An Introduction to the Study of Gastrotricha, with a Taxonomic Key to Families and Genera of the Group
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Extraction
2.3. Morphological Analysis
2.4. Taxonomic Key
3. Results
Key to Families and Genera of Gastrotricha
- 1a
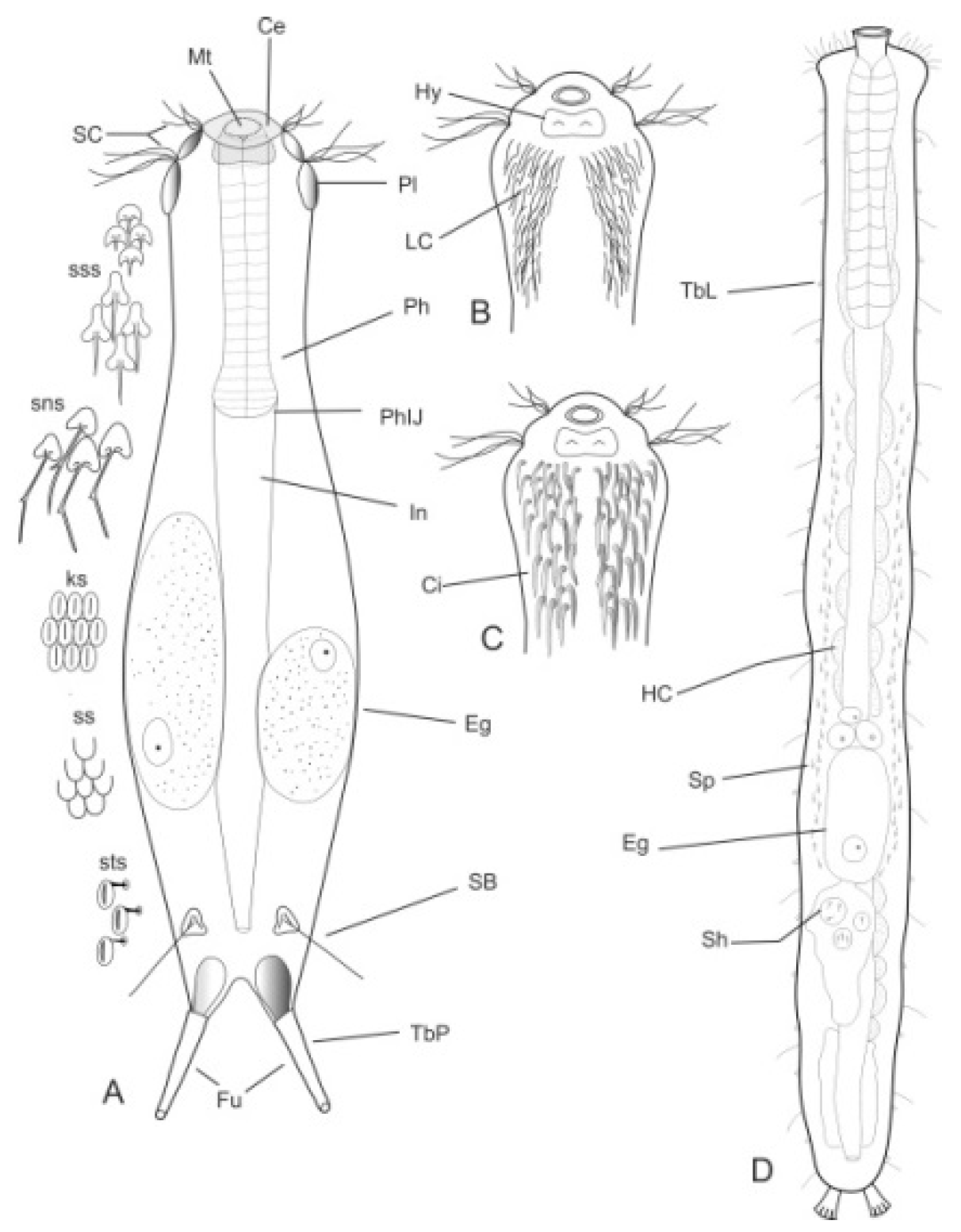
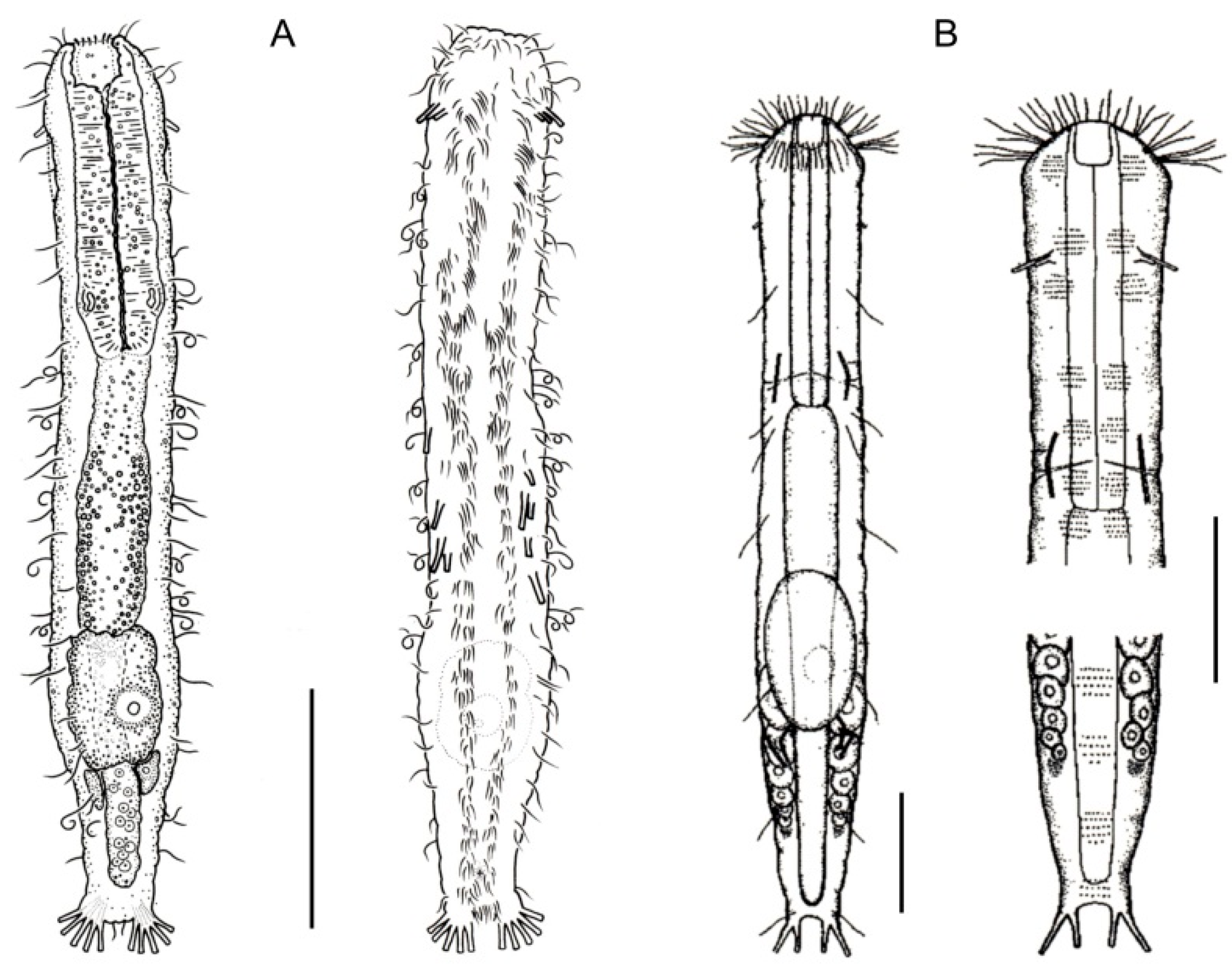
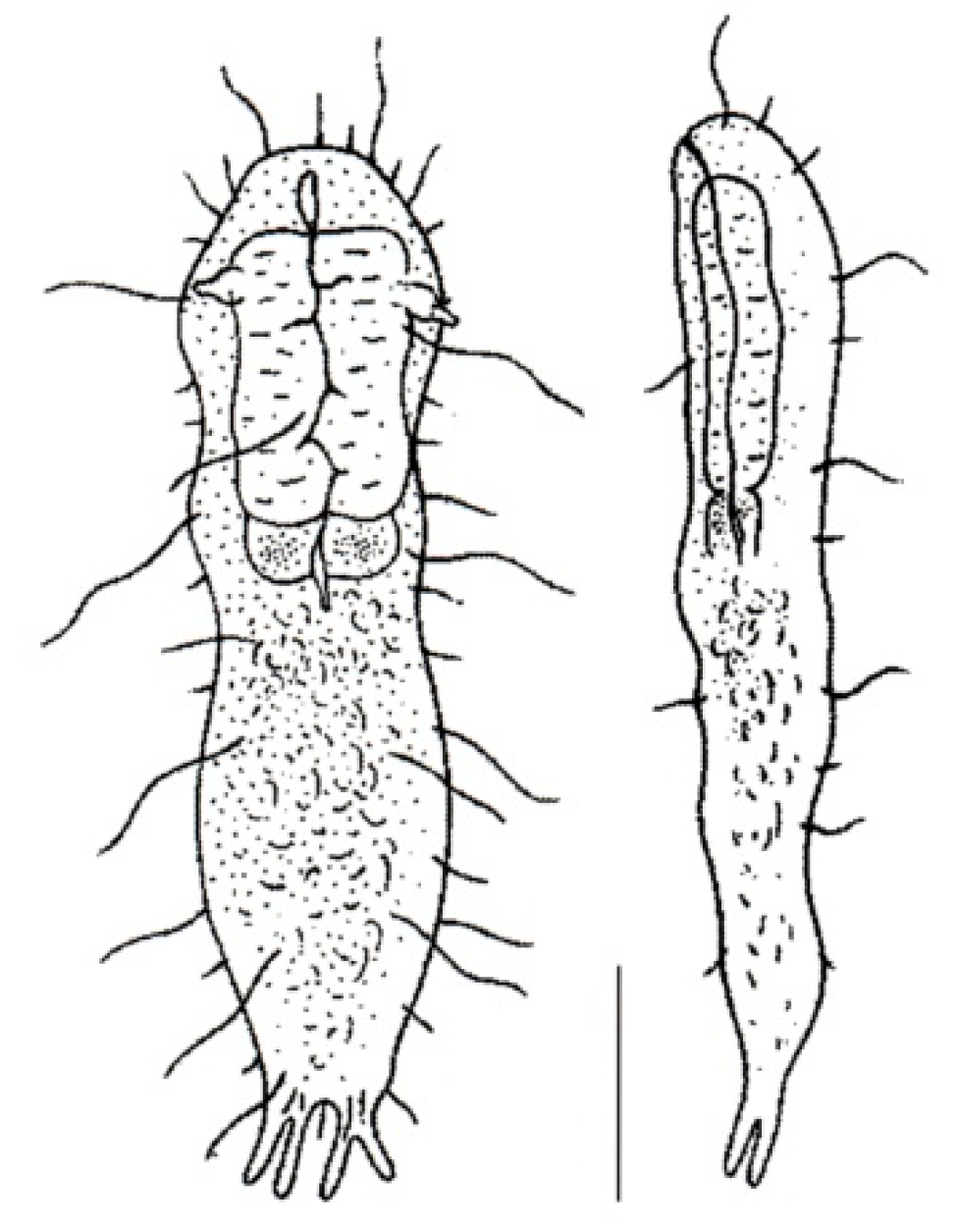
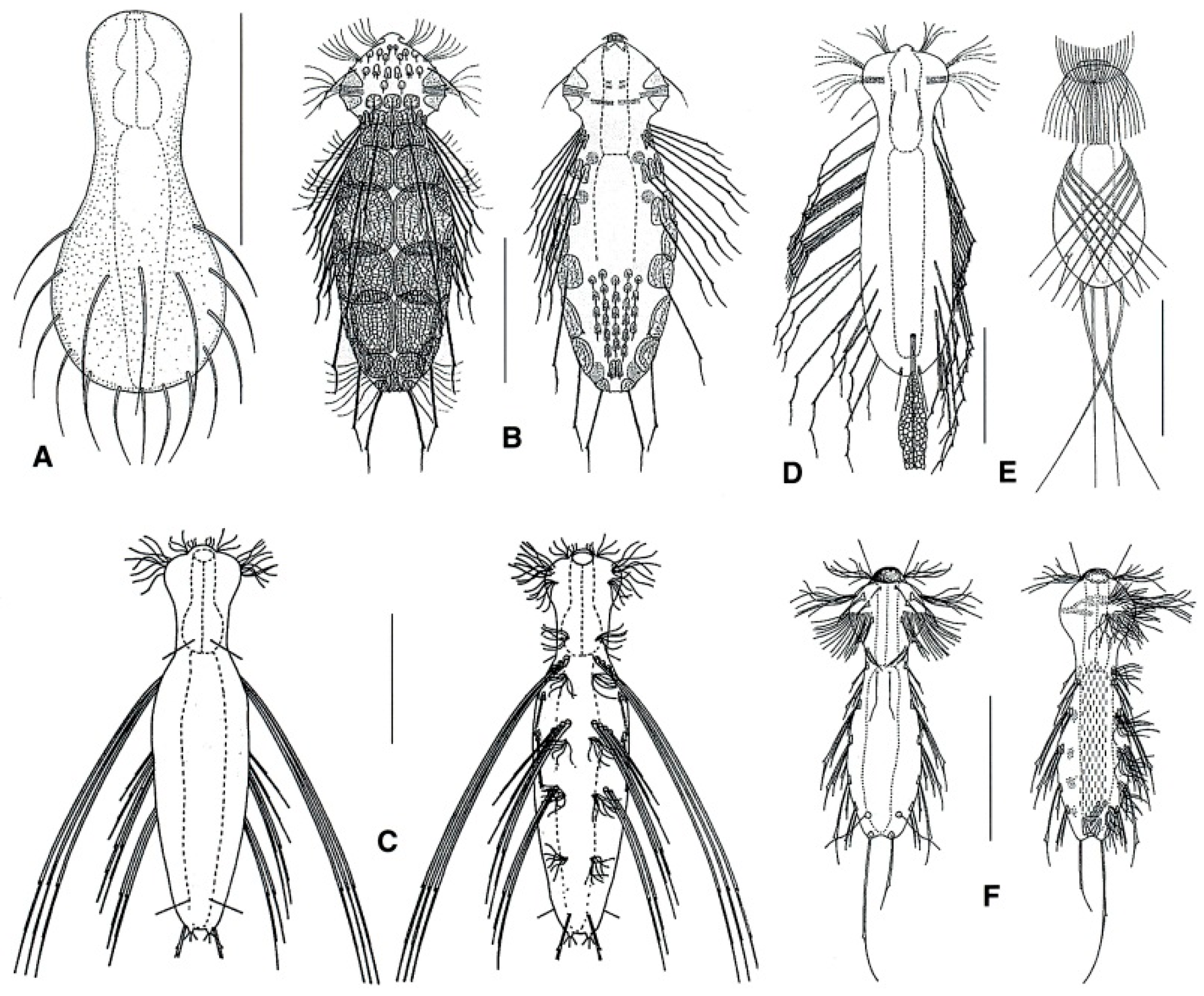
- Body flask-, bottle- or tenpin-shaped; posterior body region usually furcate (furca), less often rounded off or bifurcate; cuticle usually forming ornamentations such as scales and spines; TbA, TbD, and TbL absent; TbP present, numbering 2 (rarely 4 or 0) at the distal end of the furcal rami; mouth narrow; pharynx lacking pores. Freshwater, marine, and brackish: periphytic, epibenthic, and interstitial, occasionally semiplanktonic. Order CHAETONOTIDA, Suborder PAUCITUBULATINA (Figure 1A–C) ……………….…….... 38
- 1b
- Different from the above. …………….………..…………………………………..……....…….... 2
- 2a (1b)
- Body vermiform; cuticle naked, not forming scales and/or spines; TbA and TbD absent; TbL present in the form of numerous papillae along each side; TbP, some per side, fused at their bases forming two adhesive structures; mouth narrow, pharynx lacking pores. Uncommon; marine: interstitial. Order CHAETONOTIDA, Suborder MULTITUBULATINA, NEODASYIDAE …………………………………………………….……... Neodasys (Figure 1D)
- 2b (1b)
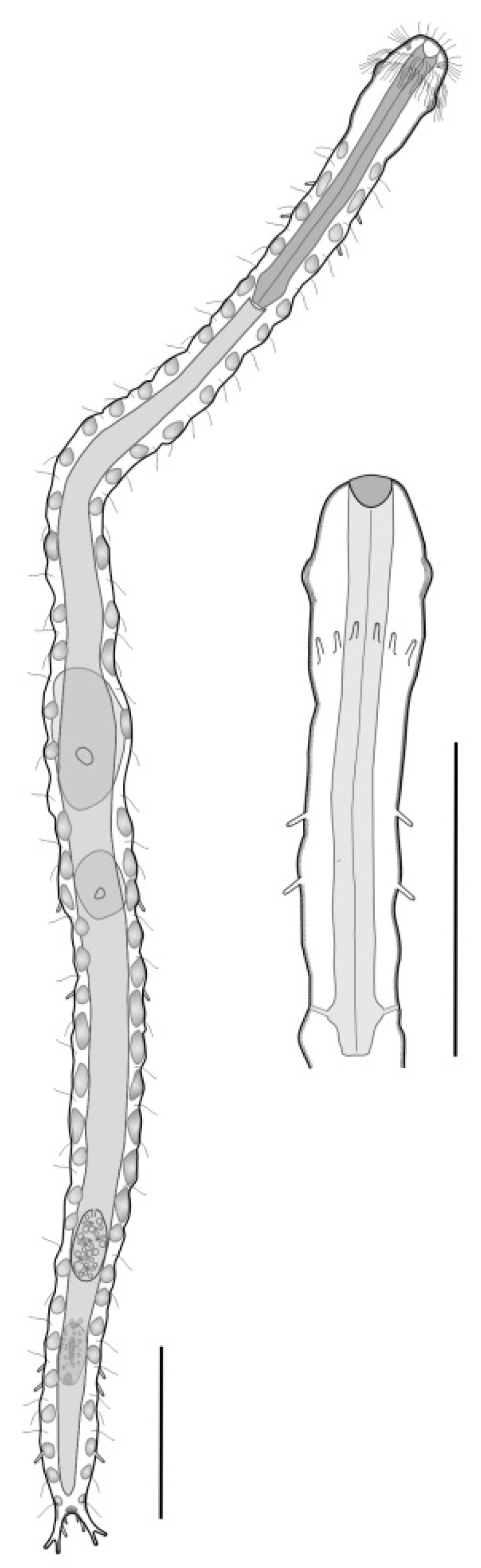
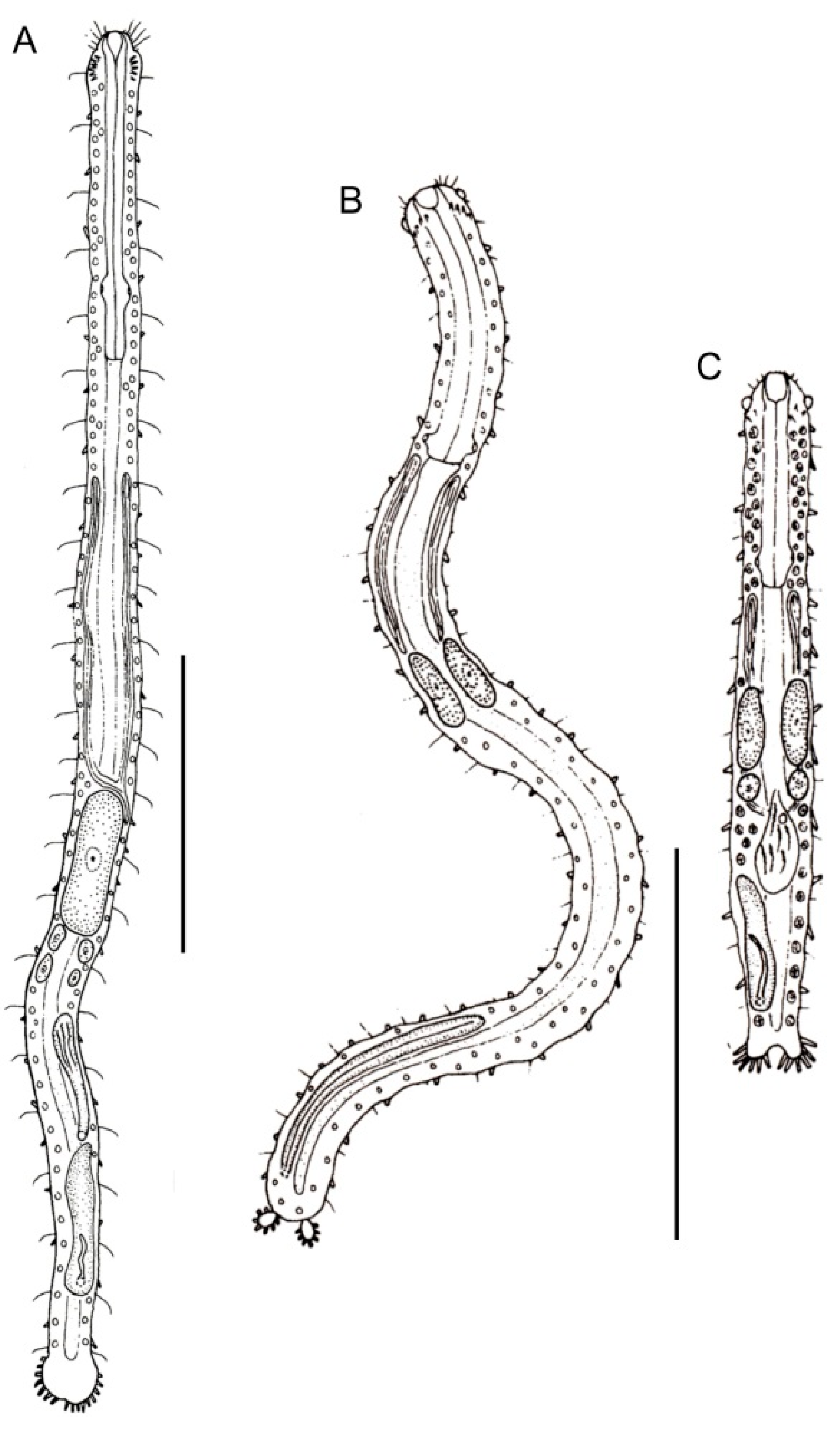
- Body usually vermiform, occasionally tenpin-shaped; cuticle naked or forming ornamentations such as plates and multi-pointed hooks; TbA, TbL, and TbP present, usually numerous; TbD present in several taxa; mouth opening narrow to broad; pharyngeal pores usually present. Marine and brackish, rarely fresh water: interstitial. Order MACRODASYIDA (Figure 2) ........... 3

- 3a (2b)
- Marine or brackish. ...……………………...……….……………………………………………… 4
- 3b (2b)
- Freshwater. ...…………...…………………....……………….......…………………....……..……37
- 4a (3a)
- Body tenpin-shaped; head well discernible, including most of pharynx; TbD absent; posterior body region lobed, furcate, or bifurcate. Cuticle bare or developing thickenings and ridges. ..……………………………………………………………………………...……...… 5
- 4b (3a)
- Body vermiform, head usually indistinct or, when distinct, includes only part of pharynx; cuticle naked or developing spines and/or scales. …...……….………………….…….....…... 9
- 5a (4a)
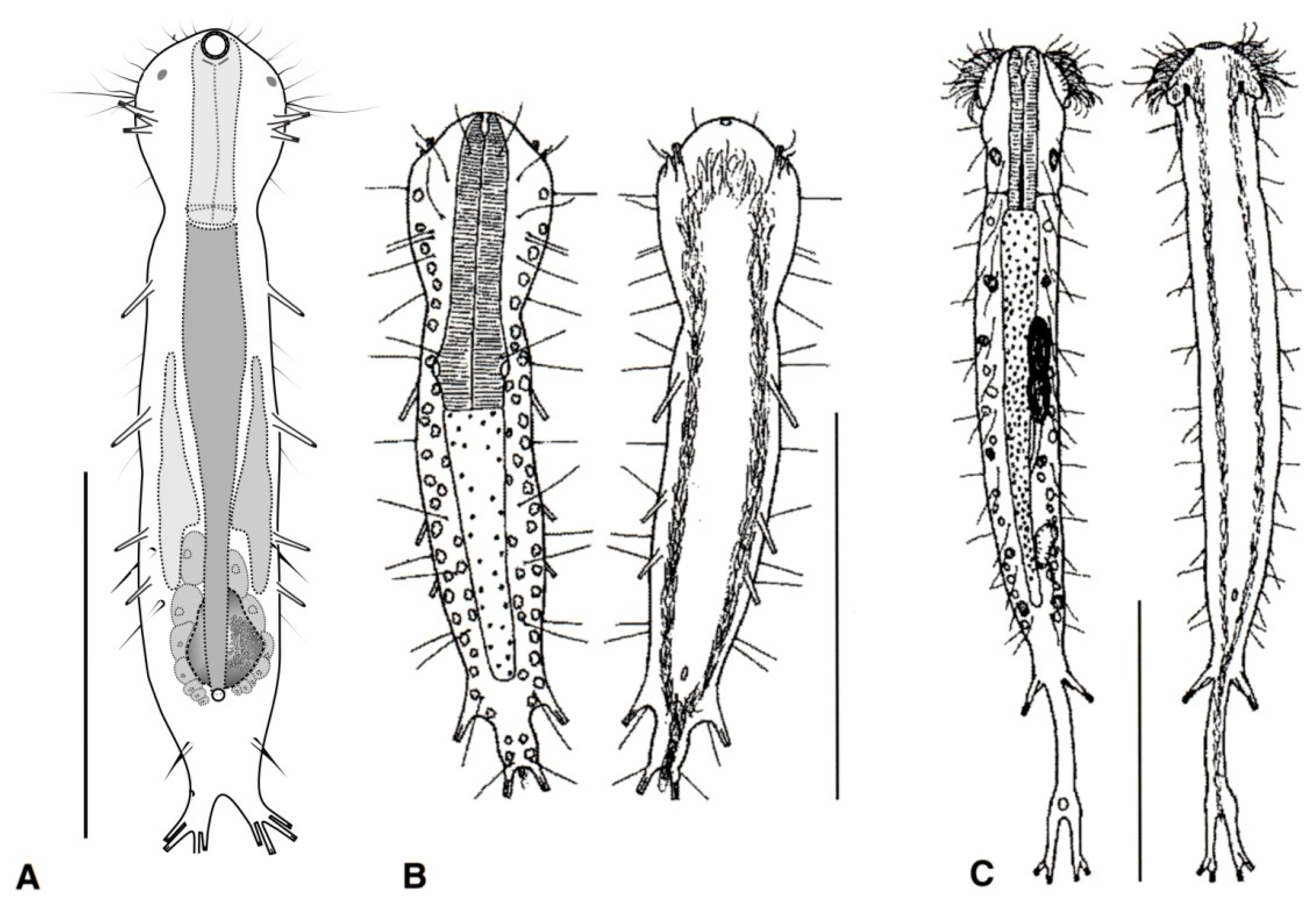
- Cuticle naked; dorsal side of the trunk naked; chordoid organ not present. Common to rare; marine and brackish: interstitial. DACTYLOPODOLIDAE (Figure 3) ….………..…...……. 6
- 5b (4a)
- Cuticle often developing thickenings and ridges; if naked, the trunk bears, on the dorsal side, long rod-like structures; chordoid organ present. Rare; marine: interstitial. XENODASYIDAE (Figure 4) ……………………………………..………………….…....…..… 8
- 6a (5a)
- Head simple or bearing two sensorial tentacles; cuticular covering bare; posterior body region bilobed; TbL present. Regionally common; marine: interstitial. .......... Dactylopodola (Figure 3A)
- 6b (5a)
- Head simple or with crenulated lateral lobes; cuticular covering bare; posterior body region bifurcate; TbL absent. ………………………….………………………………………………….. 7
- 7a (6b)
- Head simple, cuticle naked. Rare; marine: interstitial. …………….. Dendropodola (Figure 3B)
- 7b (6b)
- Head with elongate crenulated lateral lobes. Uncommon; marine: interstitial. ………………………………………………………………………………. Dendrodasys (Figure 3C)
- 8a (5b)
- Trunk region without tentacles, but presenting dented lateral sides; posterior body region furcate; distal rami, each showing a small TbP. Rare; marine: interstitial. ......................................................................................................................... Xenodasys (Figure 4A)
- 8b (5b)
- Trunk region bearing numerous tentacles; lateral sides of the trunk region parallel, lacking indentations; posterior region furcate; each ramus showing an adhesive pad at the end. Rare; marine: interstitial. ………………………………………..………........ Chordodasiopsis (Figure 4B)
- 9a (4b)
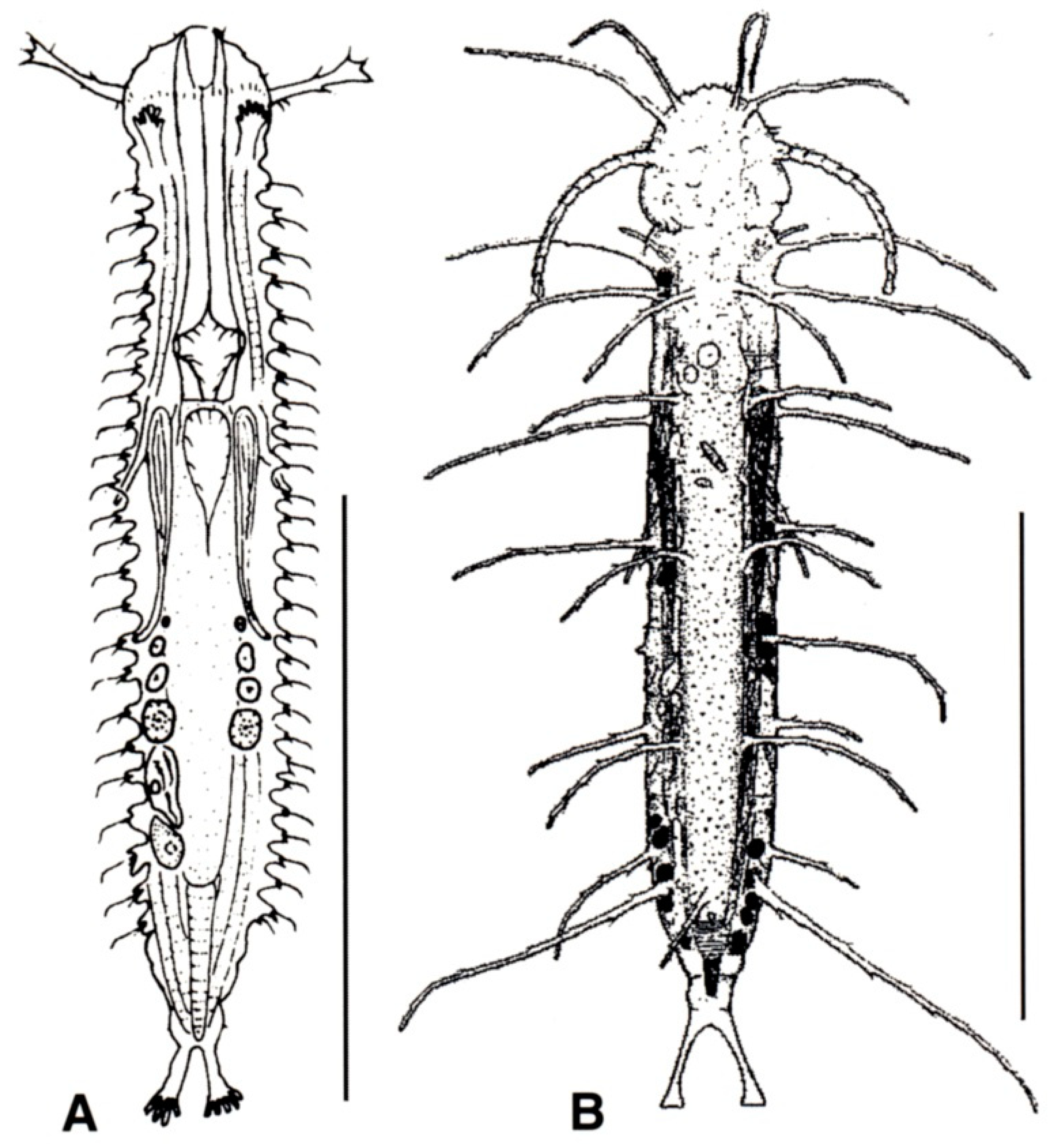
- TbA, usually 4 or more per side, occasionally 2 or 3, at the end of extensible fleshy base (Figure 2B); pharynx with pores located at the base. ................................................................. 10
- 9b (4b)
- TbA, generally 1 to 3 per side, occasionally 4 or more, arising singly and directly from the body surface; pharynx with pores at the base or in the middle. .............................................. 16
- 10a (9a)
- Head generally well demarcated posteriorly by a furrow; posterior body region tapered into a medial process, truncated, rounded, or broadly expanded, but never two-lobed. CEPHALODASYIDAE (partim) (Figure 5) .................................................................................. 11
- 10b (9a)
- Head normally not clearly delimited; posterior body region two-lobed. TURBANELLIDAE (partim) (Figure 6) ........................................................................................................................... 12
- 11a (10a)
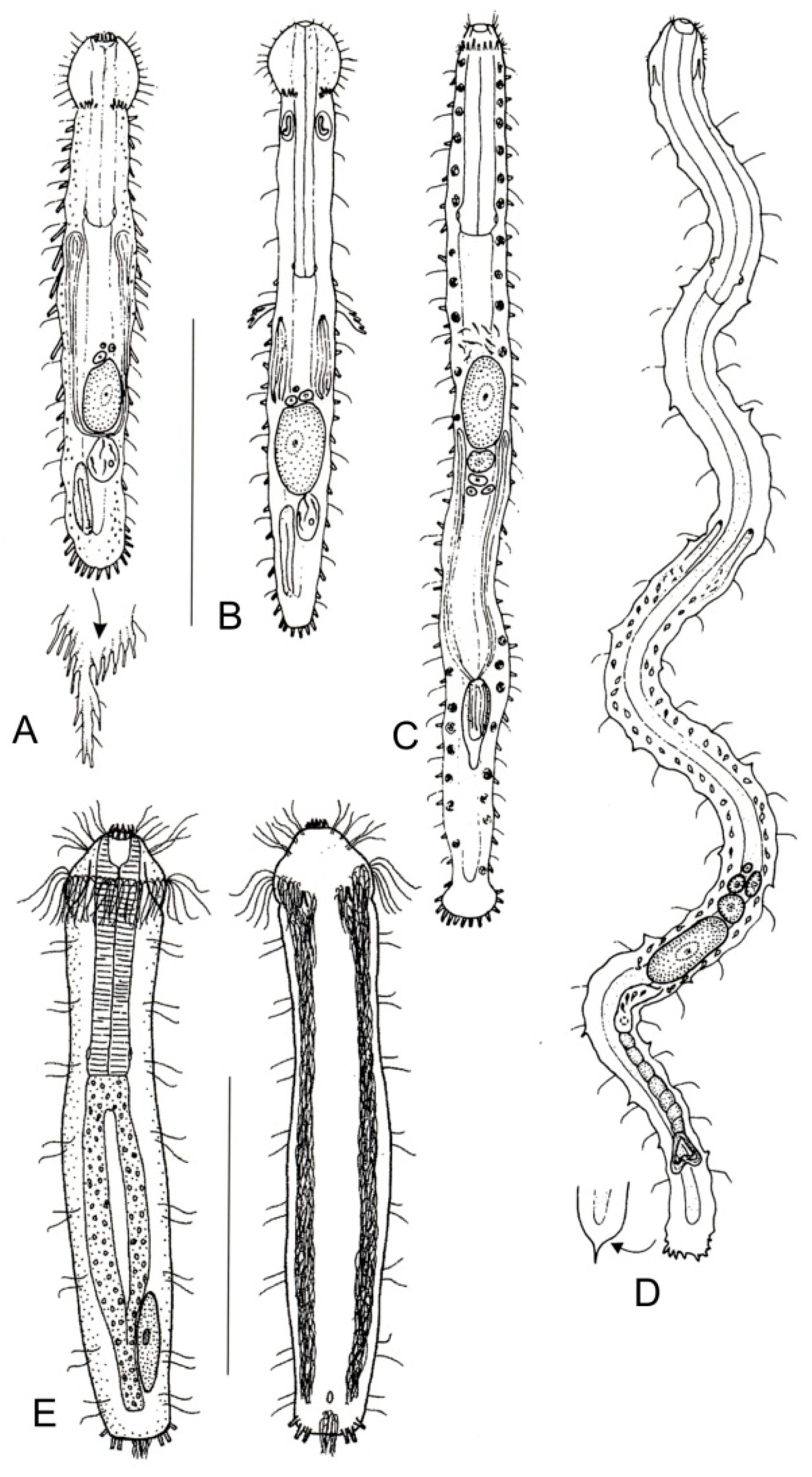
- Head surrounded by very thick and dense sensory cilia; a couple of accessory adhesive organs present near the PhIJ, laterally directed; each organ comprising 3–4 tubes of unequal length; a couple of club-shaped gravireceptor organs on the dorsal side of the posterior cephalic region may be present. Rare; marine: interstitial. ……...….. Pleurodasys (Figure 5B)
- 11b (10a)
- Cephalic sensory cilia and accessory adhesive organs described above are absent. Regionally common; marine and brackish: interstitial. ................... Cephalodasys (Figure 5A)
- 12a (10b)
- Head showing elongate lateral tentacles. …………………………………….….....………… 13
- 12b (10b)
- Head without tentacles, occasionally with conical lobes. ……………………...….......…… 14
- 13a (12a)
- TbL numerous. Uncommon; marine: interstitial. ……………..…..…. Dinodasys (Figure 6A)
- 13b (12a)
- TbL lacking, paired TbV inserted just past the PhIJ. Rare; marine: interstitial. ................. ............................................................................................................. Pseudoturbanella (Figure 6B)
- 14a (12b)
- Paired accessory adhesive organs in the anterior pharyngeal region; organs are posteriorly directed, and each is made up of two tubes of unequal lengths. Common; marine and brackish: interstitial. ........………….…..…………………..…..…… Paraturbanella (Figure 6C)
- 14b (12b)
- Accessory adhesive tubes described above are either absent or present in different body regions. ………………………………………………………………………………………...…... 15
- 15a (14b)
- Accessory adhesive tubes not present. Common; marine and brackish: interstitial. ………….……………………………………………………………….……Turbanella (Figure 6E)
- 15b (14b)
- Accessory adhesive tubes present, close to the PhIJ. Rare; marine: interstitial. …………………..…..………………...........................................…... Prostobuccantia (Figure 6D)
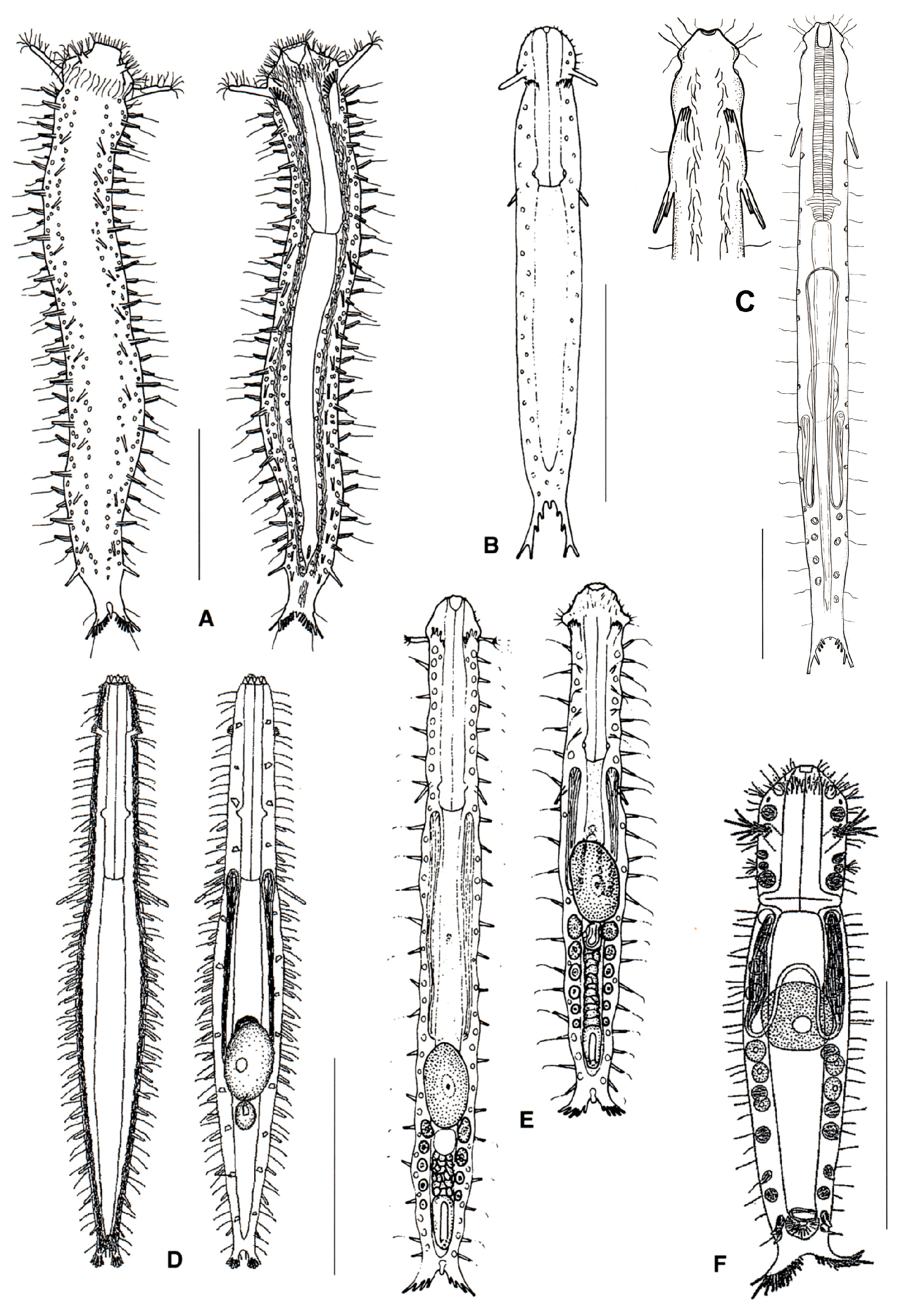
- 16a (9b)
- Pharynx with pores far from the base; posterior body region unilobed, ovoidal in shape, or tapering off. MACRODASYIDAE (Figure 7) …......................................................................…. 17
- 16b (9b)
- Pharynx with pores at the base; posterior end of body not tapering off ……………...….… 20
- 17a (16a)
- Head bearing a lateral leaf-like sensorial organ; posterior body region unilobed, ovoidal in shape. Rare; marine: interstitial. …….…………….…………………. Thaidasys (Figure 7B,C)
- 17b (16a)
- Head bearing lateral piston pit sensorial organs; posterior body region tapering into a medial process. ...……………………………………..…………………………………………… 18
- 18a (17b)
- Posterior process in the form of a long tail. Regionally common; marine: interstitial and epibenthic. …………………………………………….……………….……. Urodasys (Figure 7E)
- 18b (17b)
- Posterior process short or in the form of a short tail. ………………….……………….…… 19
- 19a (18b)
- Frontal organ posterior to the largest egg; spermatozoa filiform. Common; marine: interstitial. …..……………….…..……..………………………..…..…... Macrodasys (Figure 7D)
- 19b (18b)
- Frontal organ anterior to the largest egg; spermatozoa stout. Uncommon; marine: interstitial. ……………………...……..…………………………….…... Kryptodasys (Figure 7A)
- 20a (16b)
- Cuticle forming ornamentations such as hooks, papillae, scales, or thickenings. ..……..... 21
- 20b (16b)
- Cuticle smooth, without ornamentation such those reported above. ...……….……..….… 27
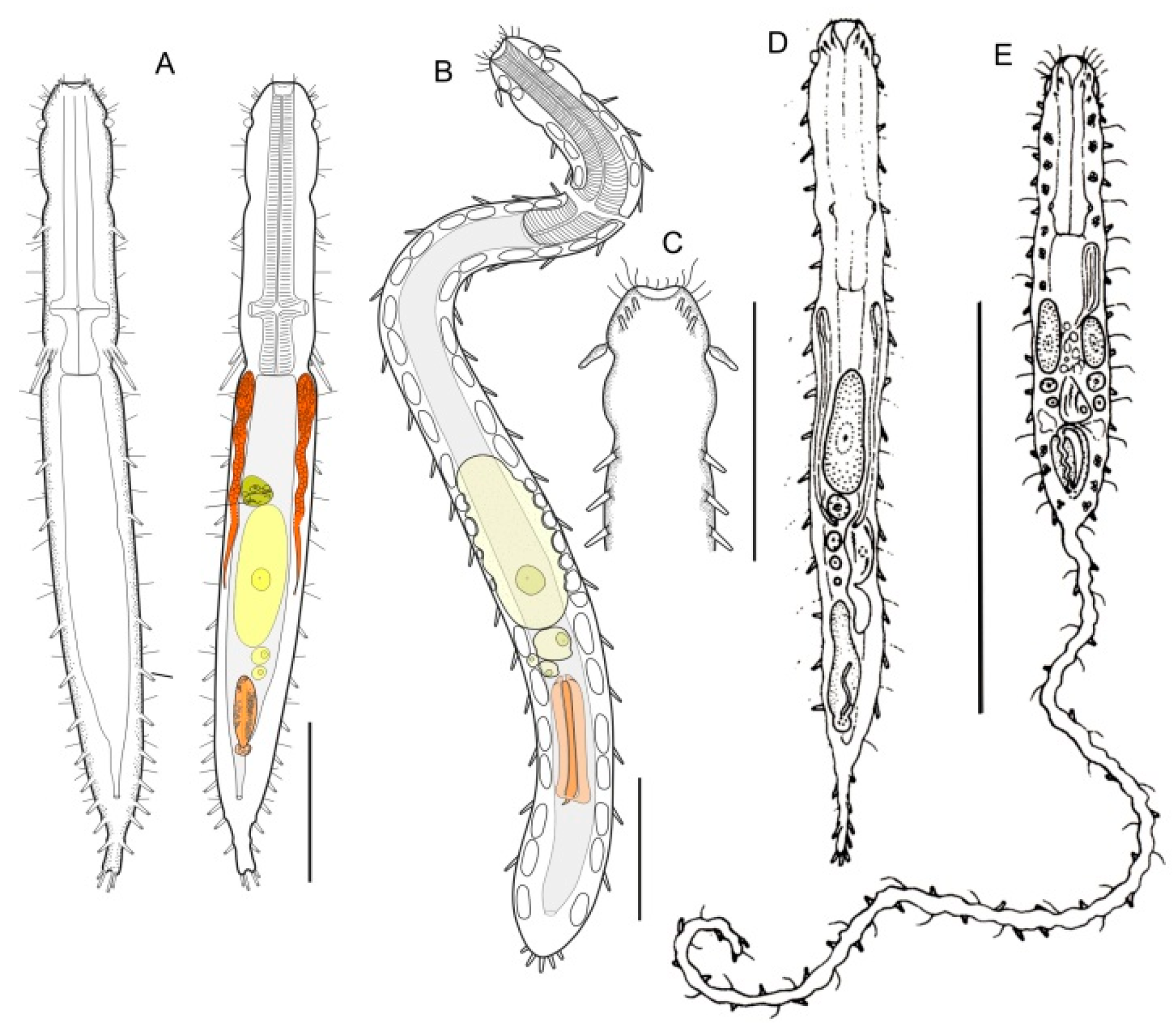
- 21a (20a)
- ) Presence of elongate scales; mouth narrow; pharynx without pores. Uncommon; marine: interstitial. LEPIDODASYIDAE ................................................…….... Lepidodasys (Figure 8)
- 21b (20a)
- Presence of variously spined hooks, large scales, or papillae; mouth opening generally broad; pharyngeal pores present. THAUMASTODERMATIDAE (partim) (Figure 9) ……………... 22
- 22a (21b)
- Presence of papillae or large scales. ...…………………..……………………….……………. 23
- 22b (21b)
- Presence of uni- or multi-spined hooks. ……………………….………………………….…. 24
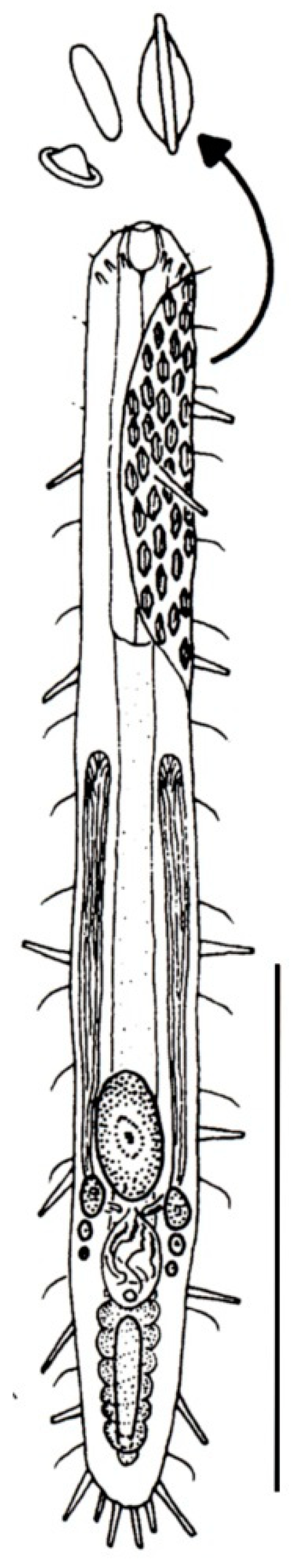
- 23a (22a)
- Cuticle with large scales, but not papillae; on either side of the body a single row of wide spines present. Regionally common; marine: interstitial. ..........….. Diplodasys (Figure 9A)
- 23b (22a)
- Cuticle with papillae, but not scales or spines. Uncommon; marine: interstitial. ....................………………………...…………………………..……..….... Oregodasys (Figure 9B)
- 24a (22b)
- Cuticle with hooks showing a single spine; right and left testicles present; Common; marine: interstitial. .....…….……...…….…………………..……....… Acanthodasys (Figure 9C)
- 24b (22b)
- Cuticle with hooks showing more than one spine; a single testicle on the right-hand body side. ……………………………………………………………………………………………….... 25
- 25a (24b)
- Anterior body region showing conspicuous, grasping structures on either side of the mouth funnel (buccal palps); hooks bearing 5, 4, or 3 spines (penta-, tetra-, or triancres). Common; marine: interstitial. …………………………………….. Pseudostomella (Figure 9D)
- 25b (24b)
- Anterior body region without buccal palps; hooks showing 5, 4, 3, or 2 spines (penta-, tetra-, tri-, or biancres). ..…………………………………………..…………………………..…. 26
- 26a (25b)
- Head bearing two pairs of sensoria tentacles on the lateral sides; mouth narrow, hooks with four spines. Common; marine: interstitial. ………….….... Thaumastoderma (Figure 9E)
- 26b (25b)
- Head bearing no or one pair of sensorial tentacles on the lateral sides; hooks with 5, 4, 3, or 2 spines. Very common; marine: interstitial. ………............. Tetranchyroderma (Figure 9F)
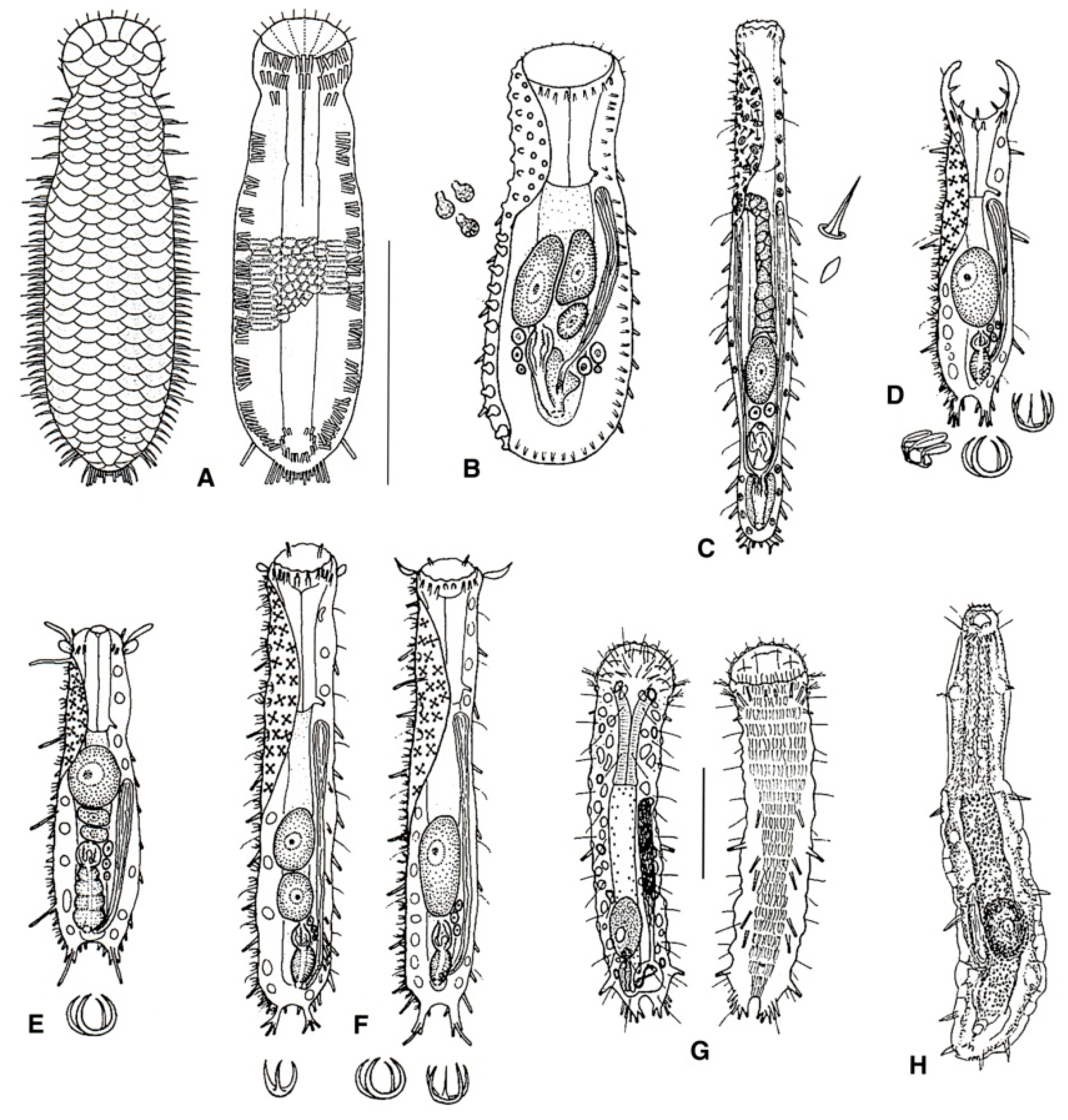
- 27a (20b)
- Male apparatus absent (i.e., parthenogenetic); TbA, two groups of three tubes per side; TbL, four or five per side, TbP up to five per side. Rare; marine: interstitial. REDUDASYIDAE (partim) ……………………………………………………………….. Anandrodasys (Figure 10A)
- 27b (20b)
- These characteristics not combined. ..……………..……...………………..………………… 28
- 28a (27b)
- TbA, several to many, arranged in two tufts; TbL absent. Rare; marine: interstitial. TURBANELLIDAE (partim) …………………………..…………….. Desmodasys (Figure 6F)
- 28b (27b)
- TbA, few to many, but not arranged in tufts; TbL normally present or, if absent, then TbA few in number. …………....…………………………………………………..……….………….. 29
- 29a (28b)
- TbA, few; TbL few; body elongate (to about 1 mm in length) and narrow; posterior end in the form of two distinct pedicles. HUMMONDASYIDAE. …….... Hummondasys(Figure 11)
- 29b (28b)
- These characteristics not combined. ……………….…………………….…….……….…….. 30
- 30a (29b)
- TbA, few to many; TbL and TbP, numerous (more than 10 per side); mouth narrow (< 0.4 × head width); posterior body region in the form of a large round lobe or clearly two-lobed. PLANODASYIDAE (Figure 12) ……………………………………………..……..……...……….. 31
- 30b (29b)
- TbA or TbL numbering fewer than six tubes per side; oral opening narrow to broad, and if narrow, then posterior body region not clearly two-lobed. ...……………………………... 33
- 31a (30a)
- TbA, present in low numbers; body very long (up to 3.5 mm in length) and rather narrow; posterior body region ending as a large lobe. Uncommon; marine: interstitial. ……………………………………………………………………….…… Megadasys (Figure 12A)
- 31b (30a)
- Posterior body region distinctly two-lobed. ………………………..……..…………………. 32
- 32a (31b)
- Posterior lobes in the form of oval appendages; most anterior TbA arranged transversely; caudal organ elongate. Rare; marine: interstitial. .……………...…… Planodasys (Figure 12B)
- 32b (31b)
- Posterior lobes in the form of furcate extensions; most anterior TbA arranged longitudinally; caudal organ ovate. Uncommon; marine: interstitial. ……………………... Crasiella (Figure 12C)
- 33a (30b)
- Oral opening, narrow (< 0.4 × head width); right and left testicles present. CEPHALODASYIDAE (partim) (Figure 5) ………………………………….……………..…... 34
- 33b (30b)
- Oral opening broad (> 0.6 × head width) or, if narrow, leading to a large buccal cavity surrounded by an oral hood; a single testicle, on the right-hand side. THAUMASTODERMATIDAE (partim) (Figure 9) ….…………………………...………………. 36
- 34a (33a)
- Total body length > 1 mm; TbA, one per side; TbL in form of numerous papillae along the body sides. Uncommon; marine: interstitial. ……………………… Dolichodasys (Figure 5D)
- 34b (33a)
- Total body length < 1 mm. …………………………………..…………….…………………… 35
- 35a (34b)
- TbA, 1–4 tubes per side, arranged in two groups; TbL, 0–6 tubes per side. Uncommon; marine: interstitial. ....................................................................................... Paradasys (Figure 5E)
- 35b (34b)
- TbA, few to several per side; TbL, several to many. Common; marine: interstitial. ……………….....……………….......………………………………....…. Mesodasys (Figure 5C)
- 36a (33b)
- Oral opening, broad; locomotor cilia extending over the entire ventral surface; male genital pore not surrounded by cuticular plates. Common; marine: interstitial. ……….…………………………………………………………......… Ptychostomella(Figure 9G)
- 36b (33b)
- Oral opening, narrow, leading to a large buccal cavity covered by an oral hood; ventral locomotor cilia restricted to the pharyngeal region; male genital pore surrounded by cuticular plates. Very rare (possibly extinct); marine: interstitial …... Hemidasys (Figure 9H)
- 37a (3b)
- Total body length 300–400 μm; TbA, 1–2 per side; pharyngeal pores present. Rare; interstitial. REDUDASYIDAE (partim) .………………..…….……..… Redudasys (Figure 10B)
- 37b (3b)
- Body length up to 220 μm; TbA, one per side; pharyngeal pores absent. Rare; interstitial. INCERTAE SEDIS. …………………………..…….………………..…… Marinellina (Figure 13)
- 38a (1a)
- 38b (1a)
- Ventral locomotor ciliation formed by single cilia, occurring in longitudinal bands or tufts, never composed of cirri (Figure 1B). Freshwater, marine, and brackish. ............................... 41
- 39a (38a)
- Cirri of the head and pharyngeal regions of two different sizes, with 1–2 transverse rows of small and short cirri anteriorly followed by transverse rows of big and longer cirri; frontal portion of pharynx with a swelling (bulb). Common; marine and brackish: interstitial. ..……………………………………….…….....….. Heteroxenotrichula (Figure 14A)
- 39b (38a)
- Cirri, all of similar size, pharynx without anterior swelling (bulb). ...………………..…… 40
- 40a (39b)
- Ovary and eggs present, testicles and spermatozoa absent; head clearly distinct; scales on the dorsal side, flat; scales of the lateral mid-trunk, pedunculated; a pair of lateral spines at the base of the furcal branches. Common; marine: interstitial. ..... Draculiciteria (Figure 14B)
- 40b (39b)
- Testicles and spermatozoa present; head in general not clearly defined; scales of the lateral mid-trunk bearing a stalk or flat; if stalked, similar to the dorsal scales. Common; marine and brackish: interstitial. ………………..……………….…. Xenotrichula (Figure 14C)
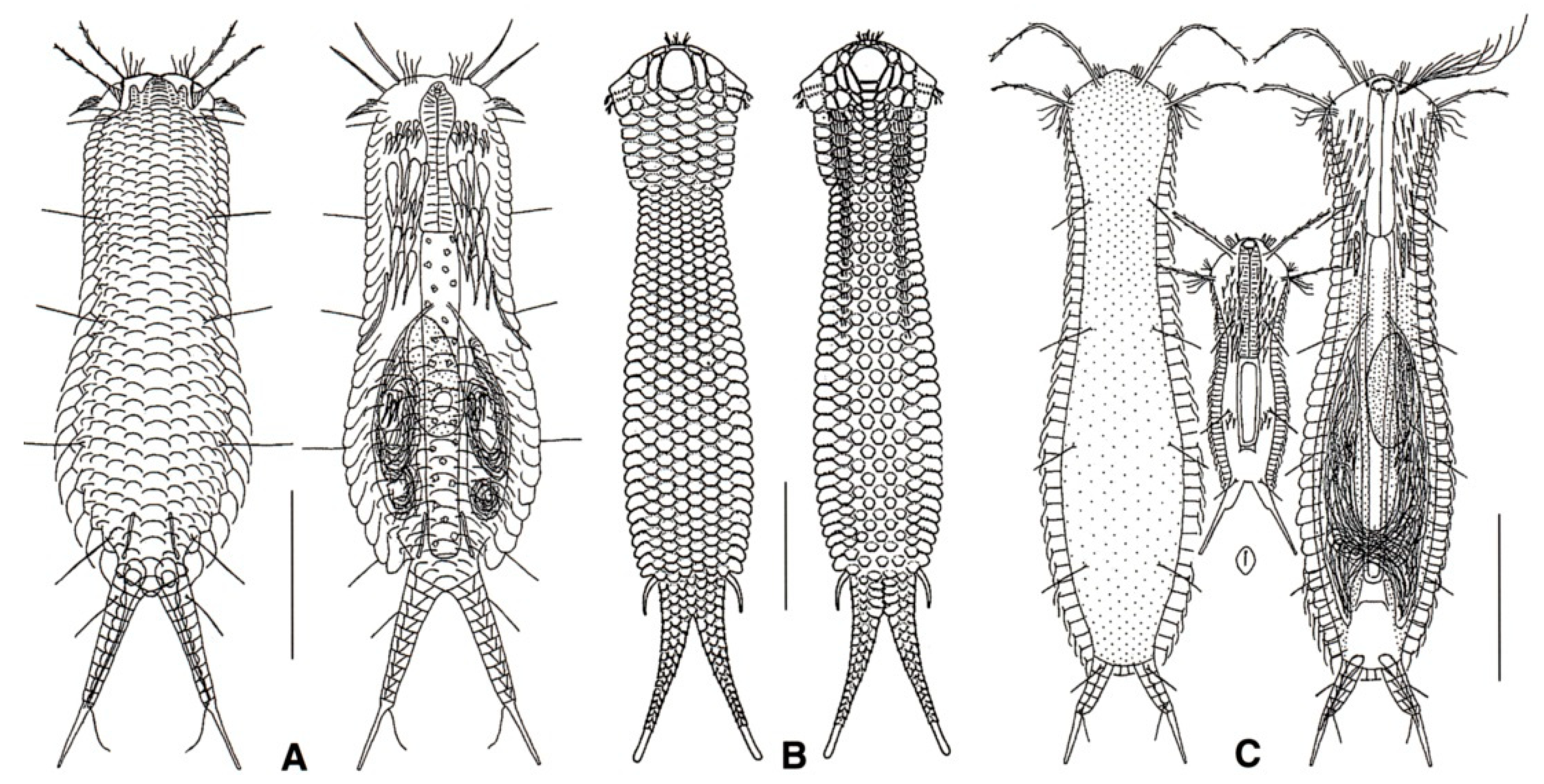
- 41a (38b)
- Posterior body region furcate or bifurcate; caudal rami with or without TbP. ……..…..... 42
- 41b (38b)
- Posterior body region rounded or truncated; perhaps showing two caudal protuberances or spines. ………………………………………………………………………………………………... 60
- 42a (41a)
- Posterior body region bifurcate, bearing four TbP or two TbP and two spiniform cuticular processes; elsewhere, cuticle smooth, not forming scales or spines. Rare; freshwater: interstitial or periphytic/epibenthic. DICHAETURIDAE …………………….….….. Dichaetura (Figure 15A)
- 42b (41a)
- Posterior body region furcate; cuticle smooth or forming spines and/or scales. ..……...... 43
- 43a (42b)
- Body cuticle smooth; caudal rami with TbP, sickle-shaped; cilia of the head not arranged into tufts. Very rare; freshwater: semiplanktonic or hyperbenthic. PROICHTHYDIIDAE (Figure 15B,C) ……………………………………..…………………………….…………..…….. 44
- 43b (42b)
- Body cuticle generally forming spines and scales; caudal rami with or without TbP; if present, caudal rami and TbP generally straight, short to very long; cilia of the head emerging as tufts or forming a continuous band around the elongate, muzzle-like frontal end. .…………………………………………………………………………………………....…… 45
- 44a (43a)
- Cilia of the head arranged as a transverse row of small elements on the dorsal side; locomotor cilia limited to head and neck, emerging as separate tufts. Freshwater: hyperbenthic …………………………………………...…….………Proichthydium (Figure 15B)
- 44b (43a)
- Cilia of the head emerging mostly from the lateral sides as single, short to very long elements; locomotor cilia distributed in two bands that run from under the head to the posterior trunk region. Freshwater: semiplanktonic. …....................................…… Proichthydioides (Figure 15C)
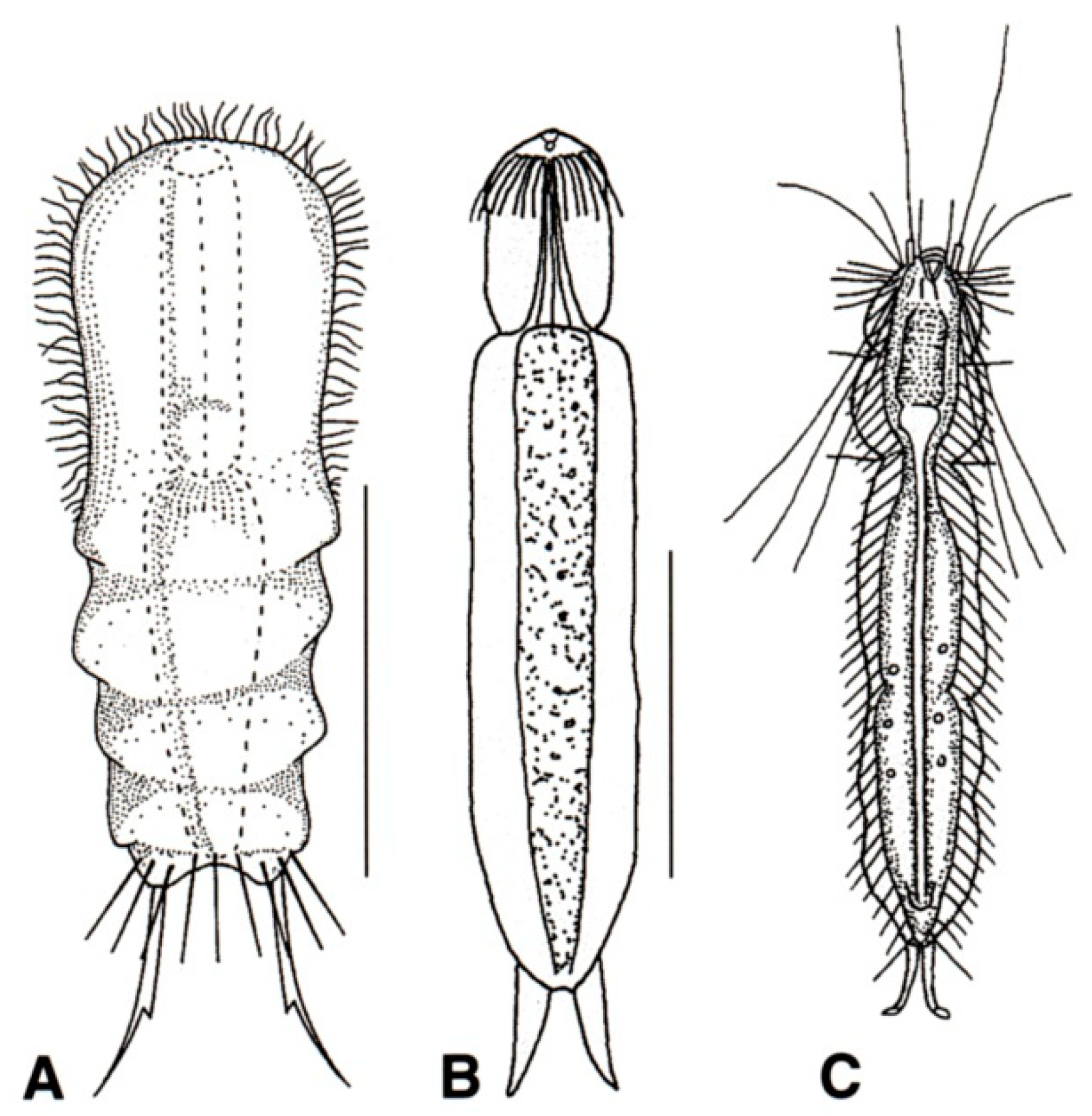
- 45a (43b)
- 45b (43b)
- Cilia of the head organized in a band, encircling a muzzle-like frontal end; TbP numbering two or four. Uncommon to rare, marine: epibenthic or interstitial. MUSELLIFERIDAE (Figure 20) …………………………………………………………………………………………………………. 67
- 46a (45a)
- TbP at the end of the furcal rami absent. Rare; freshwater: epibenthic. …. Undula (Figure 16A)
- 46b (45a)
- TbP at the end of the furcal rami present. ...……………………………………....……...…... 47
- 47b (46b)
- Furcal base narrow (pedunculated); caudal rami segmented; cephalion and hypostomion extremely large; scales without a keel, notch, or spine. Rare; freshwater: epibenthic. …………………………………………………………...……………….. Cephalionotus (Figure 16B)
- 47b (46b)
- These characteristics not combined. ……………………………………….…..……...……… 48
- 48a (47b)
- Furcal rami very long (up to one-third of the total body length), multi-segmented, bare or with tiny spines or scales. Common, freshwater: periphytic and epibenthic. ………………………………………………………………………...… Polymerurus (Figure 16C)
- 48b (47b)
- Furcal rami from very short to mid length, not segmented, scales or spines limited to the proximal portion or lacking altogether. ………………………………………..…..………….... 49
- 49a (48b)
- Cuticular covering bare (or mostly bare) or made up of scales lacking spines; seldomly, some spines may be present at the base of the furca. .……………..…………....…………..… 50
- 49b (48b)
- Cuticular covering including scales bearing spines (spined scales) and/or a keel (spined, keeled scales and keeled scales, respectively); spines from long to very short, bearing 1–2 indentations laterally (notched spines), or simple. ………………………………………………... 57

- 50a (49a)
- Cuticular covering bare, rarely a few scales and/or spines at base of the furca may be present. …………………………………………………………………………………………...... 51
- 50b (49a)
- Cuticular covering wholly or prevalently made of spineless scales. ….…….……...……... 53
- 51a (50a)
- Cuticle completely bare, very thick, obviously distinguishable from the underlying epidermal layer. Rare; freshwater: interstitial. ……...……...………..…. Arenotus (Figure 16D)
- 51b (50a)
- Cuticle thin, mostly bare, except for perhaps two terminal scales at the end ventral interciliary field; occasionally, weak striations along the body or few spines and/or scales at the furcal base may be present. Common; freshwater, rarely marine or brackish water: periphytic, epibenthic, and interstitial. …………………………………………………………………………………..…… 52
- 52a (51b)
- Furcal base pedunculated; locomotor cilia distributed in separated tufts. Uncommon; marine: interstitial. ………….……..………………..………....…. Caudichthydium (Figure 16E)
- 52b (51b)
- Furcal base not pedunculated; locomotor cilia mostly forming two longitudinal bands. Common; freshwater, rarely brackish or marine: epibenthic, periphytic, and interstitial. ………………………………………………………………….……….…Ichthydium (Figure 16F)
- 53a (50b)
- Scales, small, keeled, or stalked. ………………………………………………………………. 54
- 53b (50b)
- Scales, large and bare, round, rhomboidal, or polygonal in shape. ..………………….…... 55
- 54a (53a)
- Most scales with a stalk; occasionally, few scales may lack a stalk and bear a keel or a spine instead. Common; freshwater, brackish, and marine: epibenthic, periphytic, and interstitial. ……………………………………………………………………...…….. Aspidiophorus (Figure 16G)
- 54b (53a)
- Numerous keeled scales; occasionally, few scales may bear a spine. Common; freshwater, brackish, and marine: periphytic, epibenthic, and interstitial. ...Heterolepidoderma (Figure 16H)
- 55a (53b)
- Scales polygonal in shape. Common; freshwater, rarely brackish or marine: interstitial, epibenthic, and periphytic. ……………………………..……….....Lepidodermella (Figure 16I)
- 55b (53b)
- Scales rhomboidal or circular in shape. ………………………….………………………...… 56
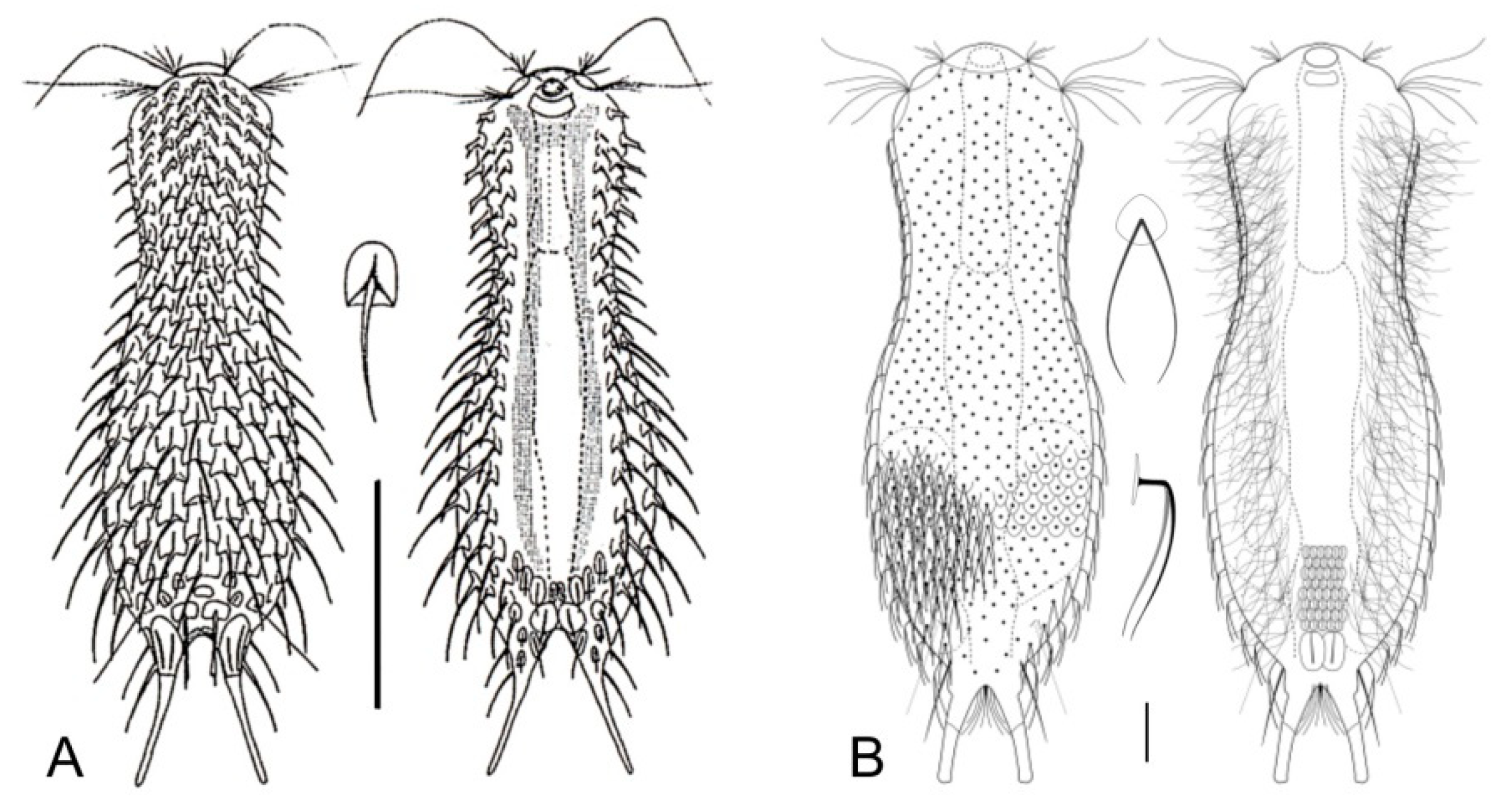
- 56a (55b)
- Scales circular. Rare; freshwater: periphytic. ..................................... Fluxiderma (Figure 16L)
- 56b (55b)
- Scales rhomboidal. Rare; freshwater: periphytic. ................. Rhomballichthys (Figure 16M)
- 57a (49b)
- Scales of the ventral interciliary field similar in shape to the scales of the dorsal side; scales of the dorsal side possessing a double edge anteriorly, with or without a spine but always deprived of a keel; several pairs of thin spines of increasing length at the lateral sides of the furcal base. Rather common; freshwater: periphytic and epibenthic. ……………………………………………………………………….... Lepidochaetus (Figure 16N)
- 57b (49b)
- Scales of the ventral interciliary field dissimilar in shape from scales of the dorsal side; scales of the dorsal side with a single edge anteriorly, keeled or keeled and spined…………………... 58
- 58a (57b)
- Scales lateral to the ventral locomotor cilia with spines bearing lamellae (hydrofoil scales); scales of the dorsal side bearing a keel; seldom presence of 1–5 scales bearing spines. Common; marine and brackish: interstitial. ……………….... Halichaetonotus (Figure 16O)
- 58b (57b)
- Scales bearing spines with lamellae normally absent; if present, dorsal scales spined. .…..…. 59
- 59a (58b)
- Dorsal scales round to suboval, without keels and/or notches but carrying distally bifurcating hairlike spines. Rare; freshwater: epibenthic……...…. Bifidochaetus (Figure 17B)
- 59b (58b)
- These characteristics not combined. Very common; freshwater, marine, and brackish: epibenthic, periphytic, and interstitial. ………...……………....…… Chaetonotus (Figure 17A)
- 60a (41b)
- Posterior body region rounded-off or truncated with paired lateral projections; head bearing a pair of rod- or club-shaped tentacles; trunk bearing small, spined scales; rarely, trunk scales restricted to a small patch on the ventral side. Uncommon to rare; freshwater: hyperbenthic and semiplanktonic. NEOGOSSEIDAE (Figure 18) ………….…….…………. 61
- 60b (41b)
- Posterior body region rounded or truncated, occasionally with a very short caudal lobe or paired postero-lateral protuberance; head without tentacles; body scales reduced or absent; trunk bearing very long and movable spines arranged into groups. Uncommon to rare; freshwater: hyperbenthic, epibenthic, and semiplanktonic. DASYDYTIDAE (Figure 19) ….…. 62
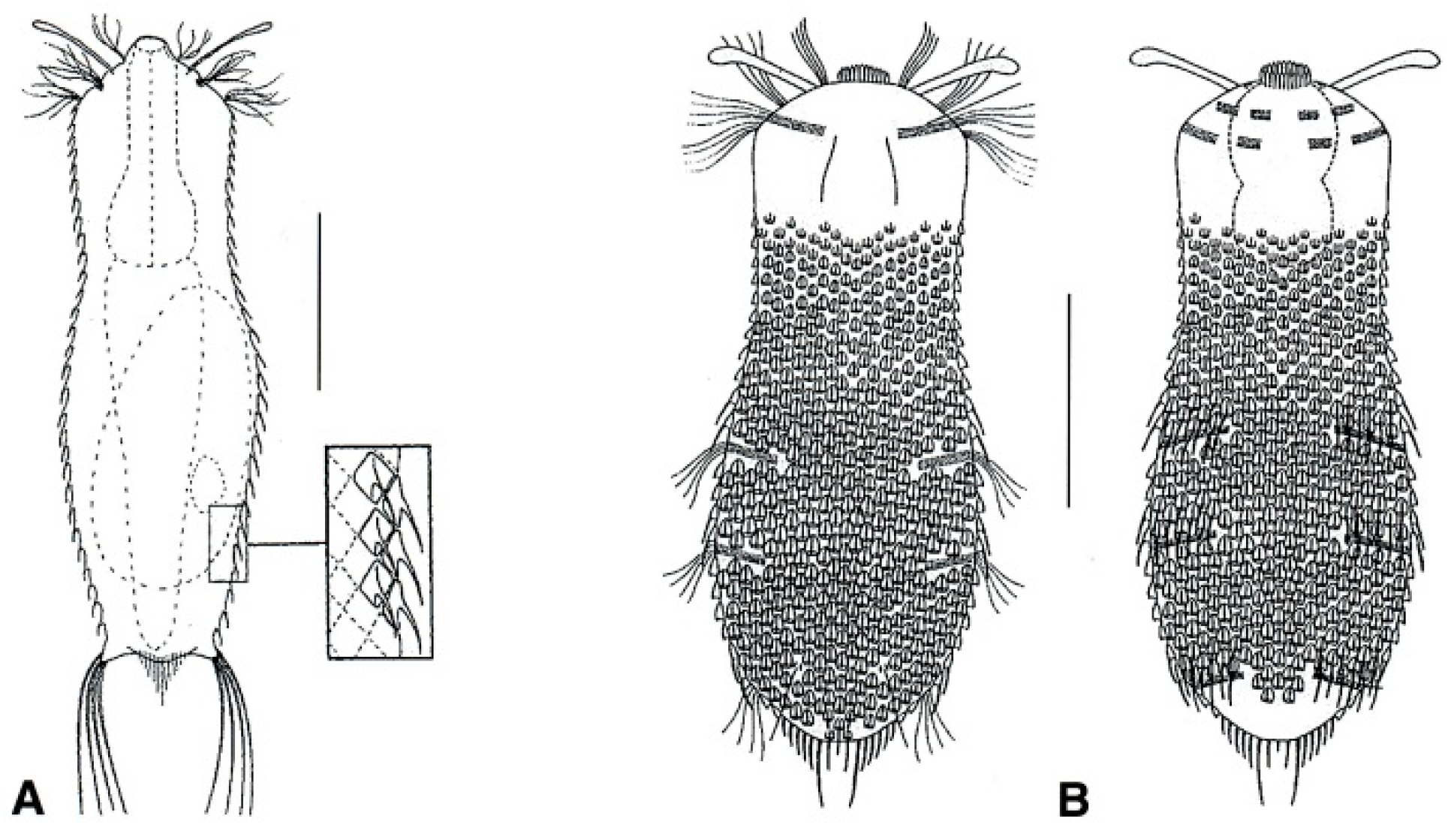
- 61a (60a)
- Posterior body region truncated, showing two lateral projections bearing a tuft of long spines; trunk with fine spined scales. Uncommon: epibenthic and semiplanktonic. ...................................................................................................................... Neogossea (Figure 18A)
- 61b (60a)
- Posterior body region rounded, with a central group of spines and no lateral projections; trunk with keeled scales, seldom reduced to a small group on the ventral side. Rare: epibenthic and semiplanktonic. …............................……………………..…………….…. Kijanebalola (Figure 18B)
- 62a (60b)
- Trunk region bearing long, scattered spines on the dorsal side or two caudal spines only; body scales absent; locomotor cilia arranged in two longitudinal bands; pharynx bearing two robust swellings (bulbs). Rare: epibenthic and semiplanktonic. …………………………………………...……………………….... Anacanthoderma (Figure 19A)
- 62b (60b
- ) Trunk region bearing long, lateral spines arranged into columns or groups; dorsal spines present or absent; locomotor cilia arranged in tufts; pharynx bearing a single swelling or cylindrical. ……………………………………………………………………………….…...…… 63
- 63a (62b)
- Lateral spines, simple or with notches; if present, scales large, elliptical in shape, and few in number; pharynx cylindrical (i.e., without bulbs). …………….……………...………….… 64
- 63b (62b)
- Lateral spines with a single lateral notch and bifurcate apex, or with 2–3 lateral notches and pointed apex; if present, numerous, small, keeled scales; pharynx bearing a swelling at the posterior end. ………………………………………………………………………………………… 65
- 64a (63a)
- Trunk showing dorsal spines; two pairs of caudal spines; all spines show a noticeable lateral notch; dorsal scales, rather large and of peculiar lace-like appearance. Rare: epibenthic, periphytic, and semiplanktonic. ……………….……... Ornamentula (Figure 19B)
- 64b (63a)
- Trunk lacking dorsal spines; a single pair of caudal spines or none; if very long, the lateral spines are thick and bent basally, becoming thinner and thinner distally; lateral notch present or absent; where present, body scales are small and feebly keeled. ………...……... 66
- 65a (65b)
- Lateral spines, robust, showing pointed tip and 2–3 lateral notches; body scales lacking; posterior body region showing two bristled protuberances on the sides. Uncommon: semiplanktonic and epibenthic.……………………..…….……………Stylochaeta (Figure 19C)
- 65b (63b)
- Lateral spines, almost straight, showing a bifurcate tip and a single lateral notch; body scales present; posterior body region rounded. Uncommon: semiplanktonic, periphytic, and epibenthic ………………………….………………………………...……Dasydytes (Figure 19D)
- 66a (63b)
- Caudal spines absent or present; if present, in general of different length; lateral spines, straight, of medium length; ventral, S-shaped, jumping spines lacking. Rare: semiplanktonic and epibenthic. ......................…………………….......................................... Setopus (Figure 19F)
- 66b (64b)
- Caudal spines absent; lateral spines very long, strongly bent crossing over the dorsal side; ventral S-shaped jumping spines present. Rare: semiplanktonic and epibenthic. ……………………………………………………………….………….…. Haltidytes (Figure 19E)
- 67a (45b)
- Furcal rami each with a single TbP; body scales bearing fine spines but lacking keels. Rare; marine: interstitial or infaunal. Uncommon: epibenthic and interstitial. .……………………………………………………………………..………. Musellifer (Figure 20A)
- 67b (45b)
- Furcal rami each with two TbP; body scales, keeled. Rare: interstitial. ...................................................................................................................... Diuronotus (Figure 20B)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Todaro, M.A. Meiofauna from the Meloria Shoals: Gastrotricha, biodiversity and seasonal dynamics. Biol. Mar. Medit. 1998, 5, 587–590. [Google Scholar]
- Gray, J.S. The effects of pollution on sand meiofauna communities. Thalass. Jugosl. 1971, 7, 79–86. [Google Scholar]
- Coull, B.S. Long-term variability of estuarine meiobenthos: An 11 year study. Mar. Ecol. Prog. Ser. 1985, 24, 205–218. [Google Scholar] [CrossRef]
- Todaro, M.A.; Fleeger, J.W.; Hummon, W.D. Marine gastrotrichs from the sand beaches of the northern Gulf of Mexico: Species list and distribution. Hydrobiologia 1995, 310, 107–117. [Google Scholar] [CrossRef]
- Hochberg, R. Spatiotemporal size-class distribution of Turbanella mustela (Gastrotricha: Macrodasyida) on a northern California beach and its effect on tidal suspension. Pacific Sci. 1999, 53, 50–60. [Google Scholar]
- Nesteruk, T. Density and biomass of Gastrotricha in sediments of different types of standing waters. Hydrobiologia 1996, 24, 205–208. [Google Scholar] [CrossRef]
- Ruppert, E.E. Gastrotricha. In Microscopic Anatomy of Invertebrates, Aschelminthes; Harrison, F.W., Ruppert, E.E., Eds.; Wiley-Liss: New York, NY, USA, 1991; Volume 4, pp. 41–109. [Google Scholar]
- Hochberg, R.; Litvaitis, M.K. A muscular double helix in gastrotricha. Zool. Anz. 2001, 240, 61–68. [Google Scholar] [CrossRef]
- Todaro, M.A.; Telford, M.J.; Lockyer, A.E.; Littlewood, D.T.J. Interrelationships of the Gastrotricha and their place among the Metazoa inferred from 18S rRNA genes. Zool. Scr. 2006, 35, 251–259. [Google Scholar] [CrossRef]
- Struck, T.H.; Wey-Fabrizius, A.R.; Golombek, A.; Hering, L.; Weigert, A.; Bleidorn, C.; Klebow, S.; Iakovenko, N.; Hausdorf, B.; Petersen, M.; et al. Platyzoan paraphyly based on phylogenomic data supports a noncoelomate ancestry of Spiralia. Mol. Biol. Evol. 2014, 31, 1833–1849. [Google Scholar] [CrossRef]
- Egger, B.; Lapraz, F.; Müller, S.; Dessimoz, C.; Girstmair, J.; Skunca, N.; Rawlinson, K.A.; Cameron, C.B.; Beli, E.; Todaro, M.A.; et al. A Transcriptomic-phylogenomic analysis of the evolutionary relationships of flatworms. Curr. Biol. 2015, 25, 1–7. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Jondelius, U.; Hochberg, R.; Hummon, W.D.; Kånneby, T.; Rocha, C.E.F. Gastrotricha: a marine sister for a freshwater puzzle. PLoS ONE 2012, 7, e31740. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Leasi, F.; Hochberg, R. A new species, genus and family of marine Gastrotricha from Jamaica, with a phylogenetic analysis of Macrodasyida based on molecular data. Syst. Biodiv. 2014, 12, 473–488. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Leasi, F. An integrated morphological and molecular approach to the description and systematisation of a novel genus and species of Macrodasyida (Gastrotricha). PLoS ONE 2015, 10, e0130278. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Dal Zotto, M.; Kånneby, T.; Hochberg, R. Integrated data analysis allows the establishment of a new, cosmopolitan genus of marine Macrodasyida (Gastrotricha). Sci. Rep. 2019, 9, 7989. [Google Scholar] [CrossRef] [PubMed]
- Leasi, F.; Todaro, M.A. The muscular system of Musellifer delamarei (Renaud-Mornant, 1968) and other chaetonotidans with implications for the phylogeny and systematisation of the Paucitubulatina (Gastrotricha). Biol. J. Linn. Soc. 2008, 94, 379–398. [Google Scholar] [CrossRef]
- Kånneby, T.; Todaro, M.A.; Jondelius, U. Phylogeny of Chaetonotidae and other Paucitubulatina (Gastrotricha: Chaetonotida) and the colonization of aquatic ecosystems. Zool. Scr. 2013, 42, 88–105. [Google Scholar] [CrossRef]
- Guidi, L.; Todaro, M.A.; Ferraguti, M.; Balsamo, M. Reproductive system and spermatozoa ultrastructure support the phylogenetic proximity of Megadasys and Crasiella (Gastrotricha, Macrodasyida). Contr. Zool. 2014, 83, 119–131. [Google Scholar] [CrossRef]
- Kånneby, T.; Todaro, M.A. The phylogenetic position of Neogosseidae (Gastrotricha: Chaetonotida) and the origin of planktonic Gastrotricha. Org. Divers. Evol. 2015, 6, 1–12. [Google Scholar]
- Balsamo, M.; Grilli, P.; Guidi, L.; d’Hondt, J.L. Gastrotricha: Biology, ecology and systematics. Families Dasydytidae, Dichaeturidae, Neogosseidae, Proichthydiidae. In Identification Guides to the Plankton and Benthos of Inland Waters; Dumont, H.J.F., Ed.; Backhuys Publisher: Leiden, The Netherlands, 2014; Volume 24, pp. 1–187. [Google Scholar]
- Kånneby, T.; Hochberg, R. Phylum Gastrotricha. In Thorp and Covich’s Freshwater Invertebrates: Ecology and General Biology; Thorp, J., Rogers, D.C., Eds.; Elsevie Academic Press: Amsterdam, The Netherlands, 2015; Volume 1, pp. 211–223. [Google Scholar]
- Kieneke, A.; Schmidt-Rhaesa, A. Gastrotricha and Gnathifera. In Handbook of Zoology; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2015; Volume 3, pp. 1–134. [Google Scholar]
- Gastrotricha Web Portal. Available online: http://www.gastrotricha.unimore.it/ (accessed on 25 June 2019).
- World Register of Marine Species (WoRMS). Available online: http://www.marinespecies.org/ (accessed on 25 June 2019).
- Hummon, W.D.; Todaro, M.A.; Balsamo, M.; Tongiorgi, P. Effects of pollution on marine Gastrotricha in the northwestern Adriatic Sea. Mar. Pollut. Bull. 1990, 21, 241–243. [Google Scholar] [CrossRef]
- Todaro, M.A.; Rocha, C.E.F. Diversity and distribution of marine Gastrotricha along the northern beaches of the state of Sao Paulo (Brazil), with description of a new species of Macrodasys (Macrodasyida, Macrodasyidae). J. Nat. Hist. 2004, 38, 1605–1634. [Google Scholar] [CrossRef]
- Todaro, M.A.; Rocha, C.E.F. Further data on marine gastrotrichs from the State of São Paulo and the first records from the State of Rio de Janeiro (Brazil). Meiofauna Mar. 2005, 14, 27–31. [Google Scholar]
- Hummon, W.D. Gastrotricha. In The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon; Carlton, J.T., Ed.; University of California Press: Berkeley, CA, USA, 2007; pp. 267–268. [Google Scholar]
- Todaro, M.A.; Leasi, F.; Bizzarri, N.; Tongiorgi, P. Meiofauna densities and gastrotrich community composition in a Mediterranean sea cave. Mar. Biol. 2006, 149, 1079–1091. [Google Scholar] [CrossRef]
- Sergeeva, N.G.; Ürkmez, D.; Todaro, M.A. Significant occurrence of Musellifer profundus Vivier, 1974 (Gastrotricha, Chaetonotida) in the Black Sea. Check List 2019, 15, 219–224. [Google Scholar] [CrossRef]
- Pfannkuche, O.; Thiel, H. Sampling processing. In Introduction to the Study of Meiofauna; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 134–145. [Google Scholar]
- Todaro, M.A. Contribution to the study of the Mediterranean meiofauna: Gastrotricha from the Island of Ponza, Italy. Boll. Zool. 1992, 59, 321–333. [Google Scholar] [CrossRef]
- Hochberg, R.; Litvaitis, M.K. Hexamethyldisilazane for scanning electron microscopy of Gastrotricha. Biotech. Histochem. 2000, 75, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Chang, C.Y. Two new marine gastrotrichs of the genus Ptychostomella (Macrodasyida, Thaumastodermatidae) from South Korea. Zool. Sci. 2003, 20, 481–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Todaro, M.A.; Hummon, W.D. An overview and a dichotomous key to genera of the phylum Gastrotricha. Meiofauna Mar. 2008, 16, 3–20. [Google Scholar]
- Kolicka, M.; Dabert, M.; Dabert, J.; Kånneby, T.; Kisielewski, J. Bifidochaetus, a new Arctic genus of freshwater Chaetonotida (Gastrotricha) from Spitsbergen revealed by an integrative taxonomic approach. Invert. Syst. 2016, 30, 398–419. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Araujo, T.Q.; Lourenço, A.P.; Guidi, L.; Balsamo, M. A new genus and new species of freshwater Chaetonotidae (Gastrotricha: Chaetonotida) from Brazil with phylogenetic position inferred from nuclear and mitochondrial DNA sequences. Syst. Biodiv. 2017, 15, 49–62. [Google Scholar] [CrossRef]
- Balsamo, M.; Todaro, M.A. Gastrotricha. In Freshwater Meiofauna Biology and Ecology; Rundle, S.D., Robertson, A.I., Schmid-Araya, J.M., Eds.; Backhuys Publisher: Leiden, The Netherlands, 2002; pp. 45–61. [Google Scholar]
- Pfannkuche, O. Gastrotricha. In Introduction to the Study of Meiofauna; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 302–311. [Google Scholar]
- Todaro, M.A.; Perissinotto, R.; Bownes, S.J. Two new marine Gastrotricha from the Indian Ocean coast of South Africa. Zootaxa 2015, 3905, 193–208. [Google Scholar] [CrossRef]
- Hummon, W.D.; Todaro, M.A.; Tongiorgi, P. Italian Marine Gastrotricha: II. One new genus and ten new species of Macrodasyida. Boll. Zool. 1993, 60, 109–127. [Google Scholar] [CrossRef]
- Hummon, W.D.; Todaro, M.A.; Tongiorgi, P.; Balsamo, M. Italian marine Gastrotricha: V. Four new and one redescribed species of Macrodasyida in the Dactylopodolidae and Thaumastodermatidae. Ital. J. Zool. 1998, 65, 109–119. [Google Scholar] [CrossRef]
- Rieger, R.M.; Ruppert, E.E.; Rieger, G.E.; Schoepfer-Sterrer, C. On the fine structure of gastrotrichs, with description of Chordodasys antennatus sp. n. Zool. Scr. 1974, 3, 219–237. [Google Scholar] [CrossRef]
- Hummon, W.D. Gastrotricha of the North Atlantic Ocean: 1. Twenty four new and two redescribed species of Macrodasyida. Meiofauna Mar. 2008, 16, 117–174. [Google Scholar]
- Todaro, M.A.; Dal Zotto, M.; Bownes, S.J.; Perissinotto, R. Two new interesting species of Macrodasyida (Gastrotricha) from KwaZulu-Natal (South Africa). Proc. Biol. Soc. Wash. 2017, 130, 139–154. [Google Scholar] [CrossRef]
- Evans, W.A.; Hummon, W.D. A new genus and species of Gastrotricha from the Atlantic coast of Florida, U.S.A. Trans. Am. Microsc. Soc. 1991, 110, 321–327. [Google Scholar] [CrossRef]
- Clausen, C. Gastrotricha Macrodasyida from the Tromsø region, northern Norway. Sarsia 2000, 85, 357–384. [Google Scholar] [CrossRef]
- Luporini, P.; Magagnini, G.; Tongiorgi, P. Contribution a la connaissance des gastrotriches des cotes de Toscane. Cah. Biol. Mar. 1971, 12, 433–455. [Google Scholar]
- Claparède, E. Miscellaneous zoologiques. III. Type d’un nouveau genere de gastrotriches. Ann. Sci. Nat. Zool. 1867, 8, 16–23. [Google Scholar]
- Kieneke, A.; Rothe, B.H.; Schmidt-Rhaesa, A. Record and description of Anandrodasys agadasys (Gastrotricha: Redudasyidae) from Lee Stocking Island (Bahamas), with remarks on populations from different geographic areas. Meiofauna Mar. 2013, 20, 39–48. [Google Scholar]
- Kisielewski, J. Two new interesting genera of Gastrotricha (Macrodasyida and Chaetonotida) from the Brazilian freshwater psammon. Hydrobiologia 1987, 153, 23–30. [Google Scholar] [CrossRef]
- Ruttner-Kolisko, A. Rheomorpha neiswestnovae und Marinellina flagellata, zwei phylogeneticsh interessante Wurmtypen aus dem Susswasserpsammon. Österr. Zool. Z. 1955, 6, 55–69. [Google Scholar]
- Luporini, P.; Magagnini, G.; Tongiorgi, P. Chaetonotoid gastrotrichs of the Tuscan Coast. Boll. Zool. 1973, 40, 31–40. [Google Scholar] [CrossRef]
- Balsamo, M. Gastrotrichi. In Guide C.N.R. per IL Riconoscimento Delle Specie Animali Delle Acque Interne Italiane; Consiglio Nazionale delle Ricerche AQ/1/199; CNR (Centro Nazionale Ricerche): Roma, Italy, 1983; Volume 7, pp. 547–571. [Google Scholar]
- Cordero, E.H. Notes sur les Gastrotriches. Physis 1918, 4, 241–244. [Google Scholar]
- Sudzuki, M. The Gastrotricha of Japan which live in the capillary water of the interstitial system: II. Bull. Biogeogr. Soc. Japan 1971, 27, 37–41. [Google Scholar]
- Kisielewski, J. Inland-water Gastrotricha from Brazil. Ann. Zool. (Warsaw) 1991, 43, 1–168. [Google Scholar]
- Mock, H. Chaetonotoidea (Gastrotricha) from the North Sea Island of Sylt. Mikrofauna. Meeres. 1980, 78, 1–107. [Google Scholar]
- Schwank, P. Gastrotricha und Nemertini. In Süsswasserfauna von Mittleuropas; Brauer, A., Ed.; G. Fisher Verlag: Stuttgart, Germany, 1990; Volume 3, pp. 1–252. [Google Scholar]
- Schrom, H. Nordadriatische Gastrotrichen. Helgoländer Wiss. Meeresunters. 1972, 23, 286–351. [Google Scholar] [CrossRef]
- Hummon, W.D.; Balsamo, M.; Todaro, M.A. Italian marine Gastrotricha: I. Six new and one redescribed species of Chaetonotida. Boll. Zool. 1992, 59, 499–516. [Google Scholar] [CrossRef]
- Kånneby, T. A redescription of Chaetonotus (Primochaetus) veronicae Kånneby, 2013 (Gastrotricha: Chaetonotidae). Zootaxa 2015, 4027, 442–446. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todaro, M.A.; Sibaja-Cordero, J.A.; Segura-Bermúdez, O.A.; Coto-Delgado, G.; Goebel-Otárola, N.; Barquero, J.D.; Cullell-Delgado, M.; Dal Zotto, M. An Introduction to the Study of Gastrotricha, with a Taxonomic Key to Families and Genera of the Group. Diversity 2019, 11, 117. https://doi.org/10.3390/d11070117
Todaro MA, Sibaja-Cordero JA, Segura-Bermúdez OA, Coto-Delgado G, Goebel-Otárola N, Barquero JD, Cullell-Delgado M, Dal Zotto M. An Introduction to the Study of Gastrotricha, with a Taxonomic Key to Families and Genera of the Group. Diversity. 2019; 11(7):117. https://doi.org/10.3390/d11070117
Chicago/Turabian StyleTodaro, M. Antonio, Jeffrey Alejandro Sibaja-Cordero, Oscar A. Segura-Bermúdez, Génesis Coto-Delgado, Nathalie Goebel-Otárola, Juan D. Barquero, Mariana Cullell-Delgado, and Matteo Dal Zotto. 2019. "An Introduction to the Study of Gastrotricha, with a Taxonomic Key to Families and Genera of the Group" Diversity 11, no. 7: 117. https://doi.org/10.3390/d11070117
APA StyleTodaro, M. A., Sibaja-Cordero, J. A., Segura-Bermúdez, O. A., Coto-Delgado, G., Goebel-Otárola, N., Barquero, J. D., Cullell-Delgado, M., & Dal Zotto, M. (2019). An Introduction to the Study of Gastrotricha, with a Taxonomic Key to Families and Genera of the Group. Diversity, 11(7), 117. https://doi.org/10.3390/d11070117






