Assessing Ecological Risks from Atmospheric Deposition of Nitrogen and Sulfur to US Forests Using Epiphytic Macrolichens
Abstract
1. Introduction
1.1. Air Pollution As A Concern of Natural Resource Managers
1.2. Air Pollution As A Human Health Concern
1.3. Critical Loads: Thresholds of Harm from Atmospheric Deposition
1.4. Lichen Critical Loads Provide Broad Environmental Protection
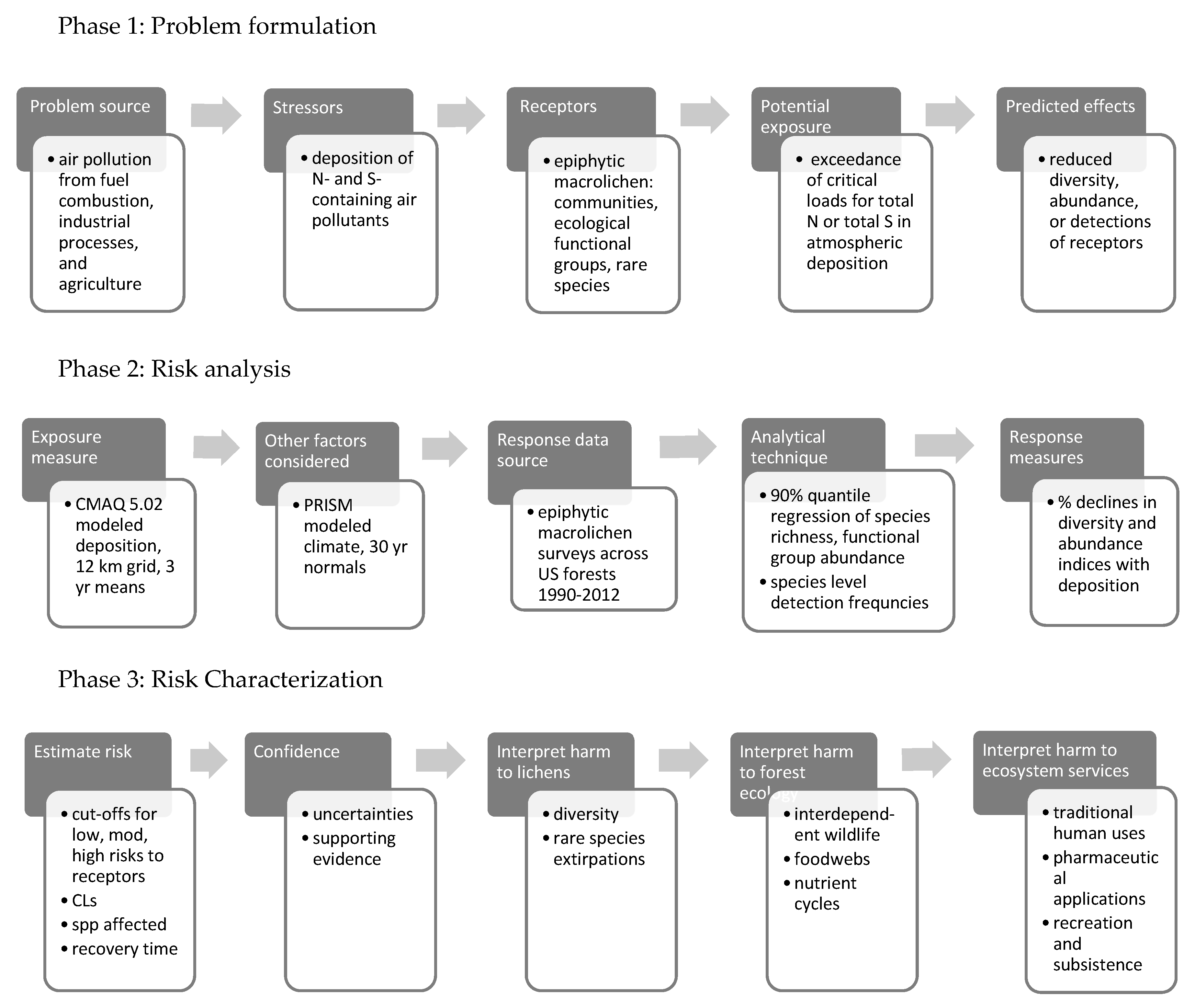
1.5. Assessing Ecological Risks from Exceedance of Lichen Critical Loads
1.5.1. Phase 1: Problem Formulation
- Conserve and promote biodiversity.
- Sustain or enhance ecosystem integrity, productivity, and services.
- Prevent extirpations of rare and conservation concern species.
1.5.2. Phase 2: Risk Analysis
- Total species richness (α-diversity) and sensitive species richness. Species counts are a direct measure of biodiversity within a site.
- Detection frequency of individual species of conservation concern—building on the data and species level sensitivity ratings from our previous study [19].
1.5.3. Phase 3: Risk Characterization
2. Materials and Methods
2.1. Lichen Data
2.1.1. Lichen Surveys
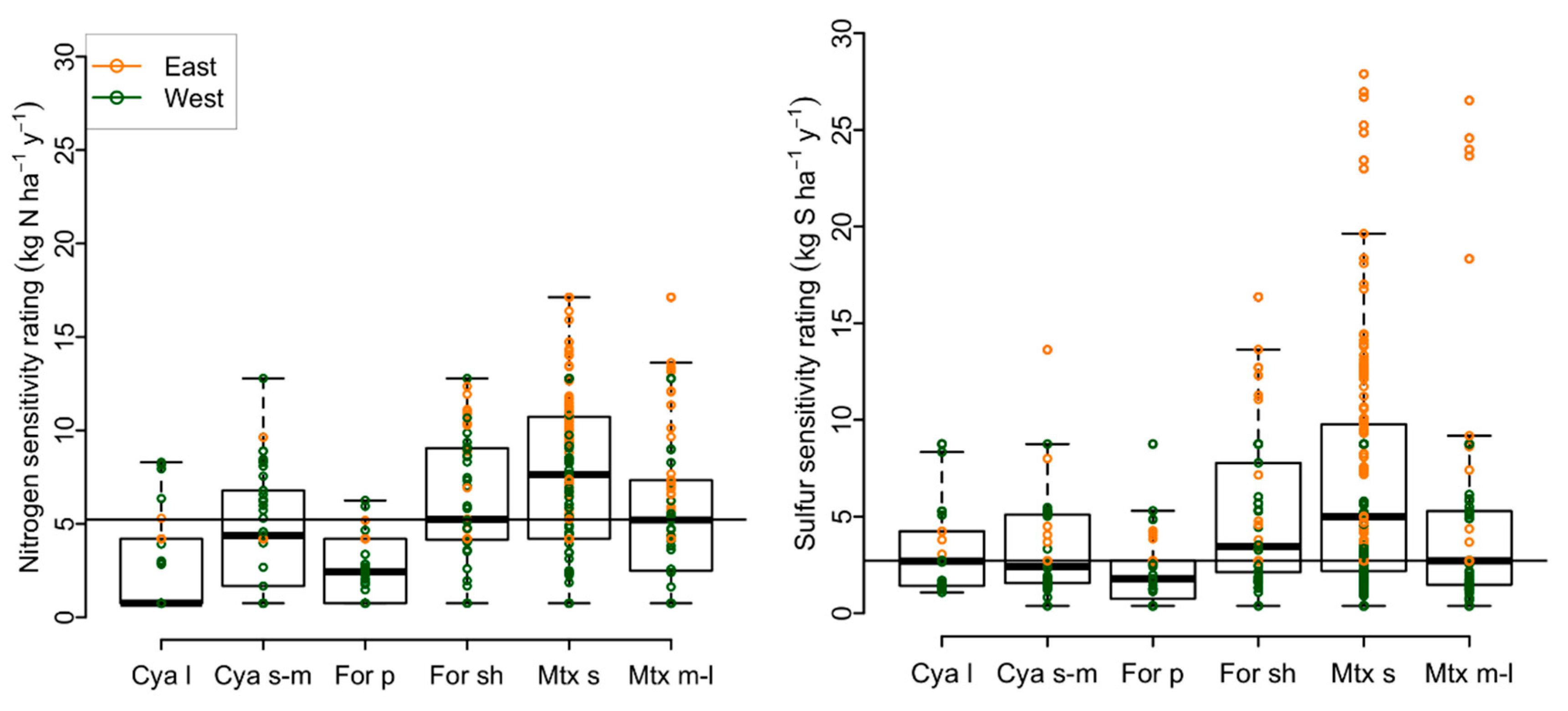
2.1.2. Air Pollution Sensitivity Ratings
2.1.3. Assigning Species to Functional Groups
2.2. Environmental Data
2.2.1. Deposition Data
2.2.2. Climate Data
2.3. Calculating Lichen Metrics
2.3.1. Biological Diversity
2.3.2. Indices of Abundance for Forage, Matrix and Cyanolichen Functional Groups
2.3.3. Diversity of S- and N-Sensitive Lichens
2.4. Statistical Analyses
2.4.1. Comparing Air Pollution Sensitivities of Species among Functional Groups
2.4.2. Rationale for 90% Quantile Regression as a Modeling Approach
2.4.3. Modeling Response of Lichen Metrics
2.4.4. Assessing Model Reliability
2.5. Quantifying Critical Loads and Risk Classes
2.6. Assessing Extirpation Risk for Species of Conservation Concern
2.7. Mapping Lichen Metric Values
3. Results
3.1. Variability in Air Pollution Sensitivity among Functional Groups and Rare Species
3.2. Response of Lichen Metrics to Deposition
3.2.1. Species Richness
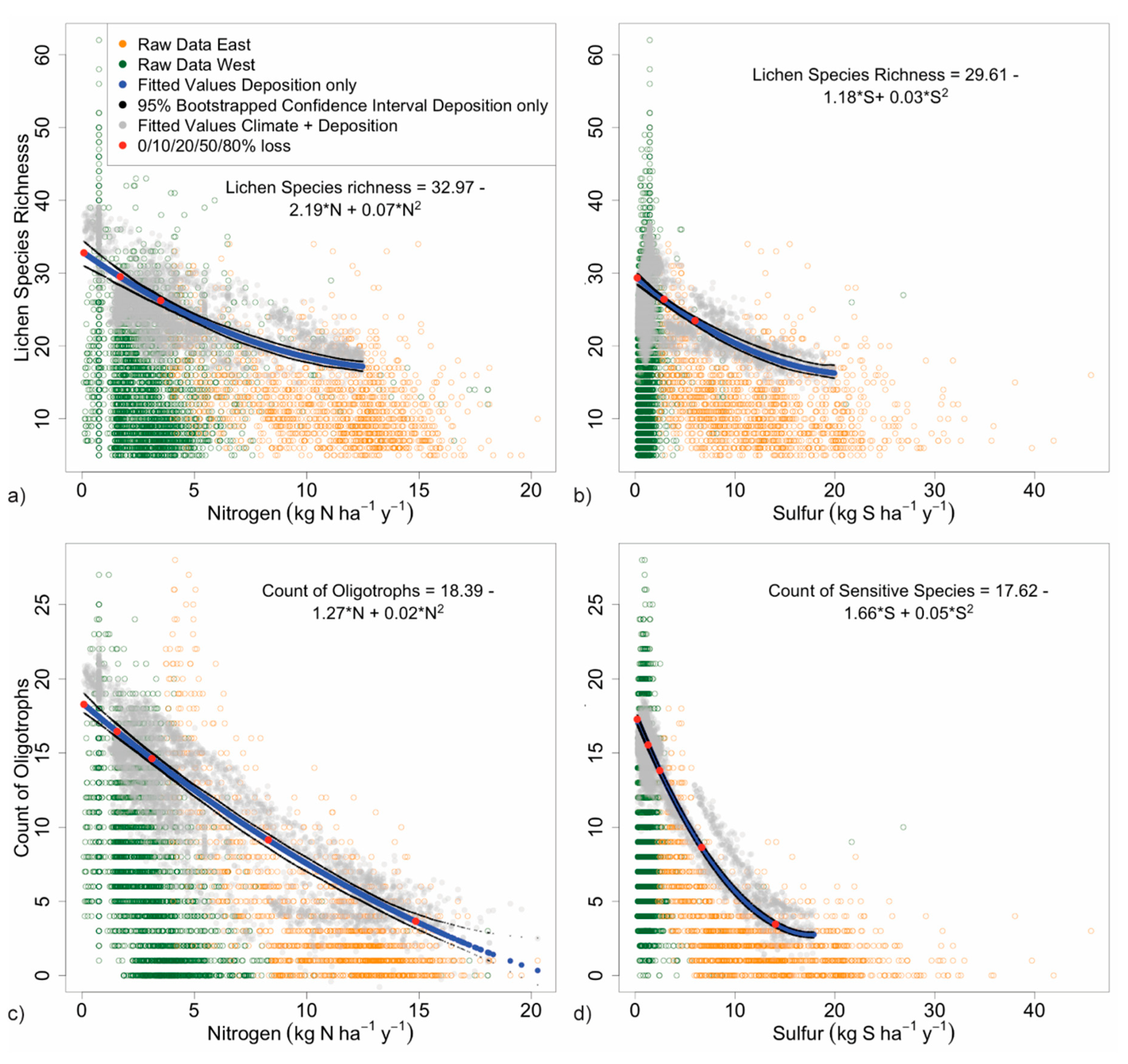
3.2.2. Forage Lichen Diversity and Abundance

3.2.3. Cyanolichen Diversity and Abundance
3.2.4. Matrix Lichen Diversity and Abundance
3.3. Response of Rare Species to Deposition
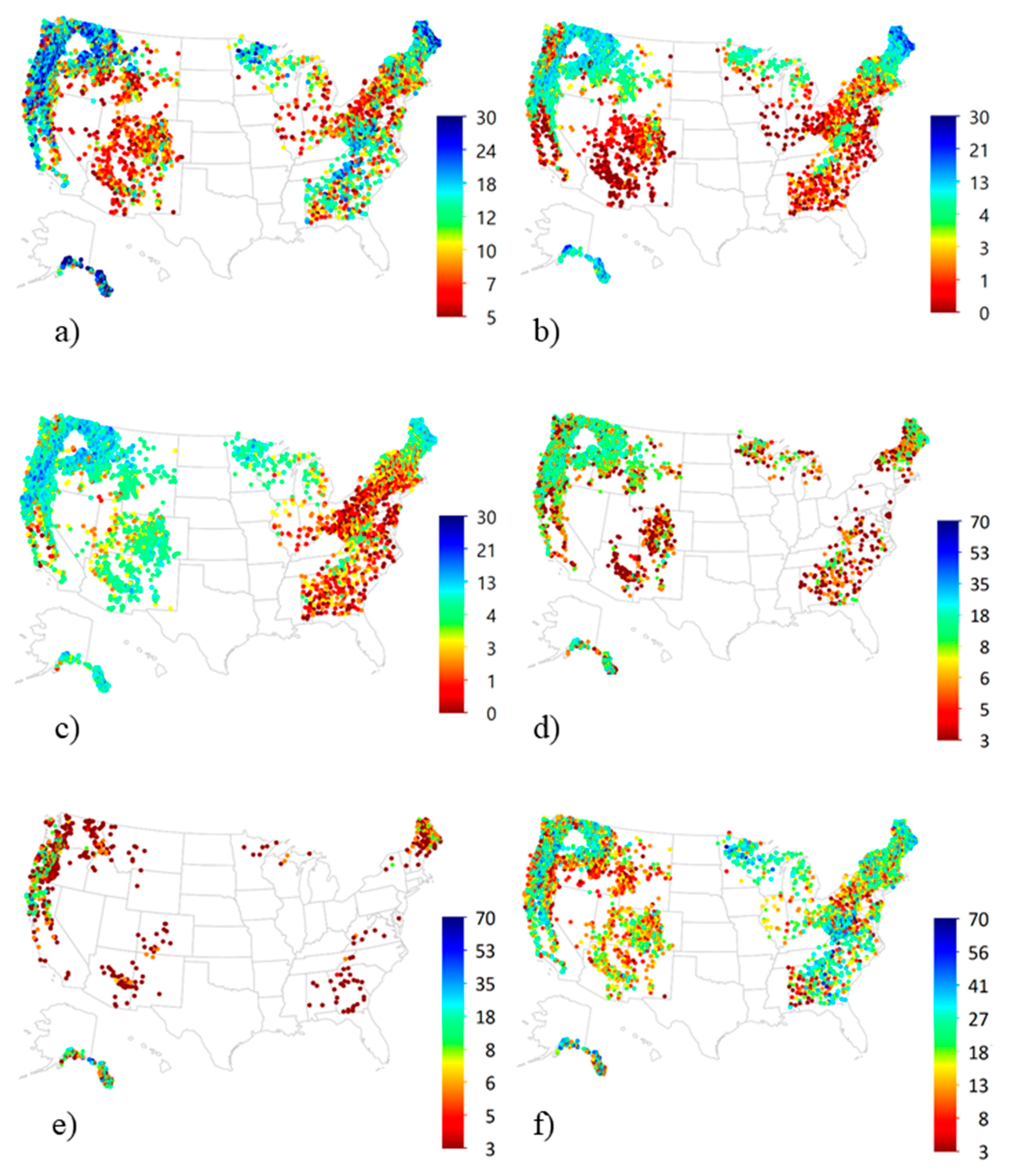
3.4. National Patterns of Lichen Diversity and Abundance
4. Discussion
4.1. Summary of Results
4.2. Estimating Risk
4.2.1. Selecting Critical Loads
4.2.2. Comparison to Other Critical Loads
4.2.3. Number of Species Affected
4.2.4. Response Time Frames
4.2.5. Assessing Uncertainties
4.3. Characterizing Risks to Lichen Biodiversity from Air Pollution
4.3.1. Total Species Richness at Community and Landscape Levels
4.3.2. Sensitive Species and Functional Groups
4.3.3. Rare Species
4.4. Characterizing Risks to Ecological Function and Integrity from Air Pollution
4.4.1. Forage Lichens
4.4.2. Cyanolichens
4.4.3. Matrix Lichens
4.5. Characterizing Risks to Ecosystem Services from Air Pollution
4.5.1. Food, Fiber, Hunting, Recreation
4.5.2. Pharmaceutical and Traditional Uses of Lichens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Economic Council Europe. Convention on Long Range Trans-boundary Pollution. Available online: https://www.unece.org/env/lrtap/welcome.html.html (accessed on 17 March 2019).
- Environment Assembly of the United Nations Environment Programme. Preventing and reducing air pollution to improve air quality globally. UNEP/EA.3/Res.8; United Nations: Nairobi, Kenya, 2018; pp. 1–4. [Google Scholar]
- Clark, C.M.; Bell, M.D.; Boyd, J.W.; Compton, J.E.; Davidson, E.A.; Davis, C.; Fenn, M.E.; Geiser, L.; Jones, L.; Blett, T.F. Nitrogen-induced terrestrial eutrophication: Cascading effects and impacts on ecosystem services. Ecosphere 2017, 8, e01877. [Google Scholar] [CrossRef]
- Blett, T.F.; Bell, M.D.; Clark, C.M.; Bingham, D.; Phelan, J.; Nahlik, A.; Landers, D.; Davis, C.; Irvine, I.; Heard, A. Air Quality and Ecosystem Services Workshop Report: Santa Monica Mountains National Recreation Area, Thousand Oaks, CA – February 24–26, 2015, Natural Resource Report NPS/NRSS/ARD/NRR--2016/1107; U.S. Department of the Interior, National Park Service: Fort Collins, CO, USA, 2016; p. 68.
- Paoletti, E.; Schaub, M.; Matyssek, R.; Wieser, G.; Augustaitis, A.; Bastrup-Birk, A.M.; Bytnerowicz, A.; Günthardt-Goerg, M.S.; Müller-Starck, G.; Serengil, Y. Advances of air pollution science: From forest decline to multiple-stress effects on forest ecosystem services. Environ. Pollut. 2010, 158, 1986–1989. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization-Europe. Review of evidence on health aspects of air pollution – REVIHAAP Project: Final Technical Report; REVIHAAP Project; WHO European Centre for Environment and Health: Bonn, Germany, 2017; p. 302. [Google Scholar]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.; Adeyi, O.; Arnold, R.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; Breysse, P.N.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Air pollution and public health; emerging hazards and improved understanding of risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef]
- Nilsson, J.; Grennfelt, P. Critical Loads for Sulphur and Nitrogen. Report from a workshop held at Skokloster, Sweden, 19–24 March 1988; Nordic Council of Ministers: Copenhagen, Denmark, 1988; p. 418. [Google Scholar]
- Porter, E.; Blett, T.; Potter, D.U.; Huber, C. Protecting resources on federal lands: Implications of critical loads for atmospheric deposition of nitrogen and sulfur. BioScience 2005, 55, 603–612. [Google Scholar] [CrossRef]
- Ferry, B.W.; Baddeley, M.S.; Hawksworth, D.L. Air Pollution and Lichens; Athlone Press, University of London: London, UK, 1973; p. 389. [Google Scholar]
- Conti, M.E.; Cecchetti, G. Biological monitoring: Lichens as bio-indicators of air pollution assessment — a review. Environ. Pollut. 2001, 114, 471–492. [Google Scholar] [CrossRef]
- Ellis, R.A.; Jacob, D.J.; Sulprizio, M.P.; Zhang, L.; Holmes, C.D.; Schichtel, B.A.; Blett, T.; Porter, E.; Pardo, L.H.; Lynch, J.A. Present and future nitrogen deposition to national parks in the United States: Critical load exceedances. Atmos. Chem. Phys. 2013, 13, 9083–9095. [Google Scholar] [CrossRef]
- Pinho, P.; Augusto, S.; Branquinho, C.; Bio, A.; Pereira, M.J.; Soares, A.; Catarino, F. Mapping lichen diversity as a first step for air quality assessment. J. Atmos. Chem. 2004, 49, 377–389. [Google Scholar] [CrossRef]
- Pardo, L.H.; Fenn, M.E.; Goodale, C.L.; Geiser, L.H.; Driscoll, C.T.; Allen, E.B.; Baron, J.S.; Bobbink, R.; Bowman, W.D.; Clark, C.M.; et al. Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecol. Appl. 2011, 21, 3049–3082. [Google Scholar] [CrossRef]
- Bobbink, R.; Braun, S.; Nordin, A.; Power, S.; Schütz, K.; Strengborn, J.; Weijters, M.; Tomassen, H. Review and Revision of Empirical Critical Loads and Dose-response Relationships: Proceedings of an expert workshop, Noordwijkerhout, 23–25 June 2010; Coordination Centre for Effects, National Institute for Health and Environment (RIVM): Bilthoven, The Netherlands, 2011; p. 246. [Google Scholar]
- Stevens, C.J.; Smart, S.M.; Henrys, P.A.; Maskell, L.C.; Crowe, A.; Simkin, J.; Cheffings, C.M.; Whitfield, C.; Gowing, D.J.G.; Rowe, E.C.; et al. Terricolous lichens as indicators of nitrogen deposition: Evidence from national records. Ecol. Indic. 2012, 20, 196–203. [Google Scholar] [CrossRef]
- Geiser, L.H.; Root, H.L.; Jovan, S.E.; St. Clair, L.L.; Dillman, K.L.; Schwede, D.B. Lichen community responses to atmospheric deposition and climate indicate protective air pollution thresholds for US forests. Environ. Pollut. (In preparation).
- Sparrius, L.B. Response of epiphytic lichen communities to decreasing ammonia air concentrations in a moderately polluted area of The Netherlands. Environ. Pollut. 2007, 146, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Wolseley, P.A.; James, P.W.; Theobald, M.R.; Sutton, M.A. Detecting changes in epiphytic lichen communities at sites affected by atmospheric ammonia from agricultural sources. Lichenologist 2006, 38, 161–176. [Google Scholar] [CrossRef]
- Geiser, L.H.; Jovan, S.E.; Glavich, D.A.; Porter, M.K. Lichen-based critical loads for atmospheric nitrogen deposition in western Oregon and Washington forests, USA. Environ. Pollut. 2010, 158, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency, Office of Research & Development. Ecological Risk Assessment. Available online: https://www.epa.gov/risk/ecological-risk-assessment (accessed on 17 March 2019).
- Fowler, J.R., III; Dearfield, K.L. Risk Characterization Handbook. EPA 100-B-00-002 2000, 189.
- CLAD. National Atmospheric Deposition Program (NADP) Critical Loads of Atmospheric Deposition (CLAD) 2016-2017 Annual Report; NADP CLAD Science Committee: Madison, WI, USA, 2018; p. 31. [Google Scholar]
- U.S. Department of Agriculture, Forest Service National Forest System, Land Management Planning Directives. Fed. Regist. 2015, 80 FR 6683, 6683–6687.
- U.S. Bureau of Land Management. Who We Are, What We Do: Our Mission. Available online: https://www.blm.gov/about/our-mission (accessed on 18 March 2019).
- U.S. National Park Service. About us. Available online: https://www.nps.gov/aboutus/index.htm (accessed on 17 March 2019).
- U.S. Fish and Wildlife Service. About the US Fish and Wildlife Service: Mission. Available online: https://www.fws.gov/help/about_us.html (accessed on 17 March 2019).
- U.S. Department of Agriculture, Forest Service, Forest Inventory and Analysis National Program. FIA Data Mart; Lichen Critical Loads. Available online: https://www.fia.fs.fed.us/tools-data/other_data/index.php (accessed on 17 March 2019).
- Giordani, P.; Incerti, G. The influence of climate on the distribution of lichens: A case study in a borderline area (Liguria, NW Italy). Plant Ecol. 2008, 195, 257–272. [Google Scholar] [CrossRef]
- Jovan, S. Lichen bioindication of biodiversity, air quality, and climate: Baseline results from monitoring in Washington, Oregon, and California, Gen. Tech. Rep. PNW-GTR-737; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2008; p. 115. [Google Scholar]
- Will-Wolf, S.; Jovan, S.; Neitlich, P.; Peck, J.E.; Rosentreter, R. Lichen-based indices to quantify responses to climate and air pollution across northeastern U.S.A. Bryologist 2015, 118, 59–82. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Forest Service Section 21. Lichen Communities. In Field Methods for Forest Health (Phase 3) Measurements; Forest Inventory and Analysis National Program: Washington, DC, USA, 2011; p. 19. [Google Scholar]
- Esslinger, T.L. A cumulative checklist for the lichen-forming, lichenicolous, and allied fungi of the continental United States and Canada. Most Recent Version (#19). North Dakota State University, Fargo, North Dakota. Available online: http://www.ndsu.edu/pubweb/~esslinge/chcklst/chcklst7.htm (accessed on 18 March 2019).
- Berryman, S.; McCune, B. Estimating epiphytic macrolichen biomass from topography, stand structure and lichen community data. J. Veg. Sci. 2006, 17, 157–170. [Google Scholar] [CrossRef]
- McCune, B. Gradients in epiphyte biomass in three Pseudotsuga-Tsuga forests of different ages in western Oregon and Washington. Bryologist 1993, 96, 405–411. [Google Scholar] [CrossRef]
- Ellis, C.J.; Coppins, B.J. Contrasting functional traits maintain lichen epiphyte diversity in response to climate and autogenic succession. J. Biogeogr. 2006, 33, 1643–1656. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Bacaro, G.; Nascimbene, J. Functional traits of epiphytic lichens as potential indicators of environmental conditions in forest ecosystems. Ecol. Indic. 2012, 18, 413–420. [Google Scholar] [CrossRef]
- Nelson, P.R.; McCune, B.; Roland, C.; Stehn, S. Non-parametric methods reveal non-linear functional trait variation of lichens along environmental and fire age gradients. J. Veg. Sci. 2015, 26, 848–865. [Google Scholar] [CrossRef]
- Matos, P.; Geiser, L.; Hardman, A.; Glavich, D.; Pinho, P.; Nunes, A.; Soares, A.M.V.M.; Branquinho, C. Tracking global change using lichen diversity: Towards a global-scale ecological indicator. Methods Ecol. Evol. 2017, 8, 788–798. [Google Scholar] [CrossRef]
- Schwede, D.B.; Lear, G.G. A novel hybrid approach for estimating total deposition in the United States. Atmos. Environ. 2014, 92, 207–220. [Google Scholar] [CrossRef]
- Root, H.T.; Geiser, L.H.; Fenn, M.E.; Jovan, S.E.; Hutten, M.A.; Ahuja, S.; Dillman, K.L.; Berryman, S.; McMurray, J.A. A simple tool for estimating through-fall nitrogen deposition in forests of western North America using lichens. For. Ecol. Manag. 2013, 306, 1–8. [Google Scholar] [CrossRef]
- Appel, K.W.; Foley, K.M.; Bash, J.O.; Pinder, R.W.; Dennis, R.L.; Allen, D.J.; Pickering, K. A multi-resolution assessment of the Community Multiscale Air Quality (CMAQ) model v4.7 wet deposition estimates for 2002–2006. Geosci. Model Dev. 2011, 4, 357–371. [Google Scholar] [CrossRef]
- Bash, J.O.; Cooter, E.J.; Dennis, R.L.; Walker, J.T.; Pleim, J.E. Evaluation of a regional air-quality model with bidirectional NH3 exchange coupled to an agroecosystem model. Biogeosciences 2013, 10, 1635–1645. [Google Scholar] [CrossRef]
- Zhang, Y.; Mathur, R.; Bash, J.O.; Hogrefe, C.; Xing, J.; Roselle, S.J. Long-term trends in total inorganic nitrogen and sulfur deposition in the U.S. from 1990 to 2010. Atmos. Chem. Phys. 2018, 18, 9091–9106. [Google Scholar] [CrossRef]
- Walker, J.T.; Bell, M.D.; Schwede, D.B.; Cole, A.; Beachley, G.; Lear, G.; Wu, Z. Assessing uncertainty in total Nr deposition estimates for North American critical load applications. Sci. Total Environ. (In preparation).
- PRISM Climate Group, Oregon State University. PRISM Climate Data. Available online: http://prism.oregonstate.edu (accessed on 4 December 2012).
- Daly, C.; Taylor, G.H.; Gibson, W.P.; Parzybok, T.W.; Johnson, G.L.; Pasteris, P. High-quality spatial climate datasets for the United States and beyond. Trans. Am. Soc. Agric. Eng. 2000, 43, 1957–1962. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.; Carroll, C. Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLOS ONE 2016, 11, e0156720. [Google Scholar] [CrossRef]
- Daly, C.; Smith, J.I.; Olson, K.V. Mapping atmospheric moisture climatologies across the conterminous United States. PLoS ONE 2015, 10, e0141140. [Google Scholar] [CrossRef] [PubMed]
- McCune, B.; Dey, J.P.; Peck, J.E.; Cassell, D.; Heiman, K.; Will-Wolf, S.; Neitlich, P.N. Repeatability of community data: Species richness versus gradient scores in large-scale lichen studies. Bryologist 1997, 100, 40–46. [Google Scholar] [CrossRef]
- Will-Wolf, S. Analyzing Lichen Indicator Data, Gen. Tech. Rept. PNW-GTR-818; Forest Inventory and Analysis Program, Pacific Northwest Research Station, U.S. Forest Service: Portland, OR, USA, 2010; pp. 1–62. [Google Scholar]
- Patterson, P.L.; Will-Wolf, S.; Trest, M.T. Section 4: Lichen Indicator. In FIA National Assessment of Data Quality for Forest Health Indicators; Westfall, J.A., Ed.; General Technical Report NRS-53; U.S. Forest Service, Northern Research Station: Newtown Square, PA, USA, 2009; pp. 40–43. [Google Scholar]
- Neitlich, P.N. Lichen Abundance and Biodiversity Along a Chronosequence from Young Managed Stands to Ancient Forest. Master of Science Thesis, Department of Botany, University of Vermont, Burlington, VT, USA, 1993; p. 90. [Google Scholar]
- Pettersson, R.B.; Ball, J.P.; Renhorn, K.-E.; Esseen, P.-A.; Sjöberg, K. Invertebrate communities in boreal forest canopies as influenced by forestry and lichens with implications for passerine birds. Biol. Conserv. 1995, 74, 57–63. [Google Scholar] [CrossRef]
- Wittmer, H.U.; McLellan, B.N.; Serrouya, R.; Apps, C.D. Changes in landscape composition influence the decline of a threatened woodland caribou population. J. Anim. Ecol. 2007, 76, 568–579. [Google Scholar] [CrossRef] [PubMed]
- SAS. JMP© Statistical Discovery software, version 11.2.0. Available online: http://www.jmp.com. (accessed on 17 March 2019).
- Brown, M.B.; Forsythe, A.B. Robust tests for the equality of variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Cade, B.S.; Noon, B.R. A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ. 2003, 1, 412–420. [Google Scholar] [CrossRef]
- Koenker, R. quantreg: Quantile Regression, R package version 5.38. Available online: https://CRAN.R-project.org/package=quantreg (accessed on 18 March 2019).
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 17 March 2019).
- Koenker, R.; Machado, J.A.F. Goodness of fit and related inference processes for quantile regression. J. Am. Stat. Assoc. 1999, 94, 1296–1310. [Google Scholar] [CrossRef]
- Koenker, R. Confidence intervals for regression quantiles. In Contributions to Statistics: Asymptotic Statistics; Mandl, P., Hušková, M., Eds.; Springer: Heidelberg, Germany, 1994; pp. 349–359. ISBN 978-3-642-57984-4. [Google Scholar]
- Omernik, J.M. Ecoregions of the conterminous United States. Ann. Assoc. Am. Geogr. 1987, 77, 118–125. [Google Scholar] [CrossRef]
- Giordani, P.; Calatayud, V.; Stofer, S.; Seidling, W.; Granke, O.; Fischer, R. Detecting the nitrogen critical loads on European forests by means of epiphytic lichens. A signal-to-noise evaluation. For. Ecol. Manag. 2014, 311, 29–40. [Google Scholar] [CrossRef]
- Gries, C.; Sanz, M.J.; Nash, T.H.I. Effect of SO2 fumigation on CO2 gas exchange, chlorophyll fluorescence and chlorophyll degradation in different lichen species from western North America. Cryptogam. Bot. 1995, 5, 239–246. [Google Scholar]
- Farmer, A.M.; Bates, J.W.; Nigel, J.; Bell, N.B. Ecophysiological effects of acid rain on bryophytes and lichens. In Bryophytes and Lichens in a Changing Environment; Bates, J.W., Farmer, A.M., Eds.; Clarendon Press: Oxford, UK, 1992; pp. 284–313. [Google Scholar]
- Hawksworth, D.L.; Rose, F. Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature 1970, 227, 145–148. [Google Scholar] [CrossRef] [PubMed]
- McNulty, S.G.; Cohen, E.C.; Moore Myers, J.A.; Sullivan, T.J.; Li, H. Estimates of critical acid loads and exceedances for forest soils across the conterminous United States. Environ. Pollut. 2007, 149, 281–292. [Google Scholar] [CrossRef]
- Lynch, J.A.; Phelan, J.; Pardo, L.H.; McDonnell, T.C.; Clark, C.M. Detailed Documentation of the National Critical Load Database (NCLD) for U.S. Critical Loads of Sulfur and Nitrogen, version 3.0; National Atmospheric Deposition Program, Illinois State Water Survey: Champaign, IL, USA, 2017; p. 93. [Google Scholar]
- Simkin, S.M.; Allen, E.B.; Bowman, W.D.; Clark, C.M.; Belnap, J.; Brooks, M.L.; Cade, B.S.; Collins, S.L.; Geiser, L.H.; Gilliam, F.S.; et al. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 4086–4091. [Google Scholar] [CrossRef]
- Clark, C.M.; Simkin, S.M.; Pardo, L.H.; Allen, E.B.; Bowman, W.D.; Belnap, J.; Brooks, M.L.; Collins, S.L.; Geiser, L.H.; Gilliam, F.S.; et al. Potential vulnerability of 348 herbaceous species to atmospheric deposition of nitrogen and sulfur in the U.S. Nat. Plants 2019, (in press).
- Horn, K.J.; Thomas, R.Q.; Clark, C.M.; Pardo, L.H.; Fenn, M.E.; Lawrence, G.B.; Perakis, S.S.; Smithwick, E.A.H.; Baldwin, D.; Braun, S.; et al. Growth and survival relationships of 71 tree species with nitrogen and sulfur deposition across the conterminous U.S. PLoS ONE 2018, 13, e0205296. [Google Scholar] [CrossRef]
- Will-Wolf, S.; Geiser, L.H.; Neitlich, P.N.; Reis, A.H. Forest lichen communities and environment—How consistent are relationships across scales? J. Veg. Sci. 2006, 17, 171–184. [Google Scholar]
- Phoenix, G.K.; Emmett, B.A.; Britton, A.J.; Caporn, S.J.M.; Dise, N.B.; Helliwell, R.; Jones, L.; Leake, J.R.; Leith, I.D.; Sheppard, L.J.; et al. Impacts of atmospheric nitrogen deposition: Responses of multiple plant and soil parameters across contrasting ecosystems in long-term field experiments. Glob. Change Biol. 2012, 18, 1197–1215. [Google Scholar] [CrossRef]
- Johansson, O.; Palmqvist, K.; Olofsson, J. Nitrogen deposition drives lichen community changes through differential species responses. Glob. Change Biol. 2012, 18, 2626–2635. [Google Scholar] [CrossRef]
- Johansson, O.; Olofsson, J.; Giesler, R.; Palmqvist, K. Lichen responses to nitrogen and phosphorus additions can be explained by the different symbiont responses. New Phytol. 2011, 191, 795–805. [Google Scholar] [CrossRef]
- Isocrono, D.; Matteucci, E.; Ferrarese, A.; Pensi, E.; Piervittori, R. Lichen colonization in the city of Turin (N Italy) based on current and historical data. Environ. Pollut. 2007, 145, 258–265. [Google Scholar] [CrossRef]
- Brodo, I.M. Lichen growth and cities: A study on Long Island, New York. Bryologist 1966, 69, 427–449. [Google Scholar] [CrossRef]
- McCune, B. Lichen communities along O3 and SO2 gradients in Indianapolis. Bryologist 1988, 91, 223–228. [Google Scholar] [CrossRef]
- Sigal, L.L.; Nash, T.H. Lichen communities on conifers in southern California mountains: An ecological survey relative to oxidant air pollution. Ecology 1983, 64, 1343–1354. [Google Scholar] [CrossRef]
- Showman, R.E. Continuing lichen recolonization in the upper Ohio River Valley. Bryologist 1997, 100, 478–481. [Google Scholar] [CrossRef]
- McClenahen, J.R.; Davis, D.D.; Hutnik, R.J. Macrolichens as bio-monitors of air-quality change in western Pennsylvania. Northeast. Nat. 2007, 14, 15–26. [Google Scholar] [CrossRef]
- McClenahen, J.R.; Showman, R.E.; Hutnik, R.J.; Davis, D.D. Temporal changes in lichen species richness and elemental composition on a Pennsylvania atmospheric deposition gradient. Evansia 2012, 29, 67–73. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; McManus, P.M. Lichen recolonization in London under conditions of rapidly falling sulphur dioxide levels, and the concept of zone skipping. Bot. J. Linn. Soc. 1989, 100, 99–109. [Google Scholar] [CrossRef]
- Cleavitt, N.L.; Hinds, J.W.; Poirot, R.L.; Geiser, L.H.; Dibble, A.C.; Leon, B.; Perron, R.; Pardo, L.H. Epiphytic macrolichen communities correspond to patterns of sulfur and nitrogen deposition in the northeastern United States. Bryologist 2015, 118, 304–324. [Google Scholar] [CrossRef]
- Forest Ecosystem Management Assessment Team. Forest Ecosystem Management: An Ecological, Economic, and Social Assessment; Appendix Table IV-A-2; U.S. Department of Agriculture, Forest Service; U.S. Department of Commerce, National Oceanic and Atmospheric Administration and National Marine Fisheries Service; U.S. Department of the Interior, Bureau of Land Management, Fish & Wildlife Service, and National Park Service; U.S. Environmental Protection Agency: Portland, OR, USA, 1993.
- U.S. Environmental Protection Agency. Air Pollutant Emissions Trends Data: Average annual emissions criteria pollutants: National Tier 1 for 1970–2017. Available online: https://www.epa.gov/air-emissions-inventories/air-pollutant-emissions-trends-data (accessed on 17 March 2019).
- Lehmann, C.M.; Gay, D.A. Monitoring long-term trends of acidic wet deposition in US precipitation: Results from the National Atmospheric Deposition Program. Power Plant Chem. 2011, 13, 378. [Google Scholar]
- Li, Y.; Schichtel, B.A.; Walker, J.T.; Schwede, D.B.; Chen, X.; Lehmann, C.M.; Puchalski, M.A.; Gay, D.A.; Collett, J.L. Increasing importance of deposition of reduced nitrogen in the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 5874–5879. [Google Scholar] [CrossRef] [PubMed]
- Brunialti, G.; Frati, L.; Malegori, C.; Giordani, P.; Malaspina, P. Do different teams produce different results in long-term lichen biomonitoring? Diversity 2019, 11, 43. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.; Butchart, S.; et al. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; United Nations: Paris, France, 2019; pp. 1–39. [Google Scholar]
- Esslinger, T.L. A cumulative checklist for the lichen-forming, lichenicolous and allied fungi of the continental United States and Canada, Version 22. Opusc. Philolichenum 2018, 17, 6–268. [Google Scholar]
- U.S. Department of Agriculture, Forest Service. National Lichens & Air Quality Database and Clearinghouse. Available online: http://gis.nacse.org/lichenair/ (accessed on 17 March 2019).
- Feuerer, T.; Hawksworth, D.L. Biodiversity of lichens, including a world-wide analysis of checklist data based on Takhtajan’s floristic regions. Biodivers. Conserv. 2007, 16, 85–98. [Google Scholar] [CrossRef]
- Seaward, M.R.D. Contributions of lichens to ecosystems. In CRC Handbook of Lichenology; Galun, M., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume II, pp. 107–144. ISBN 978-0-8493-3582-2. [Google Scholar]
- U.S. Global Change Research Program Fourth National Climate Assessment: Volume II. Impacts, Risks, and Adaptation in the United States; Washington, DC, USA, 2018; p. 1515.
- Kanakidou, M.; Myriokefalitakis, S.; Daskalakis, N.; Fanourgakis, G.; Nenes, A.; Baker, A.R.; Tsigaridis, K.; Mihalopoulos, N. Past, present, and future atmospheric nitrogen deposition. J. Atmos. Sci. Boston 2016, 73, 2039–2047. [Google Scholar] [CrossRef]
- Hayes, J.P.; Cross, S.P.; Mcintire, P.W. Seasonal variation in mycophagy by the western red-backed vole, Clethrionomys californicus, in southwestern Oregon. Northwest Sci. 1986, 60, 250–257. [Google Scholar]
- Maser, C.; Trappe, J.M.; Nussbaum, R.A. Fungal-small mammal interrelationships with emphasis on Oregon coniferous forests. Ecology 1978, 59, 799–809. [Google Scholar] [CrossRef]
- Belik, Tom. Animal Diversity Web: Myodes rutilus (northern red-backed vole). Available online: https://animaldiversity.org/accounts/Myodes_rutilus/ (accessed on 17 March 2019).
- Maser, Z.; Maser, C.; Trappe, J.M. Food habits of the northern flying squirrel (Glaucomys sabrinus) in Oregon. Can. J. Zool. 1985, 63, 1084–1088. [Google Scholar] [CrossRef]
- Antoine, M.E. An ecophysiological approach to quantifying nitrogen fixation by Lobaria oregana. Bryologist 2004, 107, 82–87. [Google Scholar] [CrossRef]
- Pike, L.H. The importance of epiphytic lichens in mineral cycling. Bryologist 1978, 81, 247–257. [Google Scholar] [CrossRef]
- Richardson, D.H.S.; Cameron, R.P. Cyanolichens: Their response to pollution and possible management strategies for their conservation in northeastern North America. Northeast. Nat. 2004, 11, 1–22. [Google Scholar] [CrossRef]
- Bokhorst, S.; Asplund, J.; Kardol, P.; Wardle, D.A. Lichen physiological traits and growth forms affect communities of associated invertebrates. Ecology 2015, 96, 2394–2407. [Google Scholar] [CrossRef] [PubMed]
- Esseen, P.-A.; Olsson, T.; Coxson, D.; Gauslaa, Y. Morphology influences water storage in hair lichens from boreal forest canopies. Fungal Ecol. 2015, 18, 26–35. [Google Scholar] [CrossRef]
- Peterson, E.B.; McCune, B. The importance of hotspots for lichen diversity in forests of western Oregon. Bryologist 2003, 106, 246–256. [Google Scholar] [CrossRef]
- Sillett, S.C. Branch epiphyte assemblages in the forest interior and on the clear-cut edge of a 700-year old Douglas fir canopy in western Oregon. Bryologist 1995, 98, 301–312. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Karp, D.S.; Mendenhall, C.D.; Sandí, R.F.; Chaumont, N.; Ehrlich, P.R.; Hadly, E.A.; Daily, G.C. Forest bolsters bird abundance, pest control and coffee yield. Ecol. Lett. 2013, 16, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Małgorzata, P. Small mammals feeding on hypogeous fungi. Acta Univ. Lodz. Folia Biol. Oecologica 2014, 10. [Google Scholar]
- Rosenberger, R.S.; White, E.M.; Kline, J.D.; Cvitanovich, C. Recreation economic values for estimating outdoor recreation economic benefits from the National Forest System, Gen. Tech. Rep. PNW-GTR-957; U.S. Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2017; pp. 1–957. [Google Scholar]
- Crawford, S.D. Lichens Used in Traditional Medicine. In Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Ranković, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 27–80. ISBN 978-3-319-13374-4. [Google Scholar]
- Crawford, S. Ethnolichenology of Bryoria fremontii: Wisdom of elders, population ecology, and nutritional chemistry. Master of Science Thesis, Department of Biology, University of Victoria, Victoria, BC, Canada, 2007. [Google Scholar]
- Sharnoff, S.D. Lichens of North America Information: Bibliographical database of the human uses of lichens. Available online: http://www.sharnoffphotos.com/lichen_info/usetaxon.html (accessed on 17 March 2019).
- Turner, N.J. Economic importance of black tree lichen (Bryoria fremontii) to the Indians of western North America. Econ. Bot. 1977, 31, 461–470. [Google Scholar] [CrossRef]
- Ranković, B. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Springer International Publishing: Cham, Switzerland, 2015; p. 202. ISBN 978-3-319-13373-7. [Google Scholar]
- Shrestha, G.; St. Clair, L.L. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E. Metabolic diversity of lichen-forming ascomycetous fungi: Culturing, polyketide and shikimate metabolite production, and PKS genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef] [PubMed]

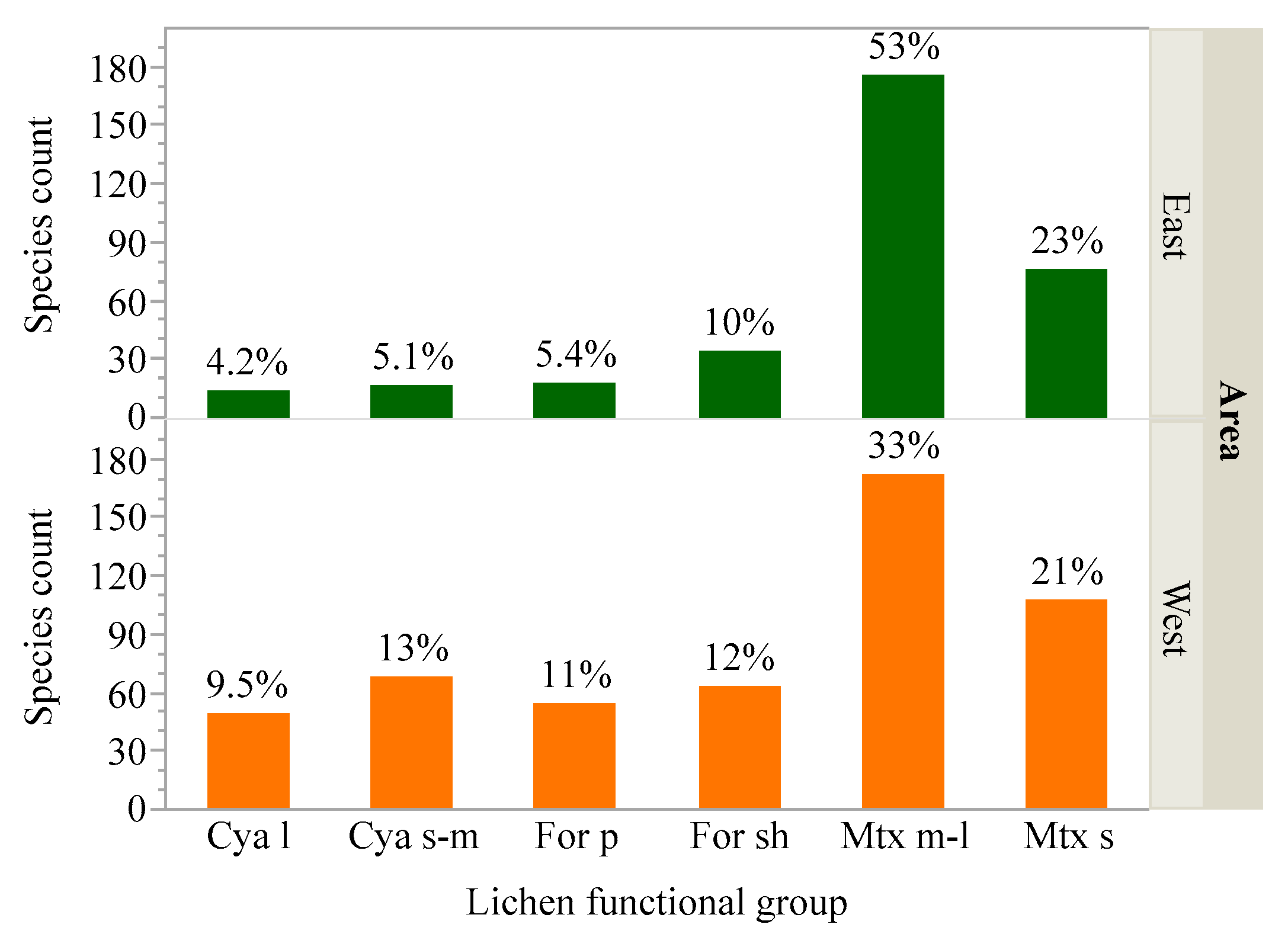
| Fxl Group | General Ecological Roles | Genera Assigned to This Functional Group 1 |
|---|---|---|
| Large cyano-lichens | Primary nitrogen-fixing epiphytes achieving high biomass in moist, temperate, old-growth forests, contributing significant amounts of new nitrogen to the forest floor. Nutrient-rich food for mollusks and other invertebrates; habitat and cover for invertebrates. | Anomolobaria, Dendriscosticta, Lobaria, Nephroma, Peltigera, Pseudocyphellaria, Sticta |
| Small to medium cyano-lichens | Nitrogen-fixing lichens, but typically low biomass, due to the small size and low abundance. Habitat and nutrient rich food for invertebrates. | Collema, Dendriscocaulon, Enchylium, Erioderma, Fuscopannaria, Lathagrium, Leioderma, Leptogium, Leptochidium, Pannaria, Scytinium, Vahliella |
| Pendant forage lichens | Critical winter forage for ungulates in areas with deep snow; primary winter forage for flying squirrels, voles, other rodents. Nesting materials for rodents and birds. Habitat and food for invertebrates. | Alectoria, Bryocaulon, Bryoria, Nodobryoria, pendant Ramalina and Usnea |
| Shrubby forage lichens | Winter forage for flying squirrels, voles, other rodents. Nesting materials for birds. Habitat and food for invertebrates. | Bunodophorun, Evernia, Letharia, Pseudevernia, Sphaerophorus, Teleoschistes, shrubby Ramalina and Usnea |
| Medium to large matrix lichens | Nesting materials for birds; habitat, cover and food for invertebrates. | Ahtiana, Canoparmelia, Cetrelia, Crespoa, Esslingeriana, Flavoparmelia, Flavopunctelia, Heterodermia, Hypogymnia, Hypotrachyna, Imshaugia, Melanelixia, Melanohalea, Menegazzia, Montanelia, Myelochroa, Niebla, Parmelia, Parmelina, Parmotrema, Physcia, Physconia, Platismatia, Punctelia, Teloschistes, Tuckermanella, Tuckermannopsis, Usnocetraria, Vulpicida |
| Small matrix lichens | Exposed habitat and food for invertebrates | Anaptychia, Bulbothrix, Candelaria, Cavernularia, Cladonia, Coccocarpia, Crespoa, Hyperphyscia, Kaernefeltia, Loxosporopsis, Parmeliella, Parmeliopsis, Phaeophyscia, Physciella, Placidium, Polycaulon, Polychidium, Pyxine, Rusavskia, Xanthomendoza, Xanthoria |
| Dep 1 | Clim | Dep + Clim 2 | (Dep + Clim) − Clim | ||||
|---|---|---|---|---|---|---|---|
| Lichen Metric | R1 | AIC | R1 | AIC | R1 | AIC | Δ AIC2 |
| Nitrogen Models | |||||||
| Species richness | 0.11 | 33265 | 0.09 | 33425 | 0.16 | 32807 | 619 |
| Oligotroph | 0.19 | 28672 | 0.11 | 29520 | 0.26 | 27887 | 1633 |
| Cyanolichens | 0.19 | 29465 | 0.21 | 29202 | 0.28 | 28419 | 783 |
| Forage lichens | 0.26 | 31982 | 0.14 | 33291 | 0.29 | 31626 | 1665 |
| Sulfur Models | |||||||
| Species richness | 0.07 | 40123 | 0.07 | 39997 | 0.13 | 39308 | 689 |
| Sensitive | 0.23 | 32588 | 0.02 | 35055 | 0.25 | 32340 | 2715 |
| Cyanolichens | 0.08 | 37265 | 0.16 | 36297 | 0.22 | 35560 | 737 |
| Forage lichens | 0.19 | 39312 | 0.11 | 39562 | 0.23 | 38026 | 1536 |
| Deposition Yielding % Decline in Count or Abundance 2 | Count or Abundance at % Decline from Maximum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lichen metric decline (%): | 0 % | 10 % | 20 % | 50 % | 80 % | 0 % | 10 % | 20 % | 50 % | 80 % |
| Nitrogen deposition | ||||||||||
| Species richness | 0.1 | 1.7 | 3.5 | -- | -- | 33 | 29 | 26 | -- | -- |
| Oligotroph richness | 0.1 | 1.5 | 3.1 | 8.3 | 14.8 | 18 | 16 | 15 | 9 | 4 |
| Forage lichen abundance | 0.1 | 1.0 | 1.9 | 5.3 | 10.4 | 30 | 27 | 24 | 15 | 6 |
| Cyanolichen abundance | 0.1 | 0.7 | 1.3 | 3.5 | 6.6 | 13 | 12 | 11 | 7 | 3 |
| Sulfur deposition | ||||||||||
| Species richness | 0.2 | 2.9 | 6.0 | -- | -- | 29 | 26 | 23 | -- | -- |
| S-sensitive species richness | 0.2 | 1.3 | 2.5 | 6.7 | 14.1 | 17 | 16 | 14 | 9 | 3 |
| Forage lichen abundance | 0.2 | 1.4 | 2.6 | 6.9 | 13 | 24 | 21 | 19 | 12 | 5 |
| Cyanolichen abundance | 0.2 | 1.2 | 2.3 | 5.9 | 11 | 10 | 9 | 8 | 5 | 2 |
| Lichen Metric | Percent Decline in Metric | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitrogen (kg ha−1 y−1) | 1 | 1.5 | 2 | 2.5 | 3 | 5 | 7.5 | 10 | 12.5 | 15 | 17.5 | 20 |
| Species richness | 6 | 9 | 12 | 15 | 17 | 27 | 37 | 44 | 48 | 49 | - | |
| Oligotroph richness | 6 | 10 | 13 | 16 | 19 | 32 | 46 | 59 | 70 | 81 | 90 | 97 |
| Forage lichen abundance 1 | 10 | 16 | 21 | 26 | 31 | 48 | 66 | 78 | 87 | 90 | - | - |
| Cyanolichen abundance 1 | 15 | 23 | 30 | 37 | 44 | 66 | 86 | 98 | 100 | - | - | - |
| Sulfur (kg ha−1 y−1) | 1 | 1.5 | 2 | 2.5 | 3 | 5 | 7.5 | 10 | 12.5 | 15 | 17.5 | 20 |
| Species richness | 3 | 5 | 7 | 9 | 10 | 17 | 24 | 31 | 36 | 40 | 43 | 45 |
| S-sensitive species richness | 7 | 12 | 16 | 20 | 24 | 39 | 55 | 67 | 76 | 82 | 84 | - |
| Forage lichen abundance 1 | 7 | 11 | 15 | 19 | 23 | 38 | 54 | 68 | 79 | 88 | 94 | 98 |
| Cyanolichen abundance 1 | 8 | 13 | 18 | 22 | 27 | 44 | 62 | 76 | 87 | 95 | 99 | 100 |
| Risk: | Low 0–20% | Hi 50–90% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mod 20–50% | V. hi >90% | ||||||||
| Rare Lichen | Poll | Sensi-tivity | Area | Fxl Gp | occ | 0 | −20% | −50% | −90% |
| Nephroma occultum | N | oligo | W | Cya l | 29 | 2.9 | 4 | 4.9 | 6 |
| Pannaria conoplea | N | oligo | E | Cya s-m | 14 | 4.2 | 5.7 | 7 | 8.8 |
| Collema subflaccidum | N | meso | W | Cya s-m | 9 | 4.6 | 5.8 | 6.5 | 12.3 |
| Ramalina sinensis | N | meso | E | For sh | 18 | 7.0 | 8.2 | 9.2 | 11 |
| Heterodermia leucomela | N | eut | W | Mtx m-l | 36 | 8.2 | 9.9 | 11 | 12.2 |
| Coccocarpia palmicola | N | eut | E | Mtx s | 13 | 9.9 | 11.6 | 14 | 17 |
| Scytinium cellulosum | S | sens | W | Cya s-m | 13 | 1.6 | 2.5 | 3.2 | 8.8 |
| Usnea longissima | S | sens | E | For p | 18 | 2.7 | 4.7 | 5.8 | 7.6 |
| Ramalina obtusata | S | intm | W | For sh | 9 | 5.3 | 6.8 | 7.7 | 8.7 |
| Pseudocyphellaria crocata | S | intm | E | Cya l | 15 | 4.2 | 5.4 | 6.5 | 8.2 |
| Cladonia cenotea | S | tol | W | Mtx s | 29 | 8.7 | |||
| Heterodermia granulifera | S | tol | E | Mtx m-l | 15 | 13.8 | 16 | 18.8 | 23.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiser, L.H.; Nelson, P.R.; Jovan, S.E.; Root, H.T.; Clark, C.M. Assessing Ecological Risks from Atmospheric Deposition of Nitrogen and Sulfur to US Forests Using Epiphytic Macrolichens. Diversity 2019, 11, 87. https://doi.org/10.3390/d11060087
Geiser LH, Nelson PR, Jovan SE, Root HT, Clark CM. Assessing Ecological Risks from Atmospheric Deposition of Nitrogen and Sulfur to US Forests Using Epiphytic Macrolichens. Diversity. 2019; 11(6):87. https://doi.org/10.3390/d11060087
Chicago/Turabian StyleGeiser, Linda H., Peter R. Nelson, Sarah E. Jovan, Heather T. Root, and Christopher M. Clark. 2019. "Assessing Ecological Risks from Atmospheric Deposition of Nitrogen and Sulfur to US Forests Using Epiphytic Macrolichens" Diversity 11, no. 6: 87. https://doi.org/10.3390/d11060087
APA StyleGeiser, L. H., Nelson, P. R., Jovan, S. E., Root, H. T., & Clark, C. M. (2019). Assessing Ecological Risks from Atmospheric Deposition of Nitrogen and Sulfur to US Forests Using Epiphytic Macrolichens. Diversity, 11(6), 87. https://doi.org/10.3390/d11060087




