Norway. Sarsia 2000, 85, 357–384. [Google Scholar] [CrossRef]
- Luporini, P.; Magagnini, G.; Tongiorgi, P. Contribution a la connaissance des gastrotriches des cotes de Toscane. Cah. Biol. Mar. 1971, 12, 433–455. [Google Scholar]
- Claparède, E. Miscellaneous zoologiques. III. Type d’un nouveau genere de gastrotriches. Ann. Sci. Nat. Zool. 1867, 8, 16–23. [Google Scholar]
- Kieneke, A.; Rothe, B.H.; Schmidt-Rhaesa, A. Record and description of Anandrodasys agadasys (Gastrotricha: Redudasyidae) from Lee Stocking Island (Bahamas), with remarks on populations from different geographic areas. Meiofauna Mar. 2013, 20, 39–48. [Google Scholar]
- Kisielewski, J. Two new interesting genera of Gastrotricha (Macrodasyida and Chaetonotida) from the Brazilian freshwater psammon. Hydrobiologia 1987, 153, 23–30. [Google Scholar] [CrossRef]
- Ruttner-Kolisko, A. Rheomorpha neiswestnovae und Marinellina flagellata, zwei phylogeneticsh interessante Wurmtypen aus dem Susswasserpsammon. Österr. Zool. Z. 1955, 6, 55–69. [Google Scholar]
- Luporini, P.; Magagnini, G.; Tongiorgi, P. Chaetonotoid gastrotrichs of the Tuscan Coast. Boll. Zool. 1973, 40, 31–40. [Google Scholar] [CrossRef]
- Balsamo, M. Gastrotrichi. In Guide C.N.R. per IL Riconoscimento Delle Specie Animali Delle Acque Interne Italiane; Consiglio Nazionale delle Ricerche AQ/1/199; CNR (Centro Nazionale Ricerche): Roma, Italy, 1983; Volume 7, pp. 547–571. [Google Scholar]
- Cordero, E.H. Notes sur les Gastrotriches. Physis 1918, 4, 241–244. [Google Scholar]
- Sudzuki, M. The Gastrotricha of Japan which live in the capillary water of the interstitial system: II. Bull. Biogeogr. Soc. Japan 1971, 27, 37–41. [Google Scholar]
- Kisielewski, J. Inland-water Gastrotricha from Brazil. Ann. Zool. (Warsaw) 1991, 43, 1–168. [Google Scholar]
- Mock, H. Chaetonotoidea (Gastrotricha) from the North Sea Island of Sylt. Mikrofauna. Meeres. 1980, 78, 1–107. [Google Scholar]
- Schwank, P. Gastrotricha und Nemertini. In Süsswasserfauna von Mittleuropas; Brauer, A., Ed.; G. Fisher Verlag: Stuttgart, Germany, 1990; Volume 3, pp. 1–252. [Google Scholar]
- Schrom, H. Nordadriatische Gastrotrichen. Helgoländer Wiss. Meeresunters. 1972, 23, 286–351. [Google Scholar] [CrossRef]
- Hummon, W.D.; Balsamo, M.; Todaro, M.A. Italian marine Gastrotricha: I. Six new and one redescribed species of Chaetonotida. Boll. Zool. 1992, 59, 499–516. [Google Scholar] [CrossRef]
- Kånneby, T. A redescription of Chaetonotus (Primochaetus) veronicae Kånneby, 2013 (Gastrotricha: Chaetonotidae). Zootaxa 2015, 4027, 442–446. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).