Biogeographical Patterns of Endolithic Infestation in an Invasive and an Indigenous Intertidal Marine Ecosystem Engineer
Abstract
1. Introduction
2. Methodology
2.1. Prevalence of Endolithic Infestation
2.2. Identification of Endolithic Organisms
2.3. Lethal Effects of Endolithic Infestation
2.4. Data Analyses
3. Results
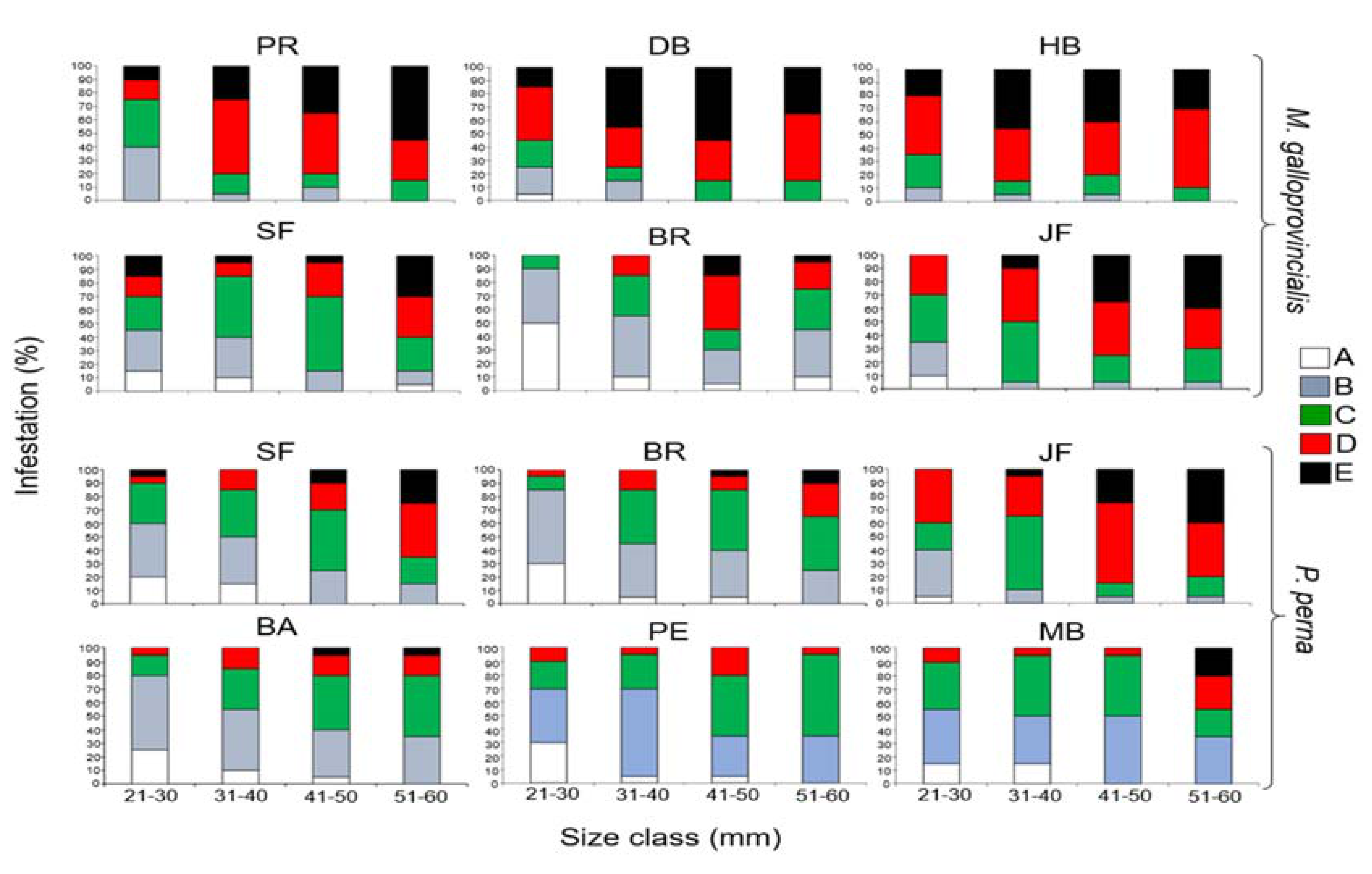
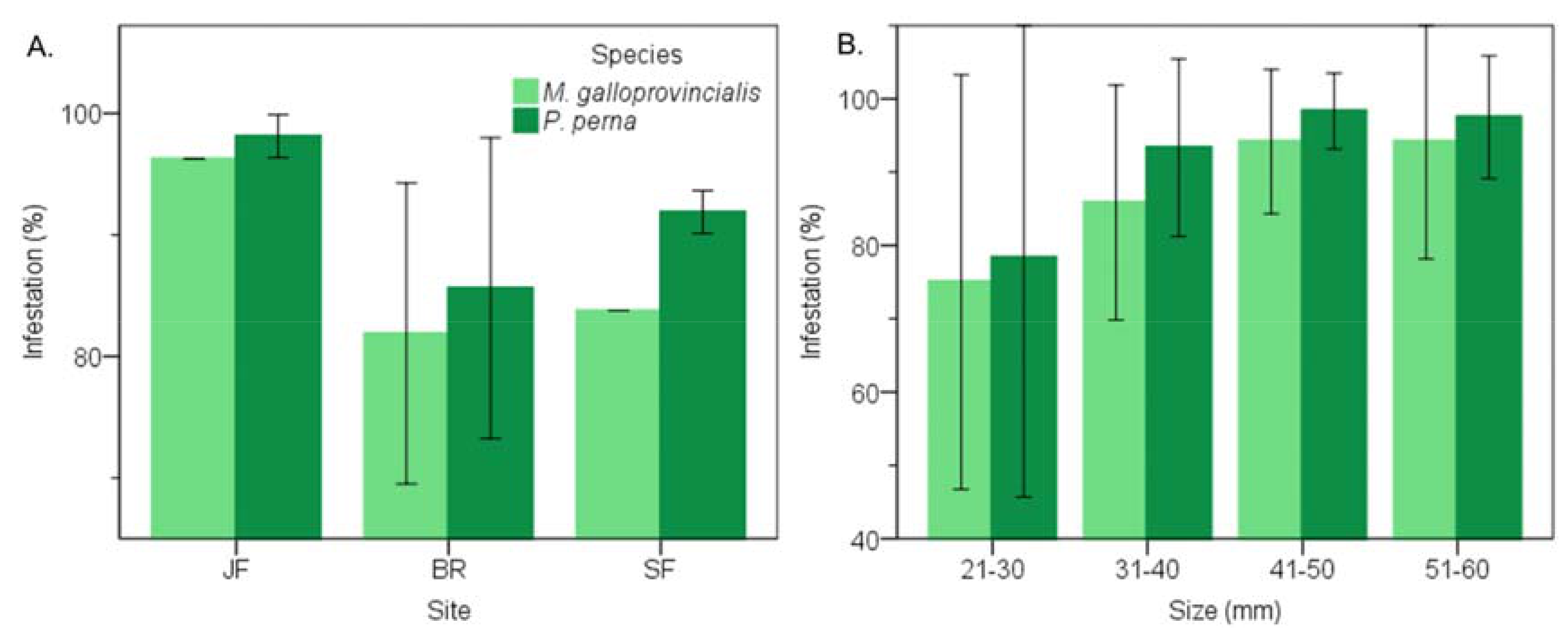
3.1. Prevalence of Infestation
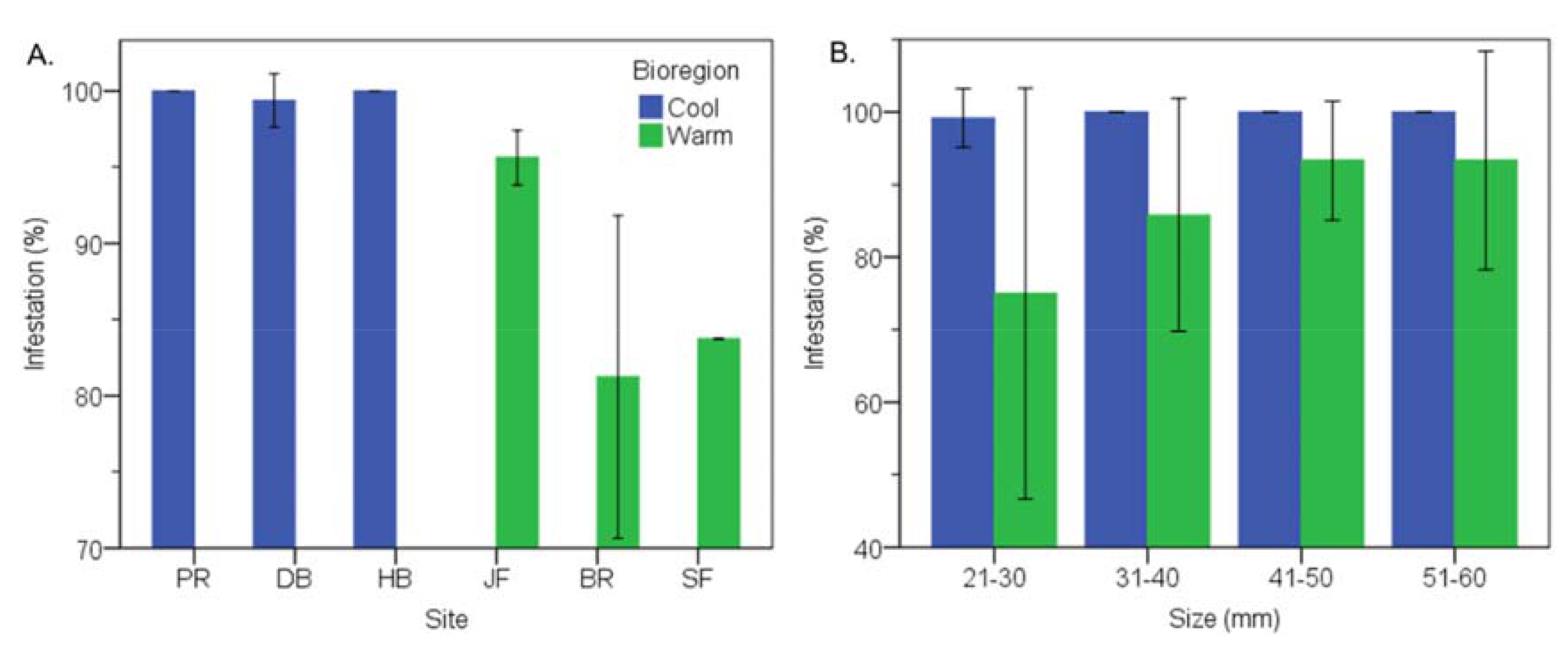
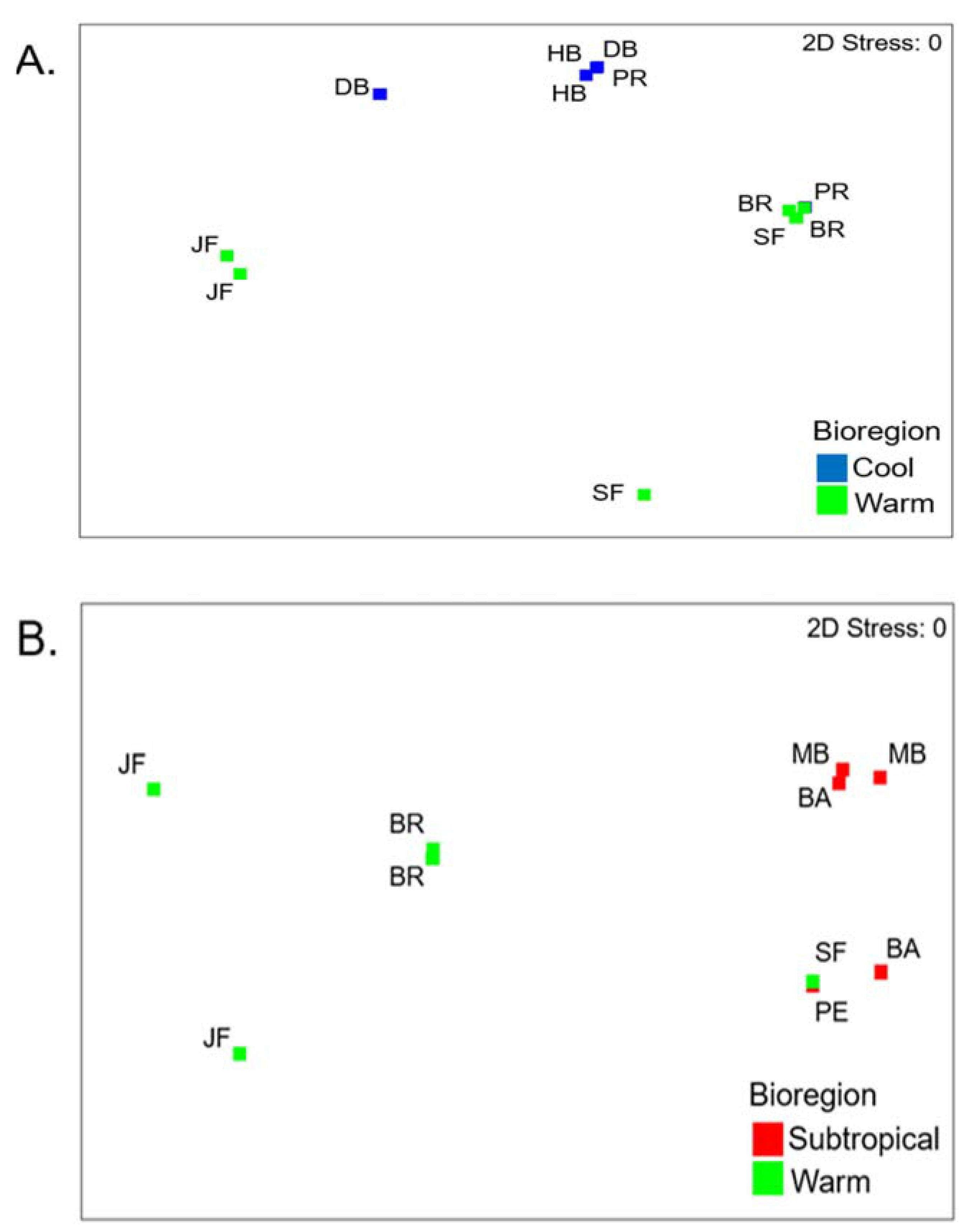
3.2. Cool Temperate vs Warm Temperate Bioregion―M. Galloprovincialis
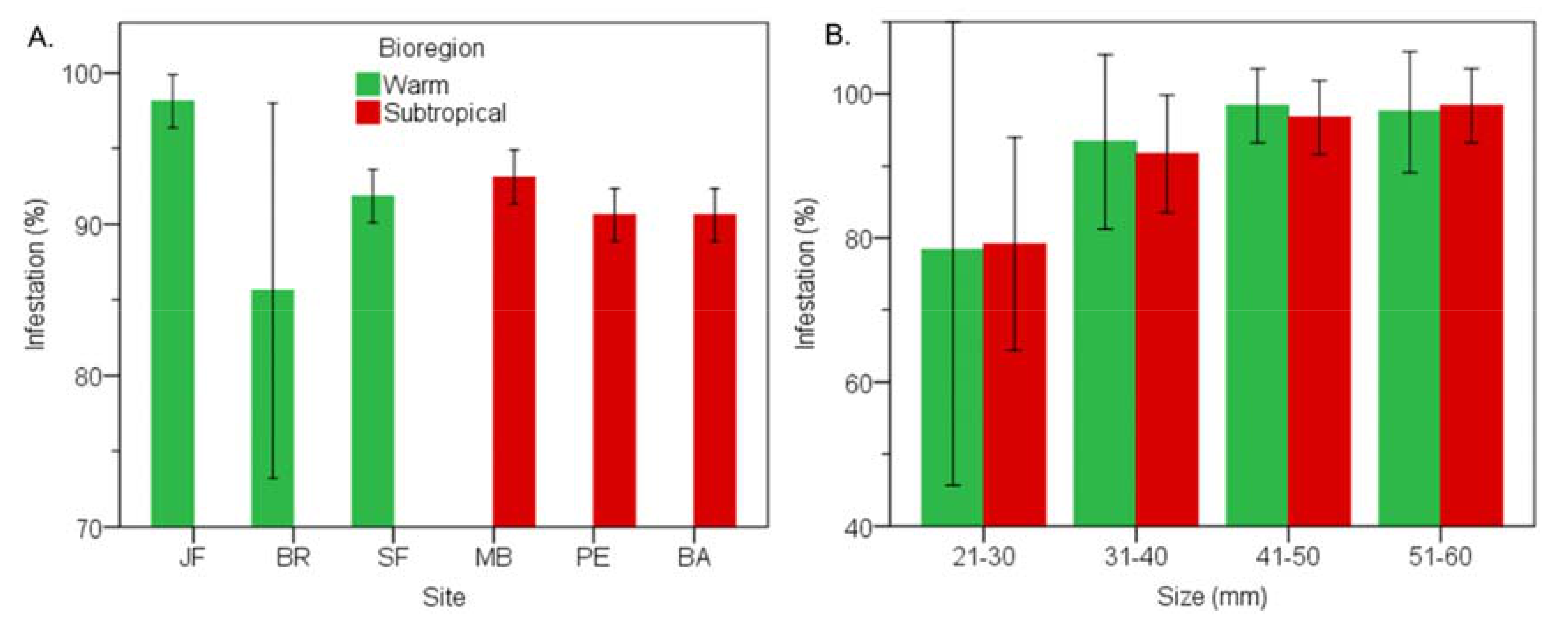
3.3. Subtropical vs Warm Temperate Bioregion - Perna Perna
3.4. Warm Temperate Bioregion―M. Galloprovincialis vs P. Perna
3.5. Cyanobacteria Species Composition
3.6. Cool Temperate vs Warm Temperate Bioregion―Mytilus Galloprovincialis
3.7. Subtropical vs Warm Temperate Bioregion―Perna Perna
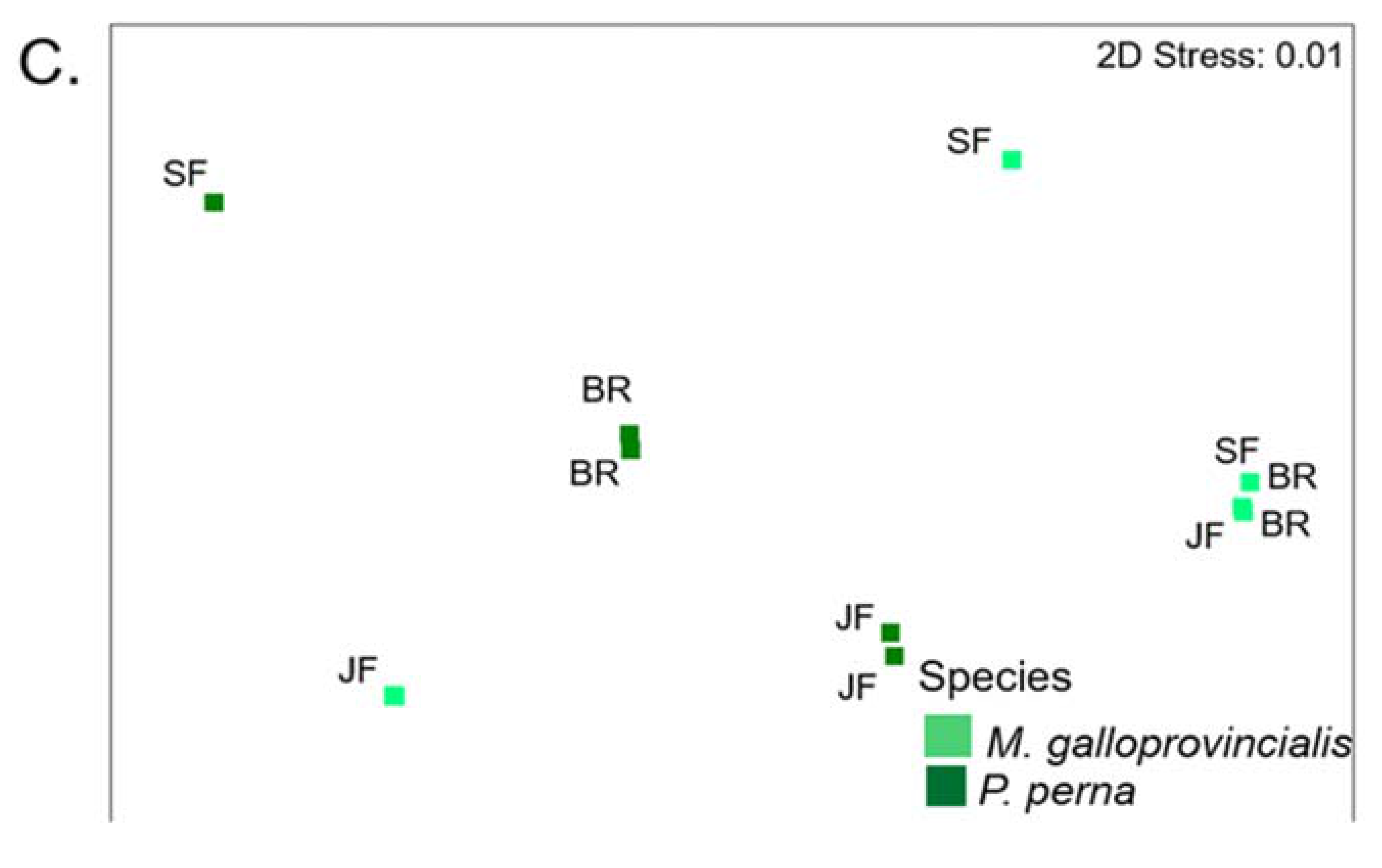
3.8. Warm Temperate Bioregion―Mytilus Galloprovincialis vs Perna Perna
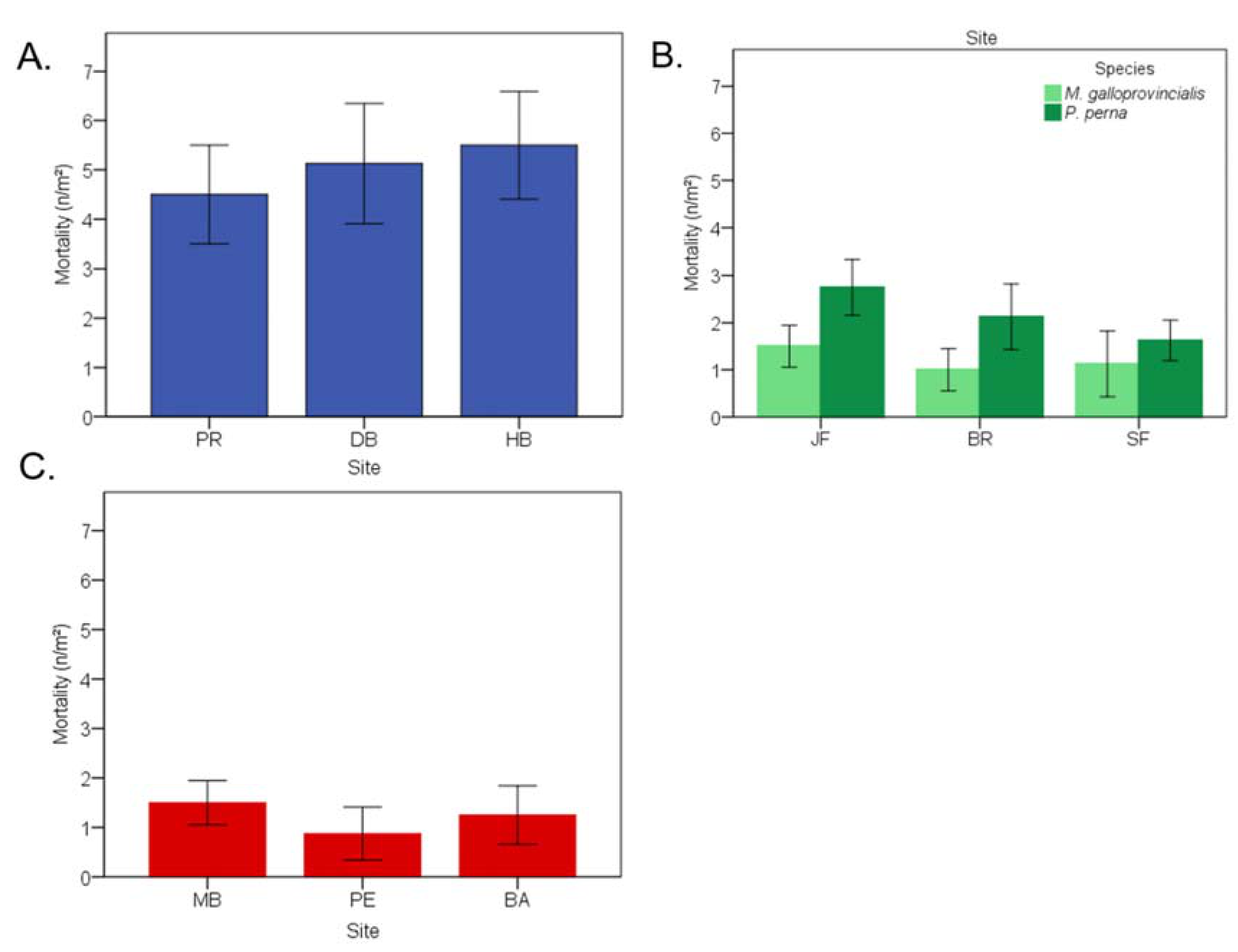
3.9. Lethal Effects of Endolithic Infestation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diels, L. Die Algen-Vegetation der Südtiroler Dolomitriffe: Ein Beitrag zur Ökologie der Lithophyten. Ber. Dtsch. Bot. Ges. 1914, 32, 507–531. [Google Scholar]
- Schneider, J.; Torunski, H. Biokarst on limestone coasts, morphogenesis and sediment production. Mar. Ecol. 1983, 4, 45–63. [Google Scholar] [CrossRef]
- Bentis, C.J.; Kaufman, L.; Golubic, S. Endolithic fungi in reef-building corals (Order: Scleractinia) are common, cosmopolitan, and potentially pathogenic. Biol. Bull. 2000, 198, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Golubic, S.; Friedmann, I.; Schneider, J. The lithobiontic ecological niche, with special reference to microorganisms. Sediment. Geol. 1981, 51, 475–478. [Google Scholar]
- Prusina, I.; Peharda, M.; Ezgeta-Balić, D.; Puljas, S.; Glamuzina, B.; Golubić, S. Life-history trait of the Mediterranean keystone species Patella rustica: Growth and microbial bioerosion. Mediterr. Mar. Sci. 2015, 16, 393–401. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Webb, S.C.; Barnaby, C. Variability of growth, health, and population turnover within mussel beds of Perna canaliculus in northern New Zealand. Mar. Biol. Res. 2008, 4, 376–383. [Google Scholar] [CrossRef]
- Kaehler, S. Incidence and distribution of phototrophic shell-degrading endoliths of the brown mussel Perna perna. Mar. Biol. 1999, 135, 505–514. [Google Scholar] [CrossRef]
- Zardi, G.I.; Nicastro, K R.; McQuaid, C.D.; Gektidis, M. Effects of endolithic parasitism on invasive and indigenous mussels in a variable physical environment. PLoS ONE 2009, 4, e6560. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Ramírez-Reinat, E.; Gao, Q. Microbial excavation of solid carbonates powered by P-type ATPase-mediated transcellular Ca2+ transport. Proc. Natl. Sci. USA 2010, 107, 21749–21754. [Google Scholar] [CrossRef]
- Grant, W.S.; Cherry, M.I. Mytilus galloprovincialis Lmk. in Southern Africa. J. Exp. Mar. Biol. Ecol. 1985, 90, 179–191. [Google Scholar] [CrossRef]
- Robinson, T.B.; Griffiths, C.L.; McQuaid, C.D.; Rius, M. Marine alien species of South Africa-status and impacts. Afr. J. Mar. Sci. 2005, 27, 297–306. [Google Scholar] [CrossRef]
- Assis, J.; Zupan, M.; Nicastro, K.R.; Zardi, G.I.; McQuaid, C.D.; Serrão, E.A. Oceanographic conditions limit the spread of a marine invader along southern African shores. PLoS ONE 2015, 10, e0128124. [Google Scholar] [CrossRef] [PubMed]
- Bownes, S.J.; McQuaid, C.D. Mechanisms of habitat segregation between an invasive and an indigenous mussel: Settlement, post-settlement mortality and recruitment. Mar. Biol. 2009, 156, 991–1006. [Google Scholar] [CrossRef]
- McQuaid, C.D.; Porri, F.; Nicastro, K.R.; Zardi, G.I. Simple, scale-dependent patterns emerge from very complex effects: An example from the intertidal mussels Mytilus galloprovincialis and Perna perna. Oceanogr. Mar.Biol. Annu. Rev. 2015, 53, 127–156. [Google Scholar]
- Marquet, N.; Nicastro, K.R.; Gektidis, M.; McQuaid, C.D.; Pearson, G.A.; Serrão, E.A.; Zardi, G.I. Comparison of phototrophic shell-degrading endoliths in invasive and native populations of the intertidal mussel Mytilus galloprovincialis. Biol. Invasions 2013, 15, 1253–1272. [Google Scholar] [CrossRef]
- Bownes, S.J.; McQuaid, C.D. Will the invasive mussel Mytilus galloprovincialis Lamarck replace the indigenous Perna perna L. on the south coast of South Africa? J. Exp. Mar. Biol. Ecol. 2006, 338, 140–151. [Google Scholar] [CrossRef]
- Kaehler, S.; McQuaid, C.D. Lethal and sub-lethal effects of phototrophic endoliths attacking the shell of the intertidal mussel Perna perna. Mar. Biol. 1999, 135, 497–503. [Google Scholar] [CrossRef]
- Geller, J.B. Reproductive responses to shell damage by the gastropod Nucella emarginata (Deshayes). J. Exp. Mar. Biol. Ecol. 1990, 136, 77–87. [Google Scholar] [CrossRef]
- Webb, S.C.; Korrubel, J.L. Shell weakening in marine mytilids attributable to blue-green alga, Mastigocoleus sp. (Nostochopsidaceae). J. Shellfish Res. 1994, 13, 11–17. [Google Scholar]
- Nicastro, K.R.; McQuaid, C.D.; Zardi, G.I. Between a rock and a hard place: Combined effect of trampling and phototrophic shell-degrading endoliths in marine intertidal mussels. Mar. Biodivers. 2018, 1–6. [Google Scholar] [CrossRef]
- Zardi, G.; Nicastro, K.; McQuaid, C.; Ng, T.; Lathlean, J.; Seuront, L. Enemies with benefits: Parasitic endoliths protect mussels against heat stress. Sci. Rep. 2016, 6, 31413. [Google Scholar] [CrossRef]
- Lourenço, C.R.; Nicastro, K.R.; McQuaid, C.D.; Sabour, B.; Zardi, G.I. Latitudinal incidence of phototrophic shell-degrading endoliths and their effects on mussel bed microclimates. Mar. Biol. 2017, 164, 129. [Google Scholar] [CrossRef]
- Margolis, L.; Esch, G.; Holmes, J.; Kuris, A.; Schad, G. The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists). J. Parasitol. 1982, 68, 131–133. [Google Scholar] [CrossRef]
- Calvo-Ugarteburu, M.G.; McQuaid, C.D. Parasitism and introduced species: Epidemiology of trematodes in the intertidal mussels Perna perna and Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 1998, 220, 47–65. [Google Scholar] [CrossRef]
- Golubic, S.; Radtke, G.; Le Campion-Alsumard, T. Endolithic fungi in marine ecosystems. Trends Microbiol. 2005, 13, 229–235. [Google Scholar] [CrossRef]
- Tribollet, A. The boring microflora in modern coral reef ecosystems: A review of its roles. In Current Developments in Bioerosion; Springer: Kaneohe, HI, USA, 2008; pp. 67–94. [Google Scholar]
- Cunha, R.L.; Nicastro, K.R.; Costa, J.; McQuaid, C.D.; Serrão, E.A.; Zardi, G.I. Wider sampling reveals a non-sister relationship for geographically contiguous lineages of a marine mussel. Ecol. Evol. 2014, 4, 2070–2081. [Google Scholar] [CrossRef]
- Zardi, G.; Nicastro, K.; McQuaid, C.; Castilho, R.; Costa, J.; Serrao, E.; Pearson, G. Intraspecific genetic lineages of a marine mussel show behavioural divergence and spatial segregation over a tropical/subtropical biogeographic transition. BMC Evol. Biol. 2015, 15, 100. [Google Scholar] [CrossRef]
- Zardi, G.; McQuaid, C.; Teske, P.; Barker, N. Unexpected genetic structure of mussel populations in South Africa: Indigenous Perna perna and invasive Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 2007, 337, 135–144. [Google Scholar] [CrossRef][Green Version]
- Andrews, W.R.H.; Hutchings, L. Upwelling in the southern Benguela Current. Prog. Oceanogr. 1980, 9, 1–81. [Google Scholar] [CrossRef]
- Lett, C.; Roy, C.; Levasseur, A.; Van Der Lingen, C.D.; Mullon, C. Simulation and quantification of enrichment and retention processes in the southern Benguela upwelling ecosystem. Fish. Oceanogr. 2006, 15, 363–372. [Google Scholar] [CrossRef]
- Pfaff, M.C.; Branch, G.M.; Wieters, E.A.; Branch, R.A.; Broitman, B.R. Upwelling intensity and wave exposure determine recruitment of intertidal mussels and barnacles in the southern Benguela upwelling region. Mar. Ecol. Prog. Ser. 2011, 425, 141–152. [Google Scholar] [CrossRef]
- Tribollet, A.; Godinot, C.; Atkinson, M.; Langdon, C. Effects of elevated pCO2 on dissolution of coral carbonates by microbial euendoliths. Global Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Reyes-Nivia, C.; Diaz-Pulido, G.; Kline, D.; Guldberg, O.-H.; Dove, S. Ocean acidification and warming scenarios increase microbioerosion of coral skeletons. Glob. Chang. Biol. 2013, 19, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Parrino, V.; Costa, G.; Cannavà, C.; Fazio, E.; Bonsignore, M.; Concetta, S.; Piccione, G.; Fazio, F. Flow cytometry and micro-Raman spectroscopy: Identification of hemocyte populations in the mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) from Faro Lake and Tyrrhenian Sea (Sicily, Italy). Fish Shellfish Immunol. 2019, 87, 1–8. [Google Scholar] [CrossRef]
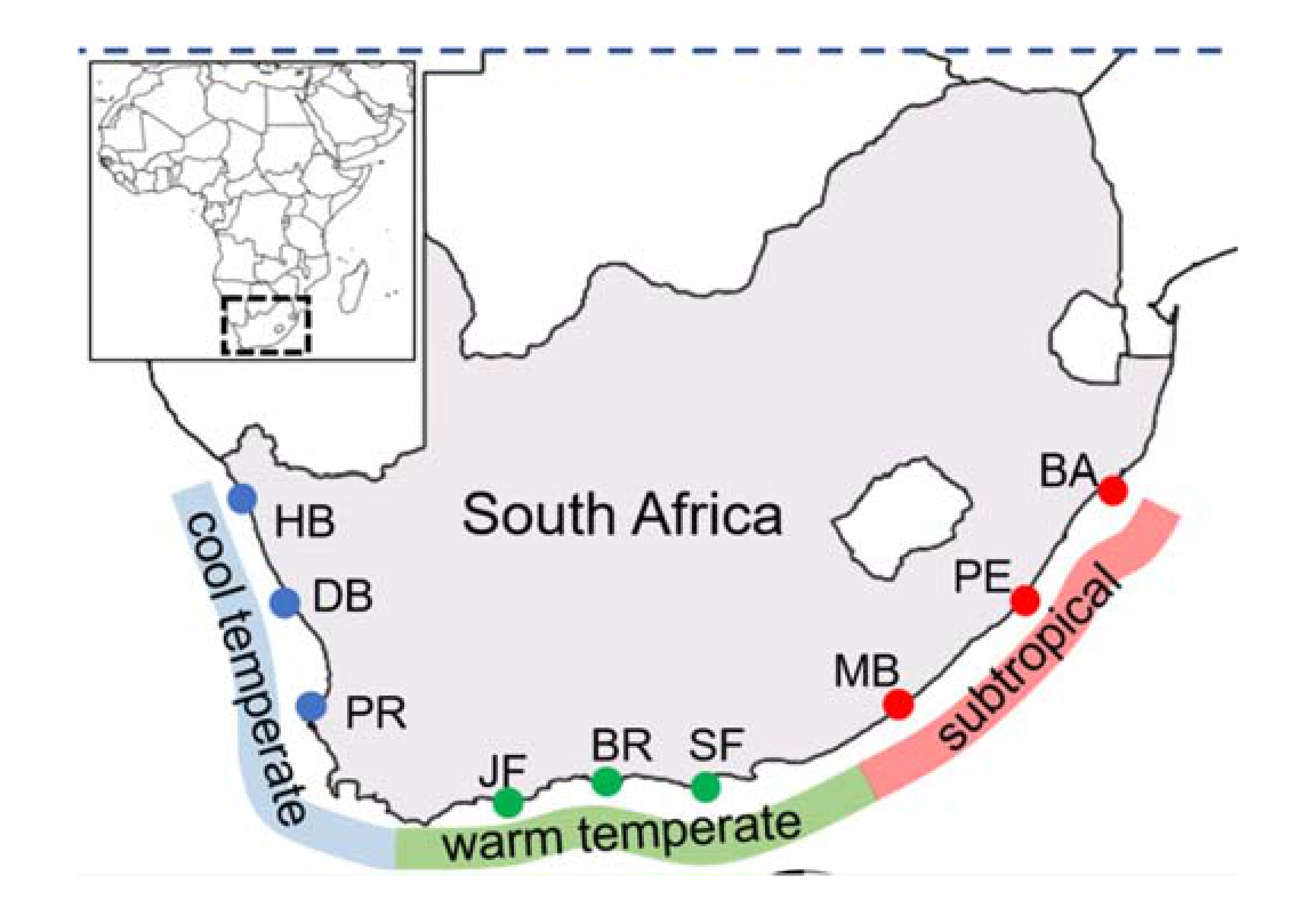
| Bioregion | Site | Codes | Coordinates |
|---|---|---|---|
| Subtropical | Ballito | BA | 29.53°N; 31.22°E |
| Port Edward | PE | 31.03°N; 30.24°E | |
| Morgans Bay | MB | 32.71° N; 28.34°E | |
| Warm temperate | St. Francis Bay | SF | 34.21°N; 24.84°E |
| Brenton-on-Sea | BR | 34.08°N; 23.02°E | |
| Jongensfontein | JF | 34.42°N; 21.38°E | |
| Cool temperate | Paternoster | PR | 32.81°N; 17.87°E |
| Doring Bay | DB | 31.78°N; 18.23°E | |
| Hondeklip Bay | HB | 30.31°N; 17.27°E |
| Cyanobacterium Species | Mytilus Galloprovincialis | |||||
|---|---|---|---|---|---|---|
| HB | DB | PR | JF | BR | SF | |
| Hyella balani (Lehman 1903) | X | X | X | X | X | X |
| Mastigocoleus testarum (Lagerheim 1886) | X | X | X | X | X | X |
| Solentia stratosa (Ercegovic 1932) | X | X | X | X | X | X |
| Plectonema terebrans (Bornet & Gomont,1889) | X | X | X | X | X | X |
| Hyella caespitosa (Bornet & Flahault 1889) | X | X | X | X | X | X |
| Hormathonema violaceo-nigrum (Ercegović 1930) | X | |||||
| Kyrthutrix dalmatica (Ercegovic 1929) | X | X | X | X | X | X |
| Hormathonema luteo brunneum (Ercegović 1930) | X | X | X | X | ||
| Cyanobacterium species | Perna perna | |||||
| JF | BR | SF | MB | PE | BA | |
| Hyella balani (Lehman 1903) | X | X | X | X | X | X |
| Mastigocoleus testarum (Lagerheim 1886) | X | X | X | X | X | X |
| Solentia stratosa (Ercegovic 1932) | X | X | X | X | X | X |
| Plectonema terebrans (Bornet & Gomont,1889) | X | X | X | X | X | X |
| Hyella caespitosa (Bornet & Flahault 1889) | X | X | X | X | X | X |
| Kyrthutrix dalmatica (Ercegovic 1929) | X | X | X | |||
| Hormathonema luteo brunneum (Ercegović 1930) | X | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndhlovu, A.; McQuaid, C.D.; Nicastro, K.; Marquet, N.; Gektidis, M.; Monaco, C.J.; Zardi, G. Biogeographical Patterns of Endolithic Infestation in an Invasive and an Indigenous Intertidal Marine Ecosystem Engineer. Diversity 2019, 11, 75. https://doi.org/10.3390/d11050075
Ndhlovu A, McQuaid CD, Nicastro K, Marquet N, Gektidis M, Monaco CJ, Zardi G. Biogeographical Patterns of Endolithic Infestation in an Invasive and an Indigenous Intertidal Marine Ecosystem Engineer. Diversity. 2019; 11(5):75. https://doi.org/10.3390/d11050075
Chicago/Turabian StyleNdhlovu, Aldwin, Christopher D. McQuaid, Katy Nicastro, Nathalie Marquet, Marcos Gektidis, Cristián J. Monaco, and Gerardo Zardi. 2019. "Biogeographical Patterns of Endolithic Infestation in an Invasive and an Indigenous Intertidal Marine Ecosystem Engineer" Diversity 11, no. 5: 75. https://doi.org/10.3390/d11050075
APA StyleNdhlovu, A., McQuaid, C. D., Nicastro, K., Marquet, N., Gektidis, M., Monaco, C. J., & Zardi, G. (2019). Biogeographical Patterns of Endolithic Infestation in an Invasive and an Indigenous Intertidal Marine Ecosystem Engineer. Diversity, 11(5), 75. https://doi.org/10.3390/d11050075





