Abstract
Composition, trophic structure, and species-substrate relationships of molluscan assemblages inhabiting different hard bottom habitats (Sabellaria alveolata reef, photophilic bottoms, Phyllophora crispa sciaphilic assemblage, and coralligenous bioconstruction) were studied in two different sites of the Tyrrhenian Sea. In particular, molluscs from the Sabellaria alvevolata (Linnaeus, 1767) reef and coralligenous concretion were investigated, testing the hypothesis that bioconstructions increase the diversity and abundance of associated biota compared to the surrounding habitats. A total of 3134 individuals belonging to the classes of Polyplacophora (5 species, 24 individuals), Bivalvia (39 sp., 2734 ind.), and Gastropoda (53 sp., 376 ind.) were identified. These three taxonomic groups showed different distribution patterns in the studied habitats. Multivariate analyses revealed significant inter-habitat differences in the composition of mollusc assemblages, especially between bioconstructions and the other habitats. S. alveolata and coralligenous host the highest rich molluscan fauna when compared to the neighboring hard bottom habitats characterized by photophilic and sciaphilic assemblages. The first ones were dominated by bivalve suspension feeders, mainly represented by sessile and sedentary organisms, which act as bio-constructors, bio-eroders, or simply inhabit the several microhabitats provided by the bioconstructions, while the second ones host a rich molluscan fauna dominated by gastropod grazers and predators. The present study increases the comparative knowledge of molluscan assemblages inhabiting habitats of littoral plans of the Mediterranean Sea, providing pivotal information regarding biodiversity of coastal zones.
1. Introduction
In marine ecosystems, any structure built by living organisms that rises from the bottom toward the surface may be defined as bioconstruction, which strongly modifies the local environment through physical and ecological processes [1].
Although corals and calcareous algae are usually indicated as the builders of biotic reefs, barnacles, molluscs, encrusting bryozoans and tubeworms also play key roles in the creation of complex biogenic structures, especially in the Mediterranean Sea [2]. Ingrosso et al. [3] reviewed the most important biogenic habitats along the Italian coasts, and found that a total of seven different formations develop in infralittoral and circalittoral bottoms. Despite their ecological and economic importance within the Mediterranean basin, the knowledge of biology and ecology of bioconstructions is far from comprehensive. The interest of the scientific community has recently increased in regard to some of them, such as coralligenous and vermetid reefs, whereas others, like Sabellaria alveolata (Linnaeus, 1767) reefs, remain poorly investigated. Similarly, the knowledge of some algae-dominated habitats is still lacking, as in the case of Phyllophora crispa (Hudson) P.S. Dixon, 1964 sciaphilic assemblages in central Tyrrhenian Sea [4].
S. alveolata (Annelida; Sabellariidae) is a gregarious polychaete worm that is able to build biogenic structures on infralittoral sandflats with laying portions of rocks, assembling tubes made by coarse sand and bioclastics. This species is considered as the most important building organism in temperate shallow environments, capable of increasing habitat heterogeneity by creating complex bioconstruction [5,6,7] and covering several square kilometers [8]. Therefore, this worm plays a pivotal role in coastal zone management and the provision of goods and services, stabilizing sediments [9,10] and filtering large volumes of water [11].
P. crispa is moderately common red alga which grows in sciaphilic habitats forming meadows or turfs. The vegetative growth of this species and the sedimentation rates likely contribute to the development of a dense twist of algal thalli, allowing for the rich presence of associated fauna and flora [4] which has recently been reported both in the Black Sea and Tyrrhenian Sea [12,13]. In the latter case, it develops mainly on hard bottoms, lying in proximity of coralligenous reefs [14]. The coralligenous habitat is the most important biogenic concretion in the Mediterranean Sea in terms of biodiversity, ecological, aesthetic, and economic values [15,16]. The primary builders are calcareous encrusting algae that grow in dim light conditions, followed by other builder species (e.g., bryozoans, polychaetes, and cnidarians) which contribute to the accretion of coralligenous reefs [15]. These concretions occur in the lower infralittoral or twilight zone, between 20 and 200 m in depth [3]. Time constraints associated with deep underwater sampling, conservation purposes, and spatial heterogeneity have contributed to the large diffusion of both photographic and visual census methods to investigate coralligenous reefs. This increased the number of studies focusing on sessile epimegabenthos assemblages, but partially overcame the lack of information regarding the real amount of species, such as vagile organisms, that thrive in coralligenous reefs [17,18,19,20,21]. Both S. alveolata and coralligenous reefs are included in the “reefs” category by the Habitat Directive (HD, 92/43/EEC), and are therefore also part of the Natura 2000 network [22].
The distribution of marine organisms is strongly influenced by environmental factors, such as depth, light, hydrodynamism, and salinity, producing a puzzle of rocky benthic communities in Mediterranean infralittoral and circalittoral bottoms [23] characterized by different habitat complexity, and defined as heterogeneity in spatial arrangement and physical structures [24]. Higher levels of habitat complexity are usually associated with bioconstructions [3]; spatial heterogeneity increases both species richness and trophic diversity of associated fauna by protection from predation [25], food supply [26], and reduction of stress [27]. In the Mediterranean Sea, the effect of algal morphology has especially been proven to help structure the associated fauna, such as polychaetes and molluscs [14,28,29,30,31].
Coll et al. [32] estimated around 2000 molluscan species in the Mediterranean Sea and identified this taxonomic group as one of the most well-known, although quantitative studies on this group occurring in peculiar Mediterranean habitats, such as S. alveolata reefs, P. crispa turf, and coralligenous concretions are still scarce. Molluscs show several adaptations to different environmental factors and food sources, resulting in being one of the main groups of benthic organisms able to colonize different marine coastal habitats [30,33,34,35,36]. In addition to their abundance and diversity, molluscan assemblages reveal a great variety of functional roles, both in bioconstructions and algae-dominated habitats [37,38,39,40]. Biodiversity is the first of eleven descriptors that have been chosen for the assessment of the Good Environmental Status (GES) within the Marine Strategy Framework Directive (MSFD, 2008/56/EC). It has a pivotal role in terms of ecosystem services and in the supply of goods and services of the investigated coastal habitats [10,41,42,43]. Therefore, solid biological and ecological knowledge of marine habitats and ecosystems is pivotal, especially in the light of the increasing seawater pollution of coastal zones.
The aim of this work is to analyze molluscan assemblages inhabiting five different Mediterranean habitats, assessing both molluscs’ diversity and their functional trophic role in order to test the effect of habitat complexity.
2. Materials and Methods
2.1. Study Area and Sampling Methods
The study was carried out in two different sites in the central Tyrrhenian Sea (Figure 1). One is located along the Latium coast (Lido di Ostia, 41°43′03.8″ N 12°18′10.5″ E), and the other at the east coast of the Giglio Island, belonging to the Tuscany Archipelago (Punta del Lazzaretto, 42°21′40.9″ N, 10°55′16.0″ E).
Figure 1.
Study areas (a) Giglio Island and (b) Latium coast, with the locations of the sampling sites (black triangles) being Punta del Lazzaretto and Lido di Ostia, respectively.
At Lido di Ostia, samplings were performed in two habitats at 3 m depth: on a continuous S. alveolata bioconstruction (SABEL), developing parallel to the shoreline [7], and on the surrounding hard bottom (HARDB), represented by rocks very poorly covered by photophilic green and brown algae. The Sabellaria alveolata reef at Lido di Ostia reaches the greatest dimension among those reported in the Mediterranean Sea [44], and therefore can be used as a model for studies concerning the associated fauna.
At Punta del Lazzaretto, samplings were performed in three habitats, which characterize the substrate from 10 m to 40 m depth [14]. The rocky bottom at 10 m depth is characterized by shallow photophilous infralittoral algal assemblages (PHOTO), dominated by Padina pavonica (Linnaeus) Thivy, 1960 and Acetabularia acetabulum (Linnaeus) P.C. Silva, 1952, while at 30 m depth, the rocky bottom is covered by Phyllophora crispa sciaphilic assemblage (PHYLL) or by coralligenous bioconstructions (CORAL). The P. crispa turf was exclusively reported by Bianchi et al. [12] and Casoli et al. [14] along the coasts of Sardinian and Tuscany; coralligenous bioconstruction at Punta del Lazzaretto, in the proximity of the P. crispa turf, is extremely relevant in dimension ([45] and references therein).
Samples were collected by SCUBA divers during late spring and summer of 2014. On SABEL, HARDB, PHOTO, and PHYLL, four replicate samples were collected by scraping the bottom within a 20 × 20 cm frame for a total thickness of 5 cm, using a hammer and chisel, and holding a 50 µm mesh bag below the sampling plot in order to minimize the loss of organisms. Sampling efforts were reduced on CORAL to four replicate samples with a surface of 10 × 10 cm and a thickness of 10 cm [14]. Then, samples were stored in 4% formaldehyde seawater solution to be transported to the laboratory, where the macrobenthos was sorted. All molluscs found were preserved in 70% alcohol solution and then identified to the lowest possible taxonomical level under a Leitz stereomicroscope.
2.2. Relation to the Substrate and Trophic Groups
Functional groups concerning molluscs’ motility (sessile, sedentary or vagile) and feeding guilds were assigned to the identified species. According to Donnarumma et al. ([35] and references therein), the following categories were considered: Micro- and Macro-grazers (MG), feeding on both diatoms and/or algae growing on rocks, on shells or on large plants; Deposit feeders (DF), feeding on both diatoms and/or microalgae of sandy sediments; Predators (P), actively feeding on sedentary or motile animals; Scavengers (SC), feeding on dead animals; Ectoparasites (E), feeding on much larger animals on which they live during their life cycle; Suspension feeders (SF), feeding on the organic particles suspended in water, which mainly comprise of bacteria, diatoms, and algal tissue, or, in coastal habitats, nano-zooplankton.
2.3. Data Analysis
Species-area curves were produced for each habitat to evaluate the efficiency of sampling. Molluscan assemblages were analyzed using sinecological indices, such as the species richness (SR), the number of individuals (N) per 2 dm3, the Pielou’s Evenness (J), and the Shannon-Weaver diversity (H’). The quantitative (DI, percentage of individuals of a given species upon the total of individuals) and qualitative (DQ, percentage of species of a given taxon upon the total of species) indices were also calculated. Further habitat comparisons were carried out by sample-based and individual-based interpolation (rarefaction) and extrapolation curves, following Colwell et al. [46] and Chao and Jost [47]. Differences on each sinecological index among habitats were assessed through a permutational analysis of variance (PERMANOVA), based on Euclidean distance and performed as univariate analysis [48]. A one-way model was used with habitat as a fixed factor. With the same design, PERMANOVA analysis based on Bray-Curtis similarity was used to test differences in the mollusc assemblages among the five habitats with four replicates. The pairwise test was performed to further highlight differences among habitats, and non-metric multidimensional scaling (n-MDS) ordination [49] was then plotted. Data were transformed using log (x+1) to reduce the effect of the dominant species in the samples [50], and 4999 permutations of the raw data units were computed to obtain p-values. A similarity percentages–species contribution analysis (SIMPER) was carried out to identify the species that mostly contribute to the similarity among habitats, as well as the species that mostly characterized each one [50].
3. Results
3.1. Composition of Mollusc Assemblages
A total of 3134 individuals were collected from the five examined habitats. The taxonomic identification yielded 96 species, 62 of which were recorded in only one of the five habitats and were represented by one or a few individuals; only two species, the gastropod Bittium reticulatum and the bivalve Musculus costulatus, were widespread in all habitats (Table 1).

Table 1.
Taxonomic list of species, occurring in each habitat (SABEL—S. alveolata reef; HARDB—hard bottom with photophilic algae; CORAL—coralligenous concretion; PHYLL—P. crispa sciaphilic assemblage; PHOTO—shallow photophilic assemblage), with their abundance (N), quantitative dominance (%DI), feeding guilds (MG Micro- and Macro-grazers, DF Deposit feeders, P Predators, E Ectoparasites, SC Scavengers, SF Suspension feeders) and relation with substrates (RS: V vagile, Sd sedentary, Ss sessile).
As for abundance, bivalves were the dominant group, with 2,734 individuals (87.23%) belonging to 38 species (39.58%). The main representative species were Mytilus galloprovincialis (1,116 ind., 40.82%) and Striarca lactea (1,088 ind., 39.80%). This group mainly occurred in HARDB (92.10%), SABEL (91.93%), and CORAL (79.95%) habitats (Figure 2a). A high number of very recently settled juveniles of M. galloprovincialis were detected in SABEL (125 ind.) and in HARDB (201 ind.), even though they were not considered for statistical analyses in order to exclude the background noise.
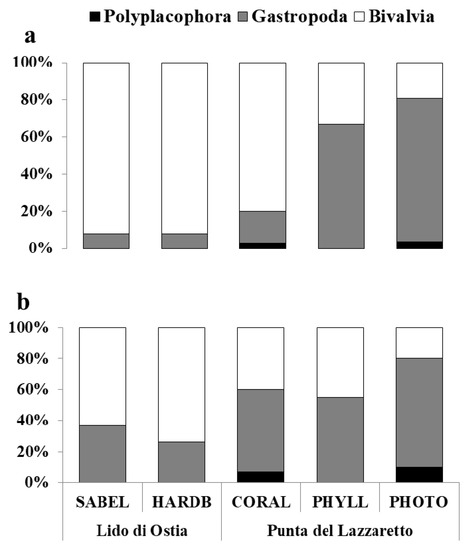
Figure 2.
Percentages of abundance (%DI—a) and species richness (%DQ—b) of Polyplacophora, Gastropoda and Bivalvia for each habitat (SABEL—S. alveolata reef; HARDB—hard bottom with photophilic algae; CORAL—coralligenous concretion; PHYLL—P. crispa sciaphilic assemblage; PHOTO—shallow photophilic assemblage) and site (Lido di Ostia; Punta del Lazzaretto).
As for species richness, gastropods were the dominant group of molluscs (Figure 2b), with 53 species (55.20%) and an abundance of 376 individuals (12%). The main representative species were B. reticulatum (103 ind., 27.39%), Ocenebra edwardsii (56 ind., 14.89%), and Bittium latreillii (53 ind., 14.10%). This group mainly occurred in algae-dominated habitats (PHOTO 76.92%; PHYLL 66.67%; Figure 2a).
Polyplacophorans were poorly represented, both in abundance (24 ind.; 0.76%) and species richness (5 sp.; 5.20%). They were only detected in two habitats of the Giglio Island (Figure 2): in PHOTO, where the species Acanthochitona crinita was represented by only one individual, and in CORAL, where four species (Acanthochitona fascicularis, Callochiton septemvalvis, Chiton corallinus, and Lepidopleurus cf cimicoides) occurred, with a total abundance of 23 individuals.
3.2. Species-Substrate Relationship
Among the 96 species belonging to the three taxonomic groups, vagile species were 53.13%, sessile species 30.21% and sedentary species 16.67%.
Bivalve assemblages were composed of both sessile (71.05%) and sedentary (28.95%) species. Sessile bivalves included 17 byssate species (e.g., M. galloprovincialis) with 2408 individuals, six shell-cemented species (e.g., Chama gryphoides) with 38 individuals, and four rocky-borer species (e.g., Hiatella arctica) with 247 individuals, which mainly occurred in SABEL, HARDB, and CORAL habitats. Sedentary bivalves were represented by seven species buried in the sediments (e.g., Parvicardium scriptum) with 36 individuals and by four free-swimming species (e.g., Flexopecten hyalinus) with five individuals, which mainly occurred in the SABEL and PHYLL habitats, respectively.
On the contrary, gastropod assemblages were more greatly represented by vagile (96.23%) than sessile (3.77%) species. The first group, with 51 species and 367 individuals, was detected in all habitats, while the second, exclusively belonging to the Vermetidae family (Thylaeodus semisurrectus and Vermetus granulatus) with 9 individuals, was found only in CORAL habitats. As for polyplacophorans, they were exclusively sedentary and dominated the CORAL habitat.
3.3. Trophic Analysis
As for trophic analysis, six feeding guilds were identified in the five habitats (Figure 3). Suspension feeders (SF) were the most dominant group (2,743 ind.; 40 sp.) and mainly occurred in habitats characterized by hard substrates (SABEL, HARDB, CORAL; DI 88.40 ± 6.26%, DQ 59.99 ± 13.97%) (Figure 3a). This guild was mainly represented by the bivalves M. galloprovincialis (1,116 ind., 40.82%SF), S. lactea (1,088 ind., 39.80%SF), and H. arctica (179 ind., 6.55%SF). The second group consisted of micro- and macro-grazers (MG; 213 ind.; 18 sp.), dominant in the PHOTO habitat (Figure 3a), with a total abundance of 65.38% and species richness of 50%. The main representative grazers were the gastropods belonging to the genus Bittium (total abundance of 156 ind., 73.24%MG). Predators (P) were the third dominant feeding guild (106 ind.; 23 sp.). They were dominant in the PHYLL habitat (Figure 3a), with a total abundance of 33.33% and species richness of 41.94%. This guild is mainly represented by the gastropod O. edwardsii (56 ind., 52.83%P), occurring in SABEL and HARDB.
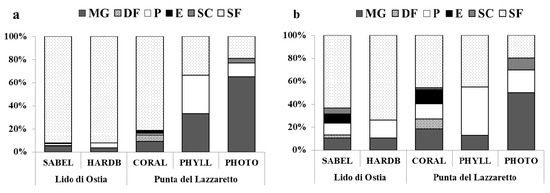
Figure 3.
Percentages of (a) abundance (%DI) and (b) species richness (%DQ) of the feeding guilds (MG Micro- and Macro-grazers, DF Deposit feeders, P Predators, E Ectoparasites, SC Scavengers, SF Suspension feeders) for each habitat and site.
The three remaining feeding guilds reached a total of 72 individuals (2.03%) and 15 species (15.46%): Deposit feeders (DF) showed the highest abundance (44 ind., 1.40%), and were mainly represented by Crisilla semistriata (25 ind., 56.82%DF) and Scissurella costata (13 ind., 29.55%DF); Ectoparasites (E) showed the highest species richness (8 sp., 8.25%), and were mainly represented by the gastropods Spiralinella incerta (7 ind., 29.17%E) and Folinella excavata (5 ind., 20.83%E). Species belonging to these two feeding guilds mainly occurred in the CORAL habitat (Figure 3b) (DF: DQ 8.62%, DI 5.46%; E: DQ 10.34%, DI 2.18%); Scavengers (SC) were only represented by the gastropods Tritia incrassata and Tritia pygmaea, with a total abundance of four individuals, which occurred in the SABEL, CORAL, and PHOTO habitats (Figure 3b).
3.4. Inter-Habitat Comparison of the Molluscan Assemblages
Species accumulation curves (supplementary material, Figure S1) showed that the molluscan assemblages were well-represented by the four replicate samples in the studied habitats. The mean values among replicates of sinecological indices (Table 2) were higher in the bioconstructions (SABEL and CORAL) when compared to the neighboring habitats, except equitability (J) at Punta del Lazzaretto. Sample-based and individual-based interpolation (rarefaction) and extrapolation curves (supplementary material, Figure S2) corroborate the results of habitat comparisons. The PERMANOVA test on the sinecological indices displayed significant differences among habitats (Table 2).

Table 2.
Mean values (± SD) of sinecological indices (SR = number of species; N = number of individuals per 2 dm3; J = evenness; H′ = Shannon-Weaver diversity) for each habitat and site, and results of permutational analysis of variance [PERMANOVA test-F: F-value; p(perm): calculated probability value; Unique perms: the number of unique permutations].
A significant difference was also detected when analyzing mollusc composition (PERMANOVA test: df = 4, F = 9.9816, p = 0.0002; Pairwise comparison, Table 3). Their spatial differences were highlighted by the n-MDS plot (Figure 4), which showed a clear separation between all the five habitats, while the four replicates of each habitat were consistent each other. The similarity percentages–species contribution analysis (SIMPER) indicated a similarity among habitats of 22.08%, due to 2 bivalves and 2 gastropods (Table 4), while for each habitat the similarity of dominant species among replicates ranged from 37.17% to 68.95% (Table 5).

Table 3.
Results of PERMANOVA pair-wise test of mollusc assemblages among the five habitats. Significant values are highlighted in bold.
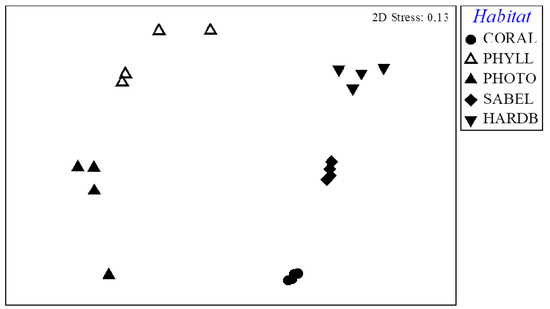
Figure 4.
Nonmetric multidimensional scaling (nMDS) plot of molluscan assemblage, based on Bray-Curtis similarity matrix, of all replicates for each habitat.

Table 4.
Results of the similarity percentages–species contribution analysis (SIMPER), showing molluscan species mostly responsible for the 22.04% similarity among habitats. (Cum%-50% cut-off).

Table 5.
Results of the SIMPER analyses, showing dominant species mostly responsible for the similarity among replicates for each habitat. (Cum%-50% cut-off).
4. Discussion
The present paper provides quantitative data on molluscan assemblages from relevant coastal habitats which are widespread in the Mediterranean Sea. Sabellaria and coralligenous bioconstructions hosted richer molluscan fauna, dominated by suspension feeder bivalves, when compared to the neighboring hard bottom habitats characterized by photophilic and sciaphilic assemblages, dominated by grazer and predator gastropods.
S. alveolata bioconstruction revealed a huge variety of associated bivalves that shed light on the complex ecological system of the reef. This grows at a very shallow depth (from the mesolittoral to around 3 m) where high-water movement contributes to the transport of sediment and suspension of organic nutrients, promoting the occurrence of associated suspension feeders which are able to remove micro-particulates from the water column [51]. The suspension feeders M. galloprovincialis, H. arctica, and S. lactea were the main components of mollusc assemblage. M. galloprovincialis is well-known as the food competitor of S. alveolata, contributing to the regression of the bioconstruction [9], while the high abundance of H. arctica and the occurrence of other species of suspension feeders, such as Parvicardium spp. and Venerupsis corrugata, are indicators of the softness of the reef that is made of poorly adherent sand grains. Indeed, H. arctica is a soft rock borer, while the latter two are a soft-bottom burrower species [52], accounting for the presence of loose sandy sediment on the reef. Furthermore, the occurrence of the rock-boring bivalve R. dubia indirectly suggests the presence of carbonate fragments, probably shells or coarse gravels, on the S. alveolata bioconstruction. Concerning gastropods, the high occurrence of micrograzers belonging to the genus Bittium have been also detected by Gravina et al. [53] for Sabellaria spinulosa (Leuckart, 1849) reefs [54,55].
Overall, molluscs abundance and species richness found in the investigated reef were markedly higher than those previously reported for other Mediterranean and Atlantic Sabellaria reefs [9,11,53,56].
As for the hard bottoms surrounding the S. alveolata reef, characterized by photophilic algae, the highest dominance of M. galloprovincialis influenced the dynamic of associated faunal assemblages. There is evidence in literature that mussels alter the bottom topography, causing both positive and negative effects on the associated biota. They offer suitable substrate for the colonization of both algae and invertebrates, but at the same time, preempt the space preventing the settlement for other kinds of sessile suspension feeder species [57,58,59,60]. In particular, the dominant species which were detected here, also associated with the suspension feeder M. galloprovincialis, were its direct predator, the shell-boring gastropod O. edwardsii [61], and the micrograzer gastropod B. reticulatum. According to Huston [62], the dominance of very few species in mollusc assemblages suggests a remarkable species competition, which, in this case, increases from the Sabellaria reef to the surrounding hard bottom, together with a decrease of species number and evenness (Sinecological indices, Table 2).
According to Gili and Coma [63], suspension feeders play a pivotal role in coralligenous reefs. Indeed, coralligenous, like in Sabellaria reef and the surrounding hard bottom, reveals a rich habitat mainly characterized by high bivalves abundance and diversity. This richness is due to species which contribute both to the biological accretion and erosion of coralligenous itself. The sessile and byssate species detected, such as Barbatia barbata and S. lactea, and cemented ones, such as Spondylus gaederopus, T. semisurrectus, and V. granulatus, act as secondary builders, due to their abundance and shell structure [64]. On the other hand, boring bivalves such as L. lithophaga, P. lithophaga, and R. dubia settled in this calcareous formation, bore the calcareous substrate by their glandular secretions and act as bio-eroders [65,66,67]. The occurrence of sand-burrowing bivalve species, such as Venus casina and Papillicardium papillosum, accounts for the heterogeneity of bioconstruction, well-known as a promoter of biodiversity [35,68,69,70].
The presence of sandy sediments occurring in the coralligenous habitat favors the accumulation of organic materials and provides optimal condition for gastropod deposit feeders, such as Alvania spinosa, Alvania tenera, S. costata, and the most abundant species, C. semistriata. According to Terlizzi et al. [71], this latter species also characterizes the coralligenous reefs along Apulian coasts. In addition, the herbivore polyplacophorans were found almost exclusively on coralligenous habitats, due to the availability of their food source, such as diatoms and red algae [72,73]. Moreover, when compared with other habitats, the coralligenous also shows a high species richness belonging to several trophic groups, such as micro- and macro-grazers, carnivores, predators, and ectoparasites, sustaining complex food webs [15,74,75].
In light of these findings, the CorMol database proposed by Poursanidis and Koutsoubas [39] could be integrated with eight additional species associated with coralligenous formations: Alvania spinosa, Cerithiopsis barleei, Fusinus dimassai, Odostomella bicincta, Thylaeodus semisurrectus, Vermetus granulatus, Vitreolina philippi, and Venus casina.
The two algal-dominated hard bottom habitats sampled at Giglio Island greatly differ from the others (Figure 4), as vagile gastropods were the main components of mollusc assemblages instead of bivalves.
From the literature, the turf of the sciaphilic red alga P. crispa provides a complex habitat due to the algal architecture, supporting several algal and animal epiphytes as food of herbivorous and carnivorous gastropods, respectively [4,14]. Zaitsev [76] and Bonifazi et al. [4] reported a rich and heterogeneous community characterized by more than 140 animal species in the Black Sea and in the Tyrrhenian Sea, respectively. Our results are consistent with these data, stressing the importance of the micrograzer B. latreillii and the predator Pusia savignyi as the main gastropods of the Phyllophora algal turf. Here, although less abundant, byssate bivalves were mainly represented by the sessile S. lactea and M. costulatus, occurring on substrate where the Phyllophora alga was attached, and the sedentary F. hyalinus, settling on the algal tallus [77].
The photophilic habitat dominated by algae P. pavonica and A. acetabulum, when compared with all the other studied habitats, shows the lowest values of both species richness and abundance of mollusc assemblages. This is consistent with some literature [37,78,79], although Donnarumma et al. [40] recently described photophilic hard bottoms from different Italian Marine Protected Areas as the richest habitat of coastal environments in terms of gastropod species and feeding guilds. This might probably be due to the different sampling procedure adopted in the field (air-lift pump rather than classical method of scraping hard bottom). Several authors reported how the complexity of algal architecture contributes to diversifying molluscan assemblages [30,80,81]. In this case, the very simple shape of tallus provided by P. pavonica and A. acetabulum affects the low abundance of molluscan fauna, mainly represented by the two congeneric micrograzers, B. latreillii and B. reticulatum and very few other species.
5. Conclusions
In conclusion, our findings clearly highlight that food availability and high habitat complexity due to biological structures growing on substrates may represent the key components determining mollusc assemblages, occurring from the infralittoral to the circalittoral plans of the Mediterranean Sea. Micro-topography and the tridimensional shape of bioconstructions (S. alveolata reef and coralligenous) promote the settlement surface and sheltered microhabitats which are mainly exploited by sessile bivalve-dominated assemblages, as well as suspension-feeding behavior. Instead, algal and their epiphytes, dominating the photophilic hard bottom, represent key factors of gastropod-dominated assemblages, mainly characterized by vagile micro- and macro-grazers.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/11/5/74/s1, Figure S1: Cumulative species-area curve for each habitat (CORAL—coralligenous concretion; PHYLL—P. crispa sciaphilic assemblage; PHOTO—shallow photophilic assemblage; SABEL—S. alveolata reef; HARDB—hard bottom with photophilic algae), Figure S2: (a) Sample-based and (b) individual-based interpolation (rarefaction) and extrapolation curves for the five habitats.
Author Contributions
Conceptualization, E.C., G.A., M.F.G., G.F.R., R.S. and L.D.; Data curation, E.C., A.B., M.F.G. and L.D.; Formal analysis, E.C., M.F.G., G.F.R. and L.D.; Methodology, E.C., A.B., G.A., M.F.G. and L.D.; Supervision, G.A., M.F.G., G.F.R. and R.S.; Writing—original draft, E.C. and L.D.; Writing—review & editing, M.F.G., G.F.R., R.S. and L.D.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge Andrea Belluscio and Daniele Ventura for the help and support during underwater sampling activities. Thanks are also due to the two anonymous reviewers for the suggestions and comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Bianchi, C.N. La biocostruzione negli ecosistemi marini e la biologia marina italiana. Biol. Mar. Mediterr. 2001, 8, 112–130. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean bioconstructions along the Italian coast. In Advances in Marine Biology, 1st ed.; Sheppard, C., Fuiman, L.A., Young, C.M., Eds.; Zoe Kruze: London, UK, 2018; Volume 79, pp. 61–136. [Google Scholar] [CrossRef]
- Bonifazi, A.; Ventura, D.; Gravina, M.F.; Lasinio, G.J.; Belluscio, A.; Ardizzone, G.D. Unusual algal turfs associated with the rhodophyta Phyllophora crispa: Benthic assemblages along a depth gradient in the Central Mediterranean Sea. Estuar. Coast. Shelf. Sci. 2017, 185, 77–93. [Google Scholar] [CrossRef]
- Dubois, S.; Retière, C.; Olivier, F. Biodiversity associated with Sabellaria alveolata (Polychaeta: Sabellariidae) reefs: Effects of human disturbances. J. Mar. Biol. Assoc. UK 2002, 82, 817–826. [Google Scholar] [CrossRef]
- La Porta, B.; Nicoletti, L. Sabellaria alveolata (Linnaeus) reefs in the central Tyrrhenian Sea (Italy) and associated polychaete fauna. Zoosymposia 2009, 2, 527–536. [Google Scholar]
- Bonifazi, A.; Ventura, D.; Mancini, E. Sabellaria reefs as reservoirs of rare species: The case of Eulalia ornata Saint-Joseph, 1888 (Annelida: Phyllodocidae). Mar. Freshw. Res. 2018, 69, 1635–1640. [Google Scholar] [CrossRef]
- Gruet, Y. Recherches sur l’ écologie des “recifs” d’Hermelles édifiés par l’annélide polychéte Sabellaria alveolata (Linné). Ph.D. Thesis, Universite de Nantes, Nantes, France, 1982. [Google Scholar]
- Dias, A.S.; Paula, J. Associated fauna of Sabellaria alveolata colonies on the central coast of Portugal. J. Mar. Biol. Assoc. UK 2001, 81, 169–170. [Google Scholar] [CrossRef]
- Salomidi, M.; Katsanevakis, S.; Borja, Á.; Braeckman, U.; Damalas, D.; Galparsoro, I.; Mifsud, R.; Mirto, S.; Pascual, M.; Pipitone, C.; et al. Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: A stepping stone towards ecosystem-based marine spatial management. Mediterr. Mar. Sci. 2012, 13, 49–88. [Google Scholar] [CrossRef]
- Dubois, S.; Commito, J.A.; Olivier, F.; Retière, C. Effects of epibionts on Sabellaria alveolata (L.) biogenic reefs and their associated fauna in the Bay of Mont Saint-Michel. Estuar. Coast. Shelf Sci. 2006, 68, 635–646. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Navone, A. I popolamenti delle scogliere rocciose sommerse dell’Area Marina Protetta di Tavolara Punta Coda Cavallo (Sardegna nord-orientale). Sci. Rep. Port-Cros Natl. Park. 2010, 24, 39–85. [Google Scholar]
- Kostylev, E.F.; Tkachenko, F.P.; Tretiak, I.P. Establishment of “Zernov’s Phyllophora field” marine reserve: Protection and restoration of a unique ecosystem. Ocean Coast. Manag. 2010, 53, 203–208. [Google Scholar] [CrossRef]
- Casoli, E.; Bonifazi, A.; Ardizzone, G.; Gravina, M.F. How algae influence sessile marine organisms: The tube worms case of study. Estuar. Coast. Shelf Sci. 2016, 178, 12–20. [Google Scholar] [CrossRef]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. In Oceanography and Marine Biology: An Annual Review, 1st ed.; Gibson, R.N., Atkinson, J.A., Gordon, J.D.M., Eds.; Taylor & Francis: Broken Sound Parkway NW, FL, USA, 2006; Volume 44, pp. 123–195. [Google Scholar]
- Tribot, A.S.; Mouquet, N.; Villéger, S.; Raymond, M.; Hoff, F.; Boissery, P.; Holon, F.; Deter, J. Taxonomic and functional diversity increase the aesthetic value of coralligenous reefs. Sci. Rep. 2016, 6, 34229. [Google Scholar] [CrossRef]
- Parravicini, V.; Micheli, F.; Montefalcone, M.; Villa, E.; Morri, C.; Bianchi, C.N. Rapid assessment of epibenthic communities: A comparison between two visual sampling techniques. J. Exp. Mar. Bio. Ecol. 2010, 395, 21–29. [Google Scholar] [CrossRef]
- Kipson, S.; Fourt, M.; Teixidó, N.; Cebrian, E.; Casas, E.; Ballesteros, E.; Zabala, M.; Garrabou, J. Rapid biodiversity assessment and monitoring method for highly diverse benthic communities: A case study of Mediterranean coralligenous outcrops. PLoS ONE 2011, 6, 11–13. [Google Scholar] [CrossRef]
- Gatti, G.; Montefalcone, M.; Rovere, A.; Parravicini, V.; Morri, C.; Albertelli, G.; Bianchi, C.N. Seafloor integrity down the harbor waterfront: The coralligenous shoals off Vado Ligure (NW Mediterranean). Adv. Oceanogr. Limnol. 2012, 3, 51–67. [Google Scholar] [CrossRef]
- Teixidó, N.; Casas, E.; Cebrián, E.; Linares, C.; Garrabou, J. Impacts on Coralligenous Outcrop Biodiversity of a Dramatic Coastal Storm. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Doxa, A.; Holon, F.; Deter, J.; Villéger, S.; Boissery, P.; Mouquet, N. Mapping biodiversity in three-dimensions challenges marine conservation strategies: The example of coralligenous assemblages in north-western Mediterranean Sea. Ecol. Indic. 2016, 61, 1042–1054. [Google Scholar] [CrossRef]
- Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Available online: https://eur-lex.europa.eu/eli/dir/1992/43/oj (accessed on 14 March 2019).
- Pérès, J.M.; Picard, J. Nouveau Manuel de Bionome benthique de la Mer Mediterranee. Recl. des Trav. la Stn. Mar. l’Endoume. 1964, 47, 5–137. [Google Scholar]
- Lassau, S.A.; Hochuli, D.F. Effects of habitat complexity on ant assemblages. Ecography 2004, 27, 157–164. [Google Scholar] [CrossRef]
- Menge, B.A.; Lubchenco, J.; Ashkenas, L.R. Diversity, heterogeneity and consumer pressure in a tropical rocky intertidal community. Oecologia 1985, 65, 394–405. [Google Scholar] [CrossRef]
- Hull, S.L. Seasonal changes in diversity and abundance of ostracods on four species of intertidal algae with differing structural complexity. Mar. Ecol. Prog. Ser. 1997, 161, 71–82. [Google Scholar] [CrossRef]
- Bertness, M.D.; Leonard, G.H.; Levine, J.M.; Schmidt, P.R.; Ingraham, A.O. Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 1999, 80, 2711–2726. [Google Scholar] [CrossRef]
- Abbiati, M.; Bianchi, C.N.; Castelli, A. Polychaete vertical zonation along a littoral cliff in the west Mediterranean. Mar. Ecol. 1987, 8, 33–48. [Google Scholar] [CrossRef]
- Giangrande, A. Polychaete zonation and its relation to algal distribution down a vertical cliff in the western Mediterranean (Italy): A structural analysis. J. Exp. Mar. Bio. Ecol. 1988, 120, 263–276. [Google Scholar] [CrossRef]
- Chemello, R.; Milazzo, M. Effect of algal architecture on associated fauna: Some evidence from phytal molluscs. Mar. Biol. 2002, 140, 981–990. [Google Scholar] [CrossRef]
- Giangrande, A.; Delos, A.L.; Musco, L.; Licciano, M.; Pierri, C. Polychaete assemblages along a rocky shore on the South Adriatic coast (Mediterranean Sea). Cah. Biol. Mar. 2004, 45, 85–95. [Google Scholar]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Milazzo, M.; Chemello, R.; Badalamenti, F.; Riggio, S. Molluscan assemblages associated with photophilic algae in the Marine Reserve of Ustica Island (Lower Tyrrhenian Sea, Italy). Ital. J. Zool. 2000, 67, 287–295. [Google Scholar] [CrossRef]
- Russo, G.F.; Patti, F.P. Early life history of two closely related gastropods, Rissoa auriscalpium and Rissoa italiensis (Caenogastropoda: Rissoidae). Mar. Boil. 2005, 147, 429–437. [Google Scholar] [CrossRef]
- Donnarumma, L.; Sandulli, R.; Apolloni, L.; Sánchez-Lizaso, J.L.; Russo, G.F. Assessment of Structural and Functional Diversity of Mollusc Assemblages within Vermetid Bioconstructions. Diversity 2018, 10, 96. [Google Scholar] [CrossRef]
- Lolas, A.; Antoniadou, C.; Vafidis, D. Spatial variation of molluscan fauna associated with Cystoseira assemblages from a semi-enclosed gulf in the Aegean Sea. Reg. Stud. Mar. Sci. 2018, 19, 17–24. [Google Scholar] [CrossRef]
- Antoniadou, C.; Koutsoubas, D.; Chintiroglou, C.C. Mollusca fauna from infralittoral hard substrate assemblages in the North Aegean Sea. Belgian J. Zool. 2005, 135, 119–126. [Google Scholar]
- Urra, J.; Rueda, J.L.; Gofas, S.; Marina, P.; Salas, C. A species-rich molluscan assemblage in a coralligenous bottom of the Alboran Sea (south-western Mediterranean): Intra-annual changes and ecological considerations. J. Mar. Biol. Assoc. UK 2012, 92, 665–677. [Google Scholar] [CrossRef]
- Poursanidis, D.; Koutsoubas, D. A computerized database (CorMol) on the molluscan fauna from the Mediterranean reef ecosystems: Part I, the coralligenous formations. Quat. Int. 2015, 390, 29–43. [Google Scholar] [CrossRef]
- Donnarumma, L.; Sandulli, R.; Appolloni, L.; Russo, G.F. Assessing molluscs functional diversity within different coastal habitats of Mediterranean marine protected areas. Ecol. Quest. 2018, 29, 35–51. [Google Scholar]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Appolloni, L.; Bevilacqua, S.; Sbrescia, L.; Sandulli, R.; Terlizzi, A.; Russo, G.F. Does full protection count for the maintenance of β-diversity patterns in marine communities? Evidence from Mediterranean fish assemblages. Aquat. Conserv. 2017, 27, 828–838. [Google Scholar] [CrossRef]
- Buonocore, E.; Picone, F.; Donnarumma, L.; Russo, G.F.; Franzese, P.P. Modeling matter and energy flows in marine ecosystems using emergy and eco-exergy methods to account for natural capital value. Ecol. Modell. 2019, 392, 137–146. [Google Scholar] [CrossRef]
- Bonifazi, A.; Lezzi, M.; Ventura, D.; Lisco, S.; Cardone, F.; Gravina, M.F. Macrofaunal biodiversity associated with different developmental phases of a threatened Mediterranean Sabellaria alveolata (Linnaeus, 1767) reef. Mar. Environ. Res. 2019. [Google Scholar] [CrossRef]
- Piazzi, L.; Gennaro, P.; Montefalcone, M.; Bianchi, C.N.; Cecchi, E.; Morri, C.; Serena, F. STAR: An integrated and standardized procedure to evaluate the ecological status of coralligenous reefs. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 1–13. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Li, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2010. [Google Scholar]
- Dame, R.F. Ecology of Marine Bivalves: An Ecosystem Approach, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 283. [Google Scholar]
- Purchon, R.D. The Biology of the Mollusca, 2nd ed.; Pergamon Press Ltd.: Oxford, UK, 1977; p. 587. [Google Scholar]
- Gravina, M.F.; Cardone, F.; Bonifazi, A.; Bertrandino, M.S.; Chimienti, G.; Longo, C.; Marzano, C.N.; Moretti, M.; Lisco, S.; Moretti, V.; et al. Sabellaria spinulosa (Polychaeta, Annelida) reefs in the Mediterranean Sea: Habitat mapping, dynamics and associated fauna for conservation management. Estuar. Coast. Shelf Sci. 2018, 200, 248–257. [Google Scholar] [CrossRef]
- Lezzi, M.; Cardone, F.; Mikac, B.; Giangrande, A. Variation and ontogenetic changes of opercular paleae in a population of Sabellaria spinulosa (Polychaeta: Sabellaridae) from the South Adriatic Sea, with remarks on larval development. Sci. Mar. 2015, 79, 1–14. [Google Scholar]
- Lisco, S.; Moretti, M.; Moretti, V.; Cardone, F.; Corriero, G.; Longo, C. Sedimentological features of Sabellaria spinulosa bioconstructions. Mar. Pet. Geol. 2017, 87, 203–212. [Google Scholar] [CrossRef]
- La Porta, B.; Targusi, M.; Lattanzi, L.; La Valle, P.; Nicoletti, L. Analisi Della Fauna Associata Alle Biocostruzioni a Sabellaria alveolata (L.) in Relazione Al Loro Stato Di Conservazione. Biol. Mar. Mediterr. 2009, 16, 36–38. [Google Scholar]
- Ardizzone, G.D.; Gravina, M.F.; Belluscio, A. Temporal development of epibenthic communities on artificial reefs in the central Mediterranean Sea. Bullletin. Mar. Sci. 1989, 44, 592–608. [Google Scholar]
- Thiel, M.; Ullrich, N. Hard rock versus soft bottom: The fauna associated with intertidal mussel beds on hard bottoms along the coast of Chile, and considerations on the functional role of mussel beds. Helgol. Mar. Res. 2002, 56, 21–30. [Google Scholar] [CrossRef]
- Maggi, E.; Bertocci, I.; Vaselli, S.; Benedetti-Cecchi, L. Effects of changes in number, identity and abundance of habitat-forming species on assemblages of rocky seashores. Mar. Ecol. Prog. Ser. 2009, 381, 39–49. [Google Scholar] [CrossRef]
- Arribas, L.P.; Donnarumma, L.; Palomo, M.G.; Scrosati, R.A. Intertidal mussels as ecosystem engineers: Their associated invertebrate biodiversity under contrasting wave exposures. Mar. Biodivers. 2014, 44, 203–211. [Google Scholar] [CrossRef]
- Tongiorgi, P.; Nardi, P.; Galleni, L.; Nigro, M.; Salghetti, U. Feeding habits of Ocinebrina edwardsi (Mollusca: Prosobranchia) a common mussel drill of the Italian coasts. Mar. Ecol. 1981, 2, 169–180. [Google Scholar] [CrossRef]
- Huston, M. A general hypothesis of species diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Gili, J.M.; Coma, R. Benthic suspension feeders: Their paramount role in littoral marine food webs. Trends Ecol. Evol. 1998, 13, 316–321. [Google Scholar] [CrossRef]
- Hrs-Brenko, M.; Legac, M. Inter-and intra-species relationships of sessile bivalves on the eastern coast of the Adriatic. Sea Nat. Croat. 2006, 15, 203–230. [Google Scholar]
- Schiaparelli, S.; Franci, G.; Albertelli, G.; Cattaneo-Vietti, R. A non destructive method to evaluate population stucture and bioerosion activity of the boring bivalve Gastrochaena dubia. J. Coast. Res. 2005, 21, 383–386. [Google Scholar] [CrossRef]
- Casoli, E.; Ricci, S.; Antonelli, F.; Perasso, S.C.; Belluscio, A.; Ardizzone, G.D. Impact and colonization dynamics of the bivalve Rocellaria dubia on limestone experimental panels in the submerged Roman city of Baiae (Naples, Italy). Int. Biodeter. Biodegr. 2016, 108, 9–15. [Google Scholar] [CrossRef]
- Fava, F.; Ponti, M.; Abbiati, M. Role of recruitment processes in structuring coralligenous benthic assemblages in the northern Adriatic continental shelf. PLoS ONE 2016, 11, e0163494. [Google Scholar] [CrossRef]
- Safriel, U.N.; Ben Eliahu, M.N. The influence of habitat structure and environmental stability on the species diversity of polychaetes in vermetid reefs. In Habitat Structure. The Physical Arrangement of Objects in Space; Bell, S.S., McCoy, E.D., Mushinsky, H.R., Eds.; Chapman and Hall, Ltd.: London, UK, 1991; pp. 349–369. [Google Scholar] [CrossRef]
- Cocito, S. Bioconstruction and biodiversity: Their mutual influence. Sci. Mar. 2004, 68, 137–144. [Google Scholar] [CrossRef]
- Kelly, N.; Metaxas, A. Diversity of invertebrate colonists on simple and complex substrates at hydrothermal vents on the Juan de Fuca Ridge. Aquat. Biol. 2008, 3, 271–281. [Google Scholar] [CrossRef]
- Terlizzi, A.; Scuderi, D.; Fraschetti, S.; Guidetti, P.; Boero, F. Molluscs on subtidal cliffs: Patterns of spatial distribution. J. Mar. Biol. Assoc. UK 2003, 83, 165–172. [Google Scholar] [CrossRef]
- Bode, A. Reproduction of the intertidal chiton Acantochitona crinita within a community of Corallina elongata (Rhodophyta). J. Moll. Stud. 1989, 55, 37–44. [Google Scholar] [CrossRef]
- Öztürk, B.; Ergen, Z.; Önen, M. Polyplacophora (Mollusca) from the Aegean coast of Turkey. Zool. Middle East. 2000, 20, 69–76. [Google Scholar] [CrossRef]
- Hong, J.S. Contribution à l’étude des peuplements d’un fond de concrétionnement coralligène dans la région marseillaise en Mediterranée Nord-occidentale. Bull. KORDI 1982, 4, 27–51. [Google Scholar]
- Boudouresque, C.F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Trav. Sci. Parc. Natl. Port-Cros. 2004, 20, 97–146. [Google Scholar]
- Zaitsev, Y. An Introduction to the Black Sea Ecology; Odessa: Smil Editing & Publishing Agency Ltd.: Istanbul, Turkey, 2008; p. 228. [Google Scholar]
- Relini, G.; Giaccone, G. Gli habitat prioritari del protocollo SPA/BIO (Convenzione di Barcellona) presenti in Italia. Schede descrittive per l’identificazione. Biol. Mar. Mediterr. 2009, 16, 1–372. [Google Scholar]
- Chintiroglou, C.; Koukouras, A. A populatrion of the Sea Anemone Anemonia viridis (Forskal, 1775) and its associated flora and fauna, in north Aegean Sea. Int. Rev. Ges. Hydrobiol. 1992, 77, 483–495. [Google Scholar] [CrossRef]
- Cosentino, A.; Giacobbe, S. Mollusc assemblages of hard bottom subtidal fringe: A comparison between two coastal typologies. Biodivers. J. 2015, 6, 353–364. [Google Scholar] [CrossRef]
- Chemello, R.; Russo, G.F. The molluscan taxocoene of photophilic algae from the Island of Lampedusa (Strait of Sicily, southern Mediterranean). Boll. Malacol. 1997, 33, 95–104. [Google Scholar]
- Torres, A.C.; Veiga, P.; Rubal, M.; Sousa-Pinto, I. The role of annual macroalgal morphology in driving its epifaunal assemblages. J. Exp. Mar. Bio. Ecol. 2015, 464, 96–106. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).