2. Materials and Methods
The methods used to apply biocide to control or eradicate IAC populations depend on a wide range of factors, including waterbody bathymetry, habitat complexity, water chemistry (temperature and pH) and time of year. The presence of species of high conservation concern, or of commercial value, may also determine whether treatment should go ahead. Combined, this means that many potential constraints will affect any project being considered and the methods used may differ between sites where projects proceed to biocide treatment. This study reviews attempts to treat a number of IAC populations in northern Europe, identifying the factors used to assess feasibility, effectiveness of treatment and overall success.
2.1. Reasons for Treatments
Thirteen potential projects in the U.K. were assessed, of which six progressed to treatment. Three projects were carried out in Sweden and two in Norway. The projects were initiated by different decision-makers in response to local detection of IAC at different times (
Table 1 and
Table 2), so were not a preplanned group of research sites. The reasons for treatment differed between countries and projects; however, the aim was complete eradication of the population in every case.
Scotland has no native crayfish. The main reason for the proposed eradication treatments was to protect catchments with high value for nature conservation and commercially important populations of salmonid fish. In England, spread of the signal crayfish to most major river catchments has led to local extinction of the native white-clawed crayfish
Austropotamobius pallipes (Lereboullet, 1858) in many catchments [
8]. The proposed eradication treatments were to protect the remaining populations of white-clawed crayfish and other species with high value for nature conservation.
In Sweden, signal crayfish were first introduced in the 1960s to improve crayfish fisheries [
33], triggering outbreaks of crayfish plague [
34]. Signal crayfish had become very widely distributed throughout southern Sweden by the mid-2000s. In order to conserve the noble crayfish
Astacus astacus (Linnaeus, 1758), the whole island of Gotland was declared as a specially protected area by the County Administrative Board. Signal crayfish eradication treatments were carried out to reinstate Gotland as an island free from IAC and so safeguard the native crayfish there. In Norway, there were no records of signal crayfish until 2006 [
35], but the species was already listed in the Norwegian black list, a government priority list of species to be targeted for rapid eradication at any sites where they were found [
36].
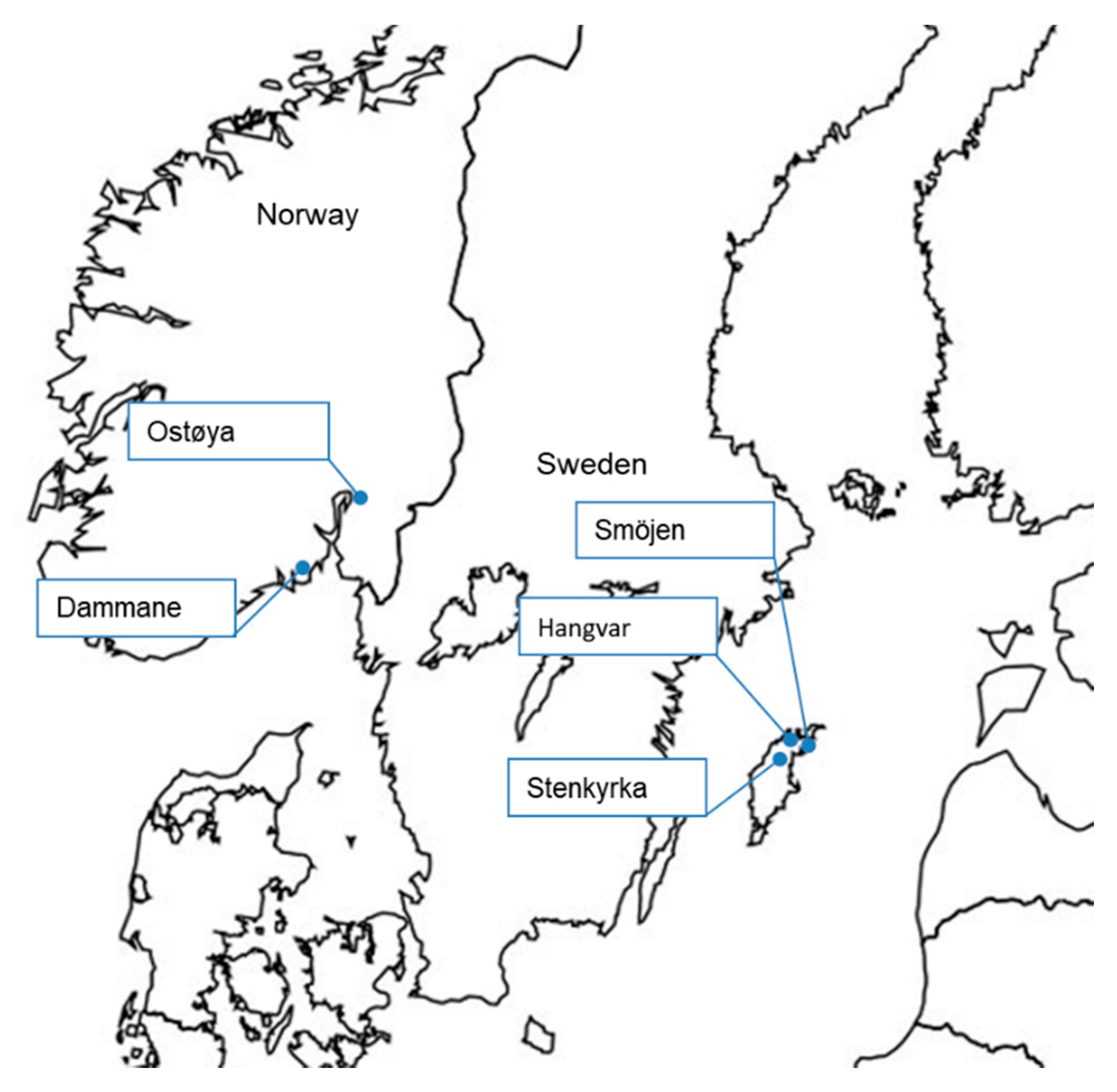
Table 1 summarises the project sites where biocide treatment was carried out and the reasons why they were chosen for treatment (see
Figure 1 and
Figure 2 for locations). Additional details of sites and treatments are given in
Table 3 below (see also
Supplementary Tables S2–S4). Projects in the U.K. where biocide treatment was considered but not carried out are included in this study (see
Table 2), because many of the problems encountered on those were shared with other projects that proceeded to biocide treatment.
2.2. Stages of the Projects
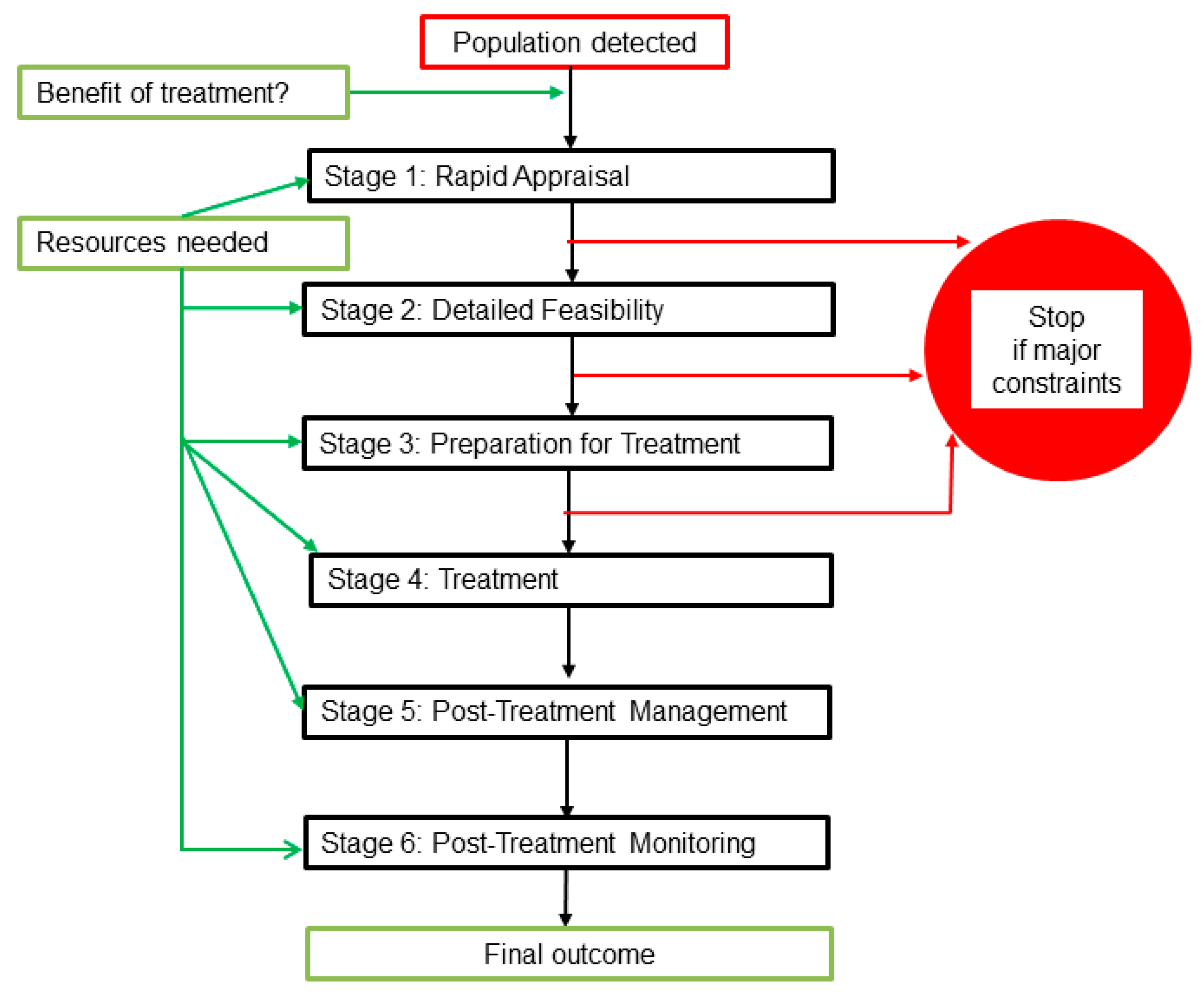
Each biocide treatment project can be considered as a six-stage process as follows: 1. an initial rapid appraisal; 2. detailed feasibility assessment; 3. preparations on site; 4. treatment; 5. post-treatment management, and 6. monitoring of the outcome in subsequent years. The stages are outlined below and additional details of work at each stage are provided in
Supplementary Table S1. The work-flow is summarised in
Figure 3, which also indicates when funding and other resources are required. It shows stop points if there are major constraints. Constraints are discussed further in
Section 2.3 and
Section 3.3, but, in summary, major constraints are factors that would prevent effective eradication treatment.
The methods were similar in all of the biocide treatment projects, with variations applied depending on individual sites and the lessons learned from preceding projects. All of the projects described here involved treatment of one or more ponds, with or without inflows and outflows. Individual sites and their treatments are summarised in
Table 3, with additional details given in
Supplementary Table S2 (U.K.) and
Table S3 (Norway and Sweden).
2.2.1. Stage 1: Rapid Appraisal
Stage 1 was a rapid appraisal to ascertain the scale of the problem and screen out projects that were too difficult to justify treatment. Any sites that were very large were quickly screened out. For these early projects, sites up to about 2 ha were considered. Three sites in Norway that were discovered to have signal crayfish after 2008 were all deep waterbodies many square kilometres in area. It was clear those were large and hence not feasible for biocide treatment; thus, they are not detailed further. Stage 1 also identified inflows or outflows that would need to be controlled during treatment, connected waterbodies and the degree of habitat complexity in the sites.
Detection of crayfish was important in determining the extent of required treatment. As well as considering whether the known location of the population could be treated, the appraisals needed to assess areas where the population could be present, undetected, at low density and hence require treatment. Two factors affecting the likelihood of detecting a population were the time since the introduction and the limits of detection by survey methods. Signal crayfish mainly hide in refuges by day, to avoid predation, so surveys are limited by the type, extent and the accessibility of refuges to physical search, or to the likelihood of crayfish entering baited traps.
Studies have shown that signal crayfish reach sexual maturity in one to three years [
40], although it may take two to six years in a cold climate [
41]. A breeding size of about 25 mm carapace length (CL) or more is reached most quickly in low density, developing populations. Signal crayfish populations have become established from low numbers of founders. In three of the projects in the U.K., the individuals responsible for the introductions of signal crayfish said they released a few 10s to a few 100s of crayfish, a single bucketful. This means a population may be difficult to detect during establishment, but the likelihood of detection increases over time and with increasing survey effort. At two of the U.K. sites (sites 2 and 3) the presence of signal crayfish was detected three years after the introduction, using only 10 trap-nights at each site.
After five years, a new population of signal crayfish would have undergone five reproductive seasons and would probably have produced two or more generations of breeding age, which would be large enough to catch in traps. Traps with a mesh size of 10 mm or less are capable of catching crayfish less than 30 mm CL [
42,
43]. A population would be expected to be detectable in a survey by trapping within five years if the site was relatively small and/or there was wide coverage of the site.
Any small inflows and outflows also needed to be assessed for their potential to support crayfish, including those too shallow for surveys by trapping. Manual survey methods [
43] are capable of detecting crayfish of smaller sizes and at a lower abundance than trapping, but only in clear shallow waterbodies with a stony substrate suitable for hand-searching or netting.
A precautionary approach was taken in determining the likely limits of population, especially upstream. As well as being important for determining the area to be treated, detection of crayfish at a low abundance was also important in determining the final outcome through post-treatment monitoring (stage 6 below).
2.2.2. Stage 2: Detailed Feasibility
Stage 2 was a more detailed feasibility study, the scope of which depended on the Stage 1 rapid appraisal. The focus in each case was on investigation of the physical area to be treated, identifying and assessing the risks. This included any environmental risks to non-target areas and the risk from any uncertainties about population distribution on the likelihood of achieving eradication. It included engaging with local stakeholders, especially landowners and occupiers and statutory authorities, securing resources for the project and carrying out project operational planning, where this stage indicated the treatment was technically feasible. At this stage, the lead government agency or agencies took the decision on whether or not to carry out the treatment.
Stage 2 included choosing a biocide for potentially feasible projects. Biocide treatments were carried out in the U.K. using natural pyrethrum (as Pyblast
®), after a preliminary study tested chlorine, ammonia, high pH, chemical deoxygenation and natural pyrethrum (at site 4) [
44]. A dosage of 50 µg/L (in pond water at pH 8.2) natural pyrethrum was the minimum to achieve 100% mortality of crayfish within 24 h. Subsequent tests ([
10] and unpublished data) showed that the presence of clay or silt in the water led to reduced toxicity and slower mortality, necessitating higher dosages in field conditions.
Synthetic pyrethroid insecticides are more toxic to crayfish [
30] and are more stable than natural pyrethrum in field conditions [
45]. Synthetic pyrethroids were not authorised for the projects in the U.K. because of previous pollution incidents with synthetic pyrethroid insecticides from careless use of sheep-dips. A precedent for use of a biocide treatment in Norwegian waterbodies had already been established [
46,
47]. The cypermethrin-based BETAMAX VET
® was used because this pharmaceutical product was already widely used as a chemo-therapeutant in Norway to treat infestations of the salmon louse
Lepeophtherius salmonis (Kroyer, 1837), a crustacean parasite on farmed Atlantic salmon (
Salmo salar (Linnaeus, 1758)). The projects on signal crayfish were an experimental extension of its use against another crustacean. In Sweden, deltamethrin was chosen, after some discussion with the environmental authorities, as a less expensive biocide than natural pyrethrum for use in large volumes of water, which would still break down fairly rapidly.
Stage 2 (or in some cases stage 3), included on-site toxicity tests using the water and substrate to be treated and signal crayfish obtained from the site. Relevant approvals or consents were obtained for experimental tests and for treatments according to national requirements. The target dosage for the waterbodies had to be high enough to make it likely that the water would achieve a concentration that would produce 100% mortality in crayfish, in field conditions (sun, vegetation and other organic matter, turbidity, temperature, etc.), in the entire treated population. At the dosage required in field conditions, the biocides were toxic to other aquatic invertebrates and fish (i.e., likely impacts on non-target species). It was desirable to minimize the quantity of biocide used to reduce the recovery time and minimize the cost of the chemical, but it was essential to avoid under-dosing. The aim of each of these projects was to achieve complete eradication. The decision-makers for the individual projects considered it important to minimize the likelihood of needing an unplanned retreatment, which would require additional resources of people, materials, time and funding.
Table 3 summarises the physical characteristics of the sites treated, the target dosages of biocides and whether hydraulic control of inflows or outflows was required.
2.2.3. Stage 3: Preparation for Treatment
On-site preparation for treatment included advance works, such as the installation of any required fencing or notices; setting up a site base or other local facilities, e.g., for storage of the biocide, other materials and equipment, and for carrying out bioassays or other biomonitoring during treatment; and temporary or permanent controls on inflows or outflows, if needed, and reduction of water level if used. It also included management of vegetation at some sites to facilitate access for the application of biocide (sites 2 and 5). Fish were removed in advance as required. Natural pyrethrum was also toxic to amphibian larvae at the dosages used, but the short-term impact of biocide treatment was mitigated by carrying out treatments after the amphibian breeding season, when most of the population would be in terrestrial habitats. Materials were also obtained in advance, including crayfish for use in monitoring cages during treatment (see stage 4 below).
Because of the difficulties of observing crayfish in a waterbody during the biocide treatment, the projects generally used caged sentinel crayfish to monitor the effectiveness of treatment during the operation. Before the start of application of biocide in stage 4, cages of crayfish were lowered to the bed of the waterbodies. Crayfish in artificial burrows were also used to monitor treatment (e.g., at site 5).
2.2.4. Stage 4: Treatment
The treatment at each site comprised application of pre-diluted biocide to the surface of each waterbody from a boat, the banks or a combination of both. Care was taken to ensure complete coverage of all areas, including the margins and any small inflows or outflows. Each waterbody was treated in its entirety on one day. There were some differences between projects in the way the biocide was applied, with methods used being mainly low-volume application of the biocide with a sprayer, or high-volume application by pump, plus a drip-feed of biocide to small inflows in some cases.
Supplementary Tables S2 and S3 provide additional details on application methods at individual sites.
Table 3 shows the target dosages and whether hydraulic control was used to prevent leakage of treated water. Sites where hydraulic control was used had biomonitoring downstream to check that there was no pollution outside the treated area. Mesh bags containing aquatic invertebrates were deployed downstream and were inspected at intervals during treatment.
The most complex treatment was 680 m of a small watercourse (site 5). This required continuous hydraulic control to prevent any leakage of treated water during and after treatment. Treatment was carried out in each of five sections in succession downstream. Each section was dammed at both ends and clean flow was diverted around it. Additional sandbags were used to create small steps in the section to ensure undercut banks were fully inundated. The section under treatment was sprayed with biocide, while flow was recirculated continuously by pumping for about four hours. Treated water was then pumped out and the section of stream was then continuously flushed with clean water and dewatered by pumping onto adjacent pasture until bioassays confirmed it was no longer toxic. Treatment progressed to subsequent sections during the flushing phase. The dosage rate in the stream sections was increased to 1 mg/L because treatment was for only four hours in recirculation. In one of the preliminary tests, adult crayfish were given a short exposure time of 15 min or two hours before being rinsed and put in clean water. A few crayfish survived 15 min exposure to 1 mg/L natural pyrethrum (affected by it, but recovered), whereas two hours exposure was lethal (Peay, personal observation).
In all of the projects, monitoring progress of the treatment using caged crayfish enabled a supplementary application to be carried out on the day after the initial application if necessary (site 6). Cages were lifted for inspection after completion of the biocide application and inspected at intervals to check for mortality. If any cages had crayfish that were unaffected on the day after treatment, it indicated that an area required better coverage with biocide (e.g., as at site 6 where active crayfish were seen in one of the cages in the deepest part of the quarry a few hours after the application).
In five of the projects, there was a second treatment with biocide after the first treatment had finished (U.K.: sites 1 and 2; Sweden: Smöjen, and Norway: both sites). At two sites, this was because survivors were detected (sites 1 and 2). Those two sites had a pre-treatment with sodium sulphite to chemically deoxygenate the water. The pre-treatment was to encourage crayfish to leave refuges and so be more readily exposed to the natural pyrethrum. In practice, residual sodium sulphite partially degraded the natural pyrethrum, reducing its effectiveness. Retreatment of the sites and subsequent treatments were carried out without preliminary deoxygenation [
14]. In Norway, the sites were given two biocide treatments, with a two-week interval, as a precaution. Those ponds were then partially drained for one winter before they were allowed to refill [
38,
39,
48]. In Sweden, the site Smöjen was given two treatments with a three-month interval, as a precaution, since the limestone bedrock had many crevices as potential refuges for crayfish.
2.2.5. Stage 5: Post-Treatment Management
Stage 5 was the management during the recovery period (i.e., the period until the treated water was no longer toxic to aquatic invertebrates). In the U.K. projects, the reduction in toxicity after completion of treatment was recorded using bioassays with freshwater shrimps Gammarus pulex (Linnaeus, 1758). In Sweden, the decrease in concentration of the biocide over time was determined by chemical analysis and by testing with sentinel cages of noble crayfish in the waterbody. In Norway, the recovery of the water was not recorded in any detail, because the ponds were partially drained before winter. At ponds with an outflow, it was necessary to prevent any discharge from the treated area until the biocide had degraded sufficiently to avoid adverse effects in untreated areas downstream. Where ponds were dewatered (site 5), there was the opportunity to search the exposed bed for any live crayfish. In one of the projects (site 5), a previously channelised 200-m length of watercourse was excavated after the treatment and the material removed was thoroughly searched for any live or dead crayfish.
The projects were carried out in accordance with risk assessments for health, safety and environment. Mitigation measures were included to avoid or minimise the risks of impacts outside the areas intended for treatment; for example, at sites with hydraulic control, contingency measures were put in place, such as additional pumps on stand-by, in case there was an increase in flow due to rainfall. Treatments and recovery periods were completed without causing pollution in untreated areas.
The three sites in Sweden were stocked with native crayfish two months to one year after treatment. One of the sites in Norway (Ostøya) was stocked with fish, as were two of the U.K. sites (sites 3 and 5).
2.2.6. Stage 6: Post-Treatment Monitoring to Determine the Outcome
The outcome of each biocide treatment was determined by a monitoring programme. The minimum was five years of annual post-treatment monitoring using extensive coverage of the site with baited crayfish traps, in some cases supplemented by additional methods. Five years without any detected presence of signal crayfish was selected as the threshold for successful eradication of a population of signal crayfish. As described in stage 1 above, it is likely that a recovering population could be detected within this time. Post-treatment monitoring was extended in Sweden and Norway to seven and six years, respectively. This was to increase the confidence in using five years without any crayfish detected as the criterion for successful eradication and to take account of the slower maturation rates in Scandinavia.
Monitoring annually after treatment enabled any failure to eradicate the population to be detected as soon as possible. If any crayfish were caught, a decision could be made quickly as to whether to carry out another session of treatment.
An intensive survey effort increased the probability of detecting of a population at a low abundance. The effective range of individual baited crayfish traps in ponds and lakes has been estimated at approximately 12.5 m
2 by Abrahamsson and Goldman [
49] and at 56.3 m
2 by Acosta and Perry [
50] in a mark recapture trial in a controlled grid. In the first five U.K. projects, the approach was to use many traps in a single trapping session. Trap coverage of sites across all post-treatment years averaged one trap per 64 m
2 (standard error (SE) ± 4.8,
n = 32). This is less than 100% coverage in individual trapping sessions, but likely to detect crayfish in repeated annual monitoring of an increasing population. Any crayfish recorded would show that eradication had not been achieved. Traps were not deployed on an entirely uniform grid at each site. Distribution was skewed where necessary to ensure good coverage of areas with potential refuges for crayfish, especially near steep banks, with reduced cover in areas with a flat, fine substrate. At site 6, 15 traps were set and lifted daily at locations around the site for a total of 200 trap nights per year. The trapping effort used in the Norwegian ponds was similarly intensive, at one trap per 16 m
2. During trapping at site 1, a note was made of the by-catch of amphibians, and other ad hoc observations of changes in conditions were recorded.
As well as the absence of signal crayfish in trap catches, an additional requirement in Norway was that no crayfish plague should be detected in sentinel native noble crayfish during the last three years of monitoring. Water samples were also taken annually using the filtration method of Strand et al. [
51] and tested for the presence of spores of crayfish plague as a potential indicator of signal crayfish, the carriers of the disease. In Sweden, trapping to confirm the presence of restocked noble crayfish was taken to be a likely indicator of absence of crayfish plague and hence absence of signal crayfish.
2.3. Assessment of Constraints on Projects
The 13 biocide projects in the U.K. (six of them treated, seven not treated) had a range of constraints that were encountered by the project teams during the appraisal and feasibility assessments (stages 1 and 2). The constraints were discussed and noted during the decision-making process in each project. After the projects had either progressed to treatment or were abandoned, the constraints for all the projects were classified by type and their relative influence on the decision-making was given a qualitative rating. There were not enough details available for this study to include constraints on the projects in Norway and Sweden in the same analysis.
Eleven types of constraint were identified, related to the environment, the benefits to be achieved from the project and issues of stakeholder acceptance and project resourcing (
Table 4). There were interactions between constraints, because the environmental categories in combination affected the likelihood of being able to treat the whole population and this influenced the views of stakeholders about the project and its benefits, but interactions were not analysed separately. Constraints were given a qualitative ranking according to their importance, or impact, on the project, on the following scale:
Minor, a matter for project planning and action, but not likely to prevent or significantly constrain the project;
Moderate, a significant constraint causing increased technical difficulty, cost and/or delay, and
Major, sufficient to stop a project on its own.
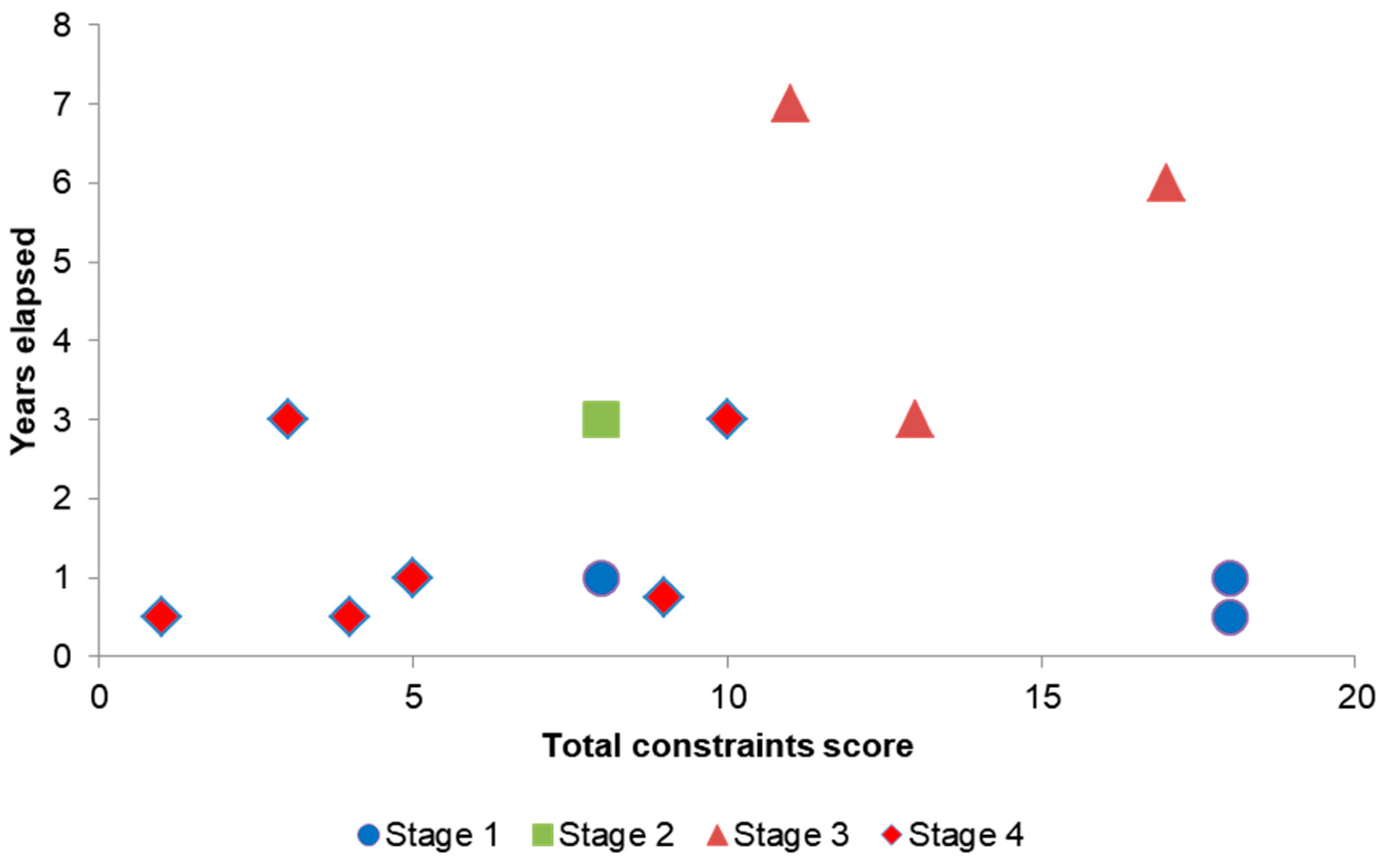
Table 5 shows the constraints on the individual projects. The constraint scores for each project were summed and the projects were ranked. The progress achieved on each project was scored according to the stage reached (1 = rapid appraisal, 2 = detailed feasibility, 3 = preparations for treatment, 4 = biocide treatment). A Spearman rank correlation test was used to determine whether there was a correlation between the total constraint score and the stage a project reached. In addition, a Spearman rank correlation test was used to test for a correlation between the time elapsed in years between first detection of signal crayfish and an appraisal (stage 1) being carried out and the time until a project either progressed to biocide treatment (stage 4) or was abandoned at an earlier stage.
4. Recommendations
Using experience gained from the projects, recommendations are given below to help those considering carrying out a biocide treatment against IAC, especially for initial appraisal and feasibility studies. Details of sites and methods of work need to be considered as early as possible in order to determine the benefits, risks, feasibility and costs of work.
4.1. Is It Worth Considering Carrying Out a Biocide Treatment Against Invasive Crayfish?
Before considering whether to make any intervention, it is worthwhile considering the benefits of doing so. The E.U. Regulation on Invasive Alien Species (Regulation No. 1143/2014) is a potential driver for future rapid eradication treatments in Europe. Prevention is the highest priority, but rapid eradication is the next intervention to be considered in the hierarchy of action. This does not mean that member states are required to eradicate IAC, and, in many states in Europe, including the U.K. and Sweden, populations are already too extensive for eradication from the state as a whole. There are still benefits in considering the issue at other scales, especially at catchment scale.
Table 8 recommends priorities for undertaking eradication projects based on the existing status of IAC. It is worth reiterating that the biocides available are not selective for crayfish, which means that there will be a temporary loss of invertebrates within the treated areas, plus a loss of amphibians and fish if any are present at the start of treatment. Where the benefits of successful eradication are high, it is more likely that the cost of treatment and its localised, recoverable environmental impacts will be accepted by stakeholders than if the benefits will be lost soon due to invasion from other sites.
For each year that a successful biocide treatment (or other intervention) prevents invasion, an annual increment of environmental impact is avoided. A cost–benefit model of biocide treatment, developed for the North Esk (sites 1–3) [
68] (pp. 179–219), used the fish restocking cost as a surrogate environmental cost to assign a value to the potential environmental impact of IAC per kilometre of invaded watercourse, annually and cumulatively. The cost of uncontrolled invasion over time was compared with the outlay cost of biocide treatment. Even when modelled with a low unit cost of environmental impact, the cost of a successful rapid eradication was calculated as being less than the impact cost within seven years.
It is recommended that regulatory authorities, or others responsible for strategy on IAS, consider priorities for a rapid eradication response, nationally and regionally, to help secure authorisations and funding quickly for priority cases when they arise.
4.2. Is There An Alternative to Use of Biocide for IAC?
Biocide treatment of IAC is intended for localised, rapid eradication. Current biocides have too much impact on non-target fauna (invertebrates, amphibians and fish) to be suitable as a recurrent control measure, or at sites where there is no reasonable likelihood of achieving eradication. Few of the projects in this study had detailed surveys of fauna and flora before and after treatment and not over the full period of monitoring for outcome. This would be worth doing in any future projects, provided that collection of baseline data did not delay treatment.
It is to be hoped that future research will find low-cost, effective eradication or control measures, with acceptably low environmental impacts, which are suitable for extensively invaded areas. In the meantime, the only confirmed successful eradications of IAC have been with biocides, or complete habitat destruction (infilling). Infilling has greater environmental impacts within sites than biocide treatment, due to the habitat loss, but the methods required are used throughout the construction industry and could be considered for small urban ponds. The cost relative to biocide treatment would depend on the volume and the cost of materials.
4.3. Is Eradication with Biocide Feasible?
The key questions are firstly, what is the extent of the population and secondly, could all of that population be treated? After the first report of a population of an IAC, some assumptions will need to be made about the likely limits of population. Recent progress in eDNA sampling [
69,
70,
71] means that this is a potentially useful tool for rapid appraisal, and specific assays for signal crayfish have already been developed (e.g., [
72]). There is uncertainty about thresholds for detection at a low abundance in different habitats, but quickly determining the detectable range of population using eDNA sampling would be a useful addition to existing survey methods.
Whether to treat additional areas where it is uncertain whether crayfish are present will have to be decided on a case-by-case basis. The maximum range upstream in any inflows to a pond is critical, because if any crayfish are untreated they will readily reinvade treated areas downstream. There is also potential for untreated crayfish downstream to reinvade. There is a much greater likelihood of being able to install temporary or permanent physical barriers to the upstream movement of crayfish than downstream. In the event that treatment has to be done in two phases, it should be done from upstream to downstream. Such factors as time since introduction, population density and habitat will have to be taken into account. Expansion of range is usually low during establishment, but more rapid thereafter. If crayfish have already reached a watercourse too large to treat, eradication is no longer feasible.
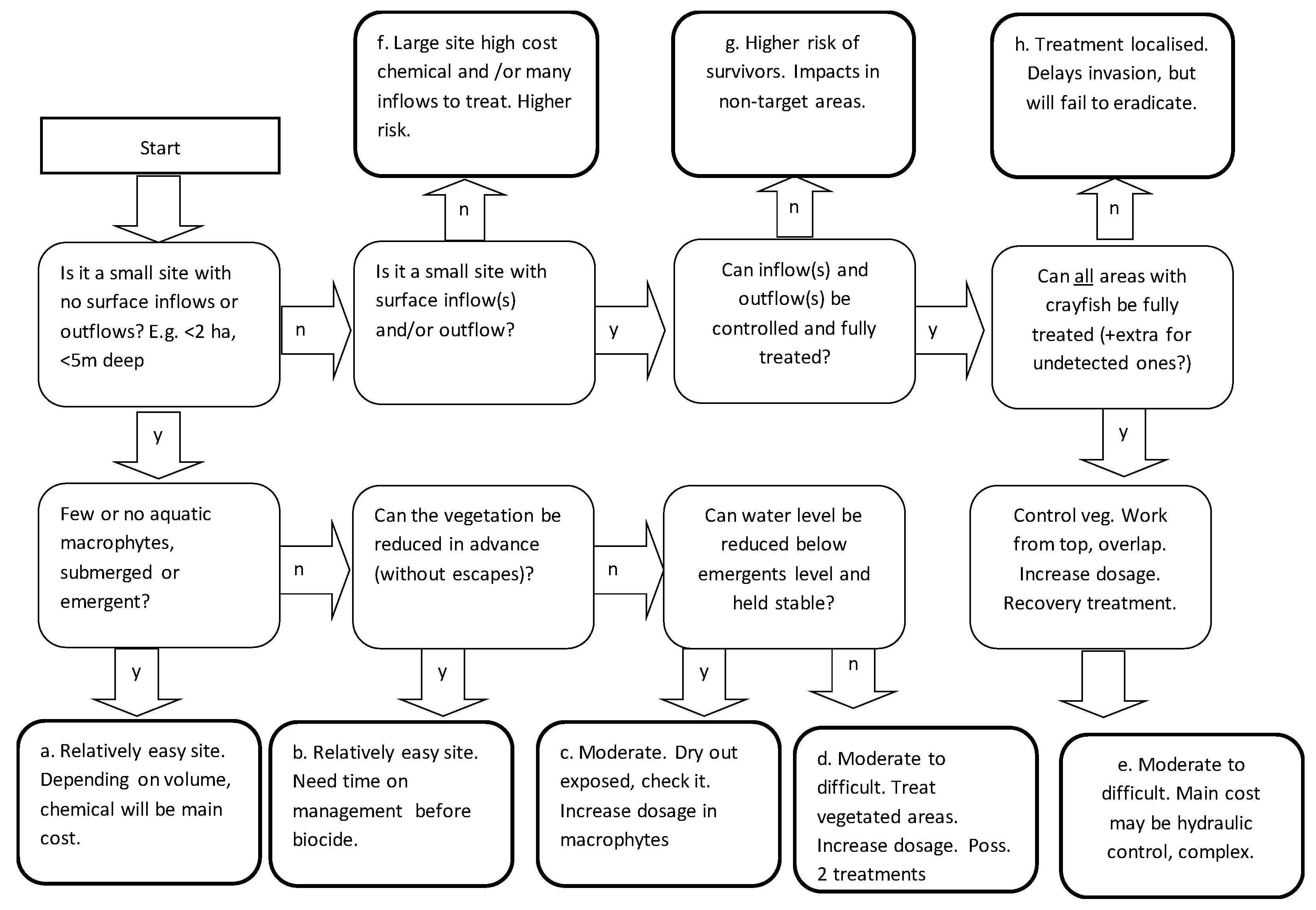
To help assess possible future projects, a decision tree (
Figure 5) has been developed for a preliminary appraisal of the feasibility of treatment of signal crayfish at a site where a population has been newly detected. It takes into account such factors as site size and habitat complexity. From the Start in the
Figure 5, making choices (y = yes, n = no) leads to summaries of risks and the potential for biocide treatment. Types a to c would be relatively straightforward. Types d to e would be more complex, but could still be successful, depending on the site. Types f and g would have greater risks of failure to achieve eradication and/or have high costs associated with them. Type h would not achieve eradication.
Securing the cooperation of all of the relevant stakeholders is essential, and if not achieved in time may prevent timely treatment. Agreement in principle should be sought from landowners and other stakeholders during the appraisal stage and reinforced as necessary during the feasibility stage, so that everyone is clear about the programme, the operations involved and the risks (environmental and operational), including any contingency measures.
To help secure cooperation of landowners in the eradication or control of priority invasive alien species, regulations are being introduced as part of the implementation of the E.U. Regulation on Invasive Alien Species (EEC/1143/2014). An example of this is the Wildlife and Natural Environment (Scotland) Act 2011, which gives statutory authorities the option of legal enforcement, if voluntary cooperation is not forthcoming.
4.4. Which Biocide and How Much to Use?
When considering which biocide to use, recommendations based on this study are as follows:
1. for a pond where outflow can be controlled without pumping: synthetic pyrethroid cypermethrin, or deltamethrin, on grounds of higher toxicity to crayfish and hence a low cost of biocide per unit volume treated (although natural pyrethrum is more readily degraded and the recovery time is shorter, hence it may be preferable for some sites).
2. for a pond with leakage: natural pyrethrum (a recovery period of one week to four months), or possibly a synthetic pyrethroid (if the risks of pollution outside the target area are manageable).
3. for a small watercourse controlled by recirculation: natural pyrethrum (a recovery period of 4–11 days with flushing). The use of a synthetic pyrethroid may be a suitable option in some cases (e.g., on a muddy site, or with post-treatment management to accelerate recovery, e.g., dewatering, or offsite disposal of treated water).
When deciding on the quantity of biocide to be applied, toxicity tests under site conditions are recommended prior to field-scale treatment, because the minimum dosage for 100% mortality may be significantly greater than in laboratory acute toxicity tests. Further allowance needs to be made for variable rates of environmental degradation of the biocide during treatment (influenced by water chemistry, temperature, sunlight, substrate, aquatic plants, organic matter, including plankton, and other suspended solids in the water). The half-life of both natural pyrethrum and synthetic pyrethroids can be less than 24 h and may be reduced to only a few hours [
29].
Natural pyrethrum may have a reduced likelihood of success if the site has a lot of burrows, which need to be fully submerged to be treated. Based on the limited number of studies so far, it is unlikely that eradication of red swamp crayfish would be achieved by natural pyrethrum alone. Destruction of burrows would also be needed.
The approach described for signal crayfish may be suitable for other IAC species with similar habitats and behaviour. There are likely to be some differences in the susceptibility of different crayfish species, as well as between biocides. In all cases, however, there will be a need for field target doses to take account of the variations in environmental conditions on the site, the method of product application, its rate of degradation and the need to fully expose all IAC to treatment.
4.5. Case for Action
When attempting to control a biological invasion, other authors have recommended considering the ease with which the species can be detected and targeted, the risks associated with management action, the likelihood of success and the extent of public concern and stakeholder involvement [
73]. Other factors mentioned include funding for the whole programme, prevention of reinvasion and awareness of possible management requirements after treatment [
74]. This study shows how such factors operate in combination when attempting to eradicate populations of invasive alien crayfish.
In this study, a delay of three or more years meant a project probably had too many difficulties to proceed. Biocide treatment within two years from detection is recommended and within one year if possible, with rapid treatment being most critical in sites with inflows or outflows.
The successful biocide treatments against signal crayfish at site-scale are encouraging, with success in eight of eleven treatments (c. 70%). There is always some uncertainty about the future success of an eradication treatment and the benefits are not guaranteed. In many cases, perhaps the majority of cases, by the time a population is detected it is already too widespread for effective eradication treatment.
Where a rapid eradication treatment is potentially feasible, the precautionary approach is to make use of the limited ‘window of opportunity’ for rapid eradication. Postponing a decision, or starting by using methods that are only likely to partially reduce the population, is likely to have irreversible consequences. It can be predicted with confidence that once a population of invasive alien crayfish is established and beyond the feasible range of rapid eradication treatment, the population will expand until it occupies all accessible areas of suitable habitat in the catchment, bringing with it the associated impacts on aquatic ecosystems. Alas, this is an all too common theme with aquatic invaders.