Abstract
Signal crayfish, as an invasive alien species in Europe, have caused impacts on aquatic communities and losses of native crayfish. Eradication of recently established populations may be possible in small ponds (<2.5 ha) and short lengths of small watercourses using a nonselective biocide. Between 2004 and 2012, a total of 13 sites in the U.K. were assessed for suitability. Six were treated with natural pyrethrum and crayfish were successfully eradicated from three. In Norway, five sites were assessed and two sites were treated with a synthetic pyrethroid, cypermethrin, both successfully. In Sweden, three sites were treated with another synthetic pyrethroid, deltamethrin, all successfully. Defining the likely extent of population was critical in determining the feasibility of treatment, as well as the ability to treat the whole population effectively. Important constraints on projects included site size, habitat complexity, environmental risks, cooperation of landowners and funding availability. Successful projects were manageably small, had good project leadership, had cooperation from stakeholders, had access to resources and were carried out within one to three years. Factors influencing success included treating beyond the likely maximum geographical extent of the population and taking care to dose the treated area thoroughly (open water, plus the banks, margins, inflows and outflows). Recommendations are given on assessing the feasibility of biocide treatments and project-planning.
1. Introduction
1.1. Biological Invasions and Eradication
The number of biological invasions that have economic and or environmental impacts is increasing annually and there is a growing need to manage them [1,2]. This is especially so in aquatic ecosystems, because of the impacts that invasive alien species can have on communities and ecosystem processes [3]. Article 8(h) of the Convention on Biological Diversity (CBD) requires States that are signatories to the Convention to “prevent the introduction of, control or eradicate those alien species which threaten ecosystems, habitats or species”. Its guiding principles outline a hierarchy of action: prevention, then early detection and eradication (eliminating the entire invading population from a given area by a time-limited campaign [4]) where prevention has failed; then management for control or mitigation, if feasible.
The European Union (E.U.) Regulation on Invasive Alien Species (EU Regulation 1143/2014) requires measures to be taken by all Member States on: prevention, early detection and rapid eradication of new invasive alien species, and management of established invasive alien species of Union concern. Based on risk assessments of individual species, an action list of Invasive Alien Species of E.U. concern was adopted (August 2016, updated in 2017) [5]. Five of 49 of the species listed are North American species of crayfish (spiny-cheek crayfish Orconectes limosus (Rafinesque, 1817), virile crayfish Orconectes virilis (Hagen, 1870), signal crayfish Pacifastacus leniusculus (Dana, 1852), red swamp crayfish Procambarus clarkii (Girard, 1852) and marbled crayfish Procambarus virginalis (Lyko, 2017), due to their impacts on native aquatic species and habitats.
Invasive alien crayfish (IAC) are known to be a major cause of loss of native crayfish species in Europe. Habitat loss and pollution have contributed to fragmentation of populations of native crayfish, but there have been both localised and widespread losses due to the transmission of crayfish plague Aphanomyces astaci (Sikora, 1906). This disease is frequently carried by North American species of crayfish, usually asymptomatically [6]. IAC are becoming increasingly widespread in mainland Europe [7] and in the United Kingdom (U.K.) [8].
A rapid response to invasion is important, because when an invasive alien species becomes widely established it is usually difficult to achieve eradication; the geographic scale at which eradication treatment is required tends to make treatment expensive, or else it may become unacceptable due to environmental impacts or social factors [9]. There is a growing catalogue of successful eradications [10,11]. Pluess et al. [12] found that about half of those reviewed had been successful. In temperate aquatic ecosystems, 64% of animal eradications were successful [13], indicating that there is potential for successful eradication treatments, but they are not always achievable.
Eradication of a population of an invasive alien crayfish potentially provides environmental, economic and/or social benefits by preventing any further impacts and wider spread of the population. The benefits of eradication must be considered against any adverse effects of the treatment on non-target organisms. Treatment that has a major, but recoverable, environmental impact may be acceptable in some circumstances if there is a single session or a short programme, but it is unlikely that such a treatment would be acceptable for use for continuing control [14].
Potential eradication or control measures against IAC have been reviewed by several authors; e.g., [4,15,16]. The methods trialed include reduction of population density by trapping [17], male sterilization [18], trapping using pheromones as an attractant [19], the introduction of predatory fish [20], infilling of ponds [21], and biocides (described in this study). A biocide, in the sense used here, is a chemical substance or pesticide intentionally used to kill living organisms. Methods involving physical removal of crayfish and use of predatory fish have reduced some populations [22]; i.e., provided a degree of control. To date, the two methods that have achieved eradication of IAC populations at one or more sites are infilling and the application of biocides.
The use of biocides as a rapid response measure against specific populations of IAC is the focus of this study. Its aim is to provide recommendations for biocide treatment as a management measure for eradicating IAC. The study presents case studies (projects) and uses the methods and outcomes of biocide treatments, by the authors and others, to identify factors that affected the success of projects of this type.
1.2. Biocide Treatments Against Crayfish
Early studies in the U.S. identified insecticides for potential use as biocides to control crayfish in fish rearing ponds [23] and in rice fields [24,25]. Fenthion, an organophosphate insecticide, was used in three field trials [23,24,26]. It appeared that dosage rates of 50–100 µg/L were effective, based on mortality in caged crayfish during treatment, although there was no long-term monitoring of the outcome. Safety concerns about organophosphate insecticides led to their withdrawal from use [27]. Plant-derived natural pyrethrum was insecticidal, but it was also photo-labile, and bound to soils and vegetation [28,29], so more stable synthetic pyrethroids were developed for agricultural use and were also found to be toxic to crayfish [30].
Bills and Marking [31] found the synthetic pyrethroid cyflutrin (as Baythroid®) to be the most effective toxicant of 19 products tested on rusty crayfish Orconectes rusticus (Girard, 1852). Laboratory static toxicity tests showed 100% mortality of crayfish at 0.05 µg/L, compared to 2.00 µg/L for fish. When on-site tests were carried out, however, the minimum concentration to achieve 100% mortality was 25 µg/L, which was also toxic to fish. Treatment of the pond at this dosage killed all of the caged crayfish within 24 h and the pond ceased to be toxic to fish within 6 weeks [31]. No crayfish were trapped in the following spring, but there was no further monitoring to check for total eradication.
Kozak and Policar [32] carried out the first reported biocide treatment on signal crayfish by applying chlorinated lime to a fish farm pond. In field conditions, however, the chlorine was rapidly deactivated by substrate and organic matter, becoming sub-lethal in 1–24 h after application, even with the resulting high pH (pH 9.3), and so did not achieve eradication.
This study presents a series of eradication projects using biocide treatment on crayfish in Europe. These projects are the first use of natural pyrethrum as a biocide in ponds and against crayfish, together with the first use of synthetic pyrethroids as a biocide against signal crayfish. The study is the first exploration of this type of treatment across a diverse range of sites. Importantly, for the first time, the study describes both the immediate mortality of crayfish from biocide treatment and the overall outcomes, verified by long-term monitoring. A series of 11 biocide treatment projects has been carried out against signal crayfish in ponds in Scotland (U.K.), England (U.K.), Norway and Sweden, which are the basis of this study. Biocide treatment was considered in a further seven projects in the U.K., but not carried out and these are also presented here, because they help to illustrate the effects of constraints on projects. The technical details and the lessons learned from all of the projects are intended to provide guidance for future field-scale biocide treatment on populations of IAC.
2. Materials and Methods
The methods used to apply biocide to control or eradicate IAC populations depend on a wide range of factors, including waterbody bathymetry, habitat complexity, water chemistry (temperature and pH) and time of year. The presence of species of high conservation concern, or of commercial value, may also determine whether treatment should go ahead. Combined, this means that many potential constraints will affect any project being considered and the methods used may differ between sites where projects proceed to biocide treatment. This study reviews attempts to treat a number of IAC populations in northern Europe, identifying the factors used to assess feasibility, effectiveness of treatment and overall success.
2.1. Reasons for Treatments
Thirteen potential projects in the U.K. were assessed, of which six progressed to treatment. Three projects were carried out in Sweden and two in Norway. The projects were initiated by different decision-makers in response to local detection of IAC at different times (Table 1 and Table 2), so were not a preplanned group of research sites. The reasons for treatment differed between countries and projects; however, the aim was complete eradication of the population in every case.

Table 1.
Biocide treatment projects against signal crayfish 2004–2012 and locations and reasons for site selection.

Table 2.
A summary of potential biocide projects in the U.K. that did not proceed to completion.
Scotland has no native crayfish. The main reason for the proposed eradication treatments was to protect catchments with high value for nature conservation and commercially important populations of salmonid fish. In England, spread of the signal crayfish to most major river catchments has led to local extinction of the native white-clawed crayfish Austropotamobius pallipes (Lereboullet, 1858) in many catchments [8]. The proposed eradication treatments were to protect the remaining populations of white-clawed crayfish and other species with high value for nature conservation.
In Sweden, signal crayfish were first introduced in the 1960s to improve crayfish fisheries [33], triggering outbreaks of crayfish plague [34]. Signal crayfish had become very widely distributed throughout southern Sweden by the mid-2000s. In order to conserve the noble crayfish Astacus astacus (Linnaeus, 1758), the whole island of Gotland was declared as a specially protected area by the County Administrative Board. Signal crayfish eradication treatments were carried out to reinstate Gotland as an island free from IAC and so safeguard the native crayfish there. In Norway, there were no records of signal crayfish until 2006 [35], but the species was already listed in the Norwegian black list, a government priority list of species to be targeted for rapid eradication at any sites where they were found [36].
Table 1 summarises the project sites where biocide treatment was carried out and the reasons why they were chosen for treatment (see Figure 1 and Figure 2 for locations). Additional details of sites and treatments are given in Table 3 below (see also Supplementary Tables S2–S4). Projects in the U.K. where biocide treatment was considered but not carried out are included in this study (see Table 2), because many of the problems encountered on those were shared with other projects that proceeded to biocide treatment.
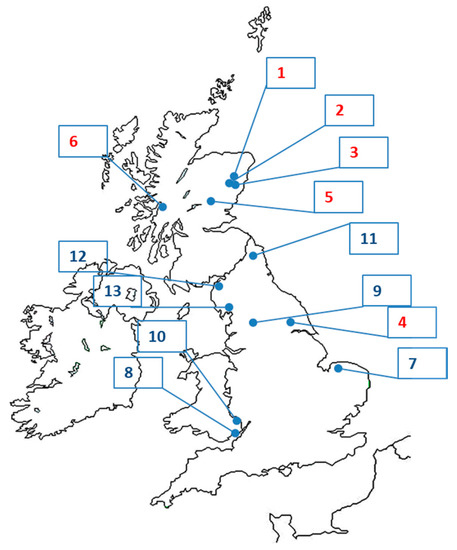
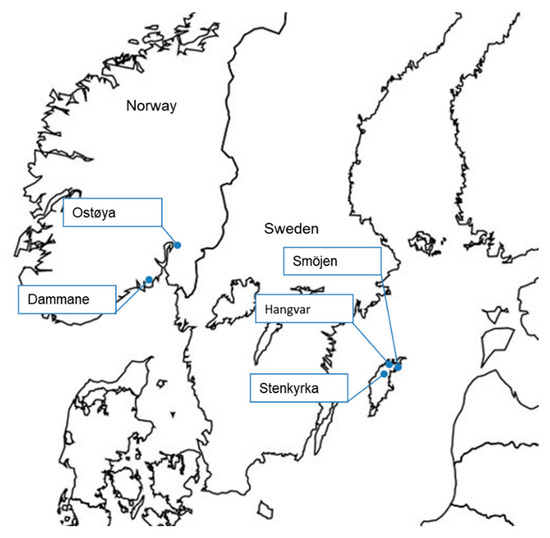
Figure 2.
The locations of biocide treatments against signal crayfish in Norway and Sweden (see Table 1).

Table 3.
A summary of biocide treatments against signal crayfish at sites in the U.K., Sweden and Norway.
2.2. Stages of the Projects
Each biocide treatment project can be considered as a six-stage process as follows: 1. an initial rapid appraisal; 2. detailed feasibility assessment; 3. preparations on site; 4. treatment; 5. post-treatment management, and 6. monitoring of the outcome in subsequent years. The stages are outlined below and additional details of work at each stage are provided in Supplementary Table S1. The work-flow is summarised in Figure 3, which also indicates when funding and other resources are required. It shows stop points if there are major constraints. Constraints are discussed further in Section 2.3 and Section 3.3, but, in summary, major constraints are factors that would prevent effective eradication treatment.
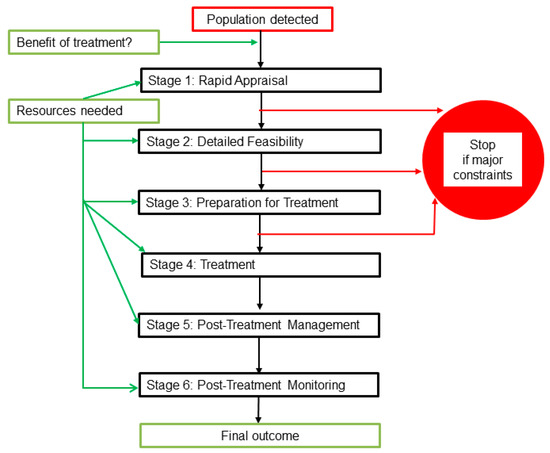
Figure 3.
A summary of the work stages for biocide treatments against signal crayfish.
The methods were similar in all of the biocide treatment projects, with variations applied depending on individual sites and the lessons learned from preceding projects. All of the projects described here involved treatment of one or more ponds, with or without inflows and outflows. Individual sites and their treatments are summarised in Table 3, with additional details given in Supplementary Table S2 (U.K.) and Table S3 (Norway and Sweden).
2.2.1. Stage 1: Rapid Appraisal
Stage 1 was a rapid appraisal to ascertain the scale of the problem and screen out projects that were too difficult to justify treatment. Any sites that were very large were quickly screened out. For these early projects, sites up to about 2 ha were considered. Three sites in Norway that were discovered to have signal crayfish after 2008 were all deep waterbodies many square kilometres in area. It was clear those were large and hence not feasible for biocide treatment; thus, they are not detailed further. Stage 1 also identified inflows or outflows that would need to be controlled during treatment, connected waterbodies and the degree of habitat complexity in the sites.
Detection of crayfish was important in determining the extent of required treatment. As well as considering whether the known location of the population could be treated, the appraisals needed to assess areas where the population could be present, undetected, at low density and hence require treatment. Two factors affecting the likelihood of detecting a population were the time since the introduction and the limits of detection by survey methods. Signal crayfish mainly hide in refuges by day, to avoid predation, so surveys are limited by the type, extent and the accessibility of refuges to physical search, or to the likelihood of crayfish entering baited traps.
Studies have shown that signal crayfish reach sexual maturity in one to three years [40], although it may take two to six years in a cold climate [41]. A breeding size of about 25 mm carapace length (CL) or more is reached most quickly in low density, developing populations. Signal crayfish populations have become established from low numbers of founders. In three of the projects in the U.K., the individuals responsible for the introductions of signal crayfish said they released a few 10s to a few 100s of crayfish, a single bucketful. This means a population may be difficult to detect during establishment, but the likelihood of detection increases over time and with increasing survey effort. At two of the U.K. sites (sites 2 and 3) the presence of signal crayfish was detected three years after the introduction, using only 10 trap-nights at each site.
After five years, a new population of signal crayfish would have undergone five reproductive seasons and would probably have produced two or more generations of breeding age, which would be large enough to catch in traps. Traps with a mesh size of 10 mm or less are capable of catching crayfish less than 30 mm CL [42,43]. A population would be expected to be detectable in a survey by trapping within five years if the site was relatively small and/or there was wide coverage of the site.
Any small inflows and outflows also needed to be assessed for their potential to support crayfish, including those too shallow for surveys by trapping. Manual survey methods [43] are capable of detecting crayfish of smaller sizes and at a lower abundance than trapping, but only in clear shallow waterbodies with a stony substrate suitable for hand-searching or netting.
A precautionary approach was taken in determining the likely limits of population, especially upstream. As well as being important for determining the area to be treated, detection of crayfish at a low abundance was also important in determining the final outcome through post-treatment monitoring (stage 6 below).
2.2.2. Stage 2: Detailed Feasibility
Stage 2 was a more detailed feasibility study, the scope of which depended on the Stage 1 rapid appraisal. The focus in each case was on investigation of the physical area to be treated, identifying and assessing the risks. This included any environmental risks to non-target areas and the risk from any uncertainties about population distribution on the likelihood of achieving eradication. It included engaging with local stakeholders, especially landowners and occupiers and statutory authorities, securing resources for the project and carrying out project operational planning, where this stage indicated the treatment was technically feasible. At this stage, the lead government agency or agencies took the decision on whether or not to carry out the treatment.
Stage 2 included choosing a biocide for potentially feasible projects. Biocide treatments were carried out in the U.K. using natural pyrethrum (as Pyblast®), after a preliminary study tested chlorine, ammonia, high pH, chemical deoxygenation and natural pyrethrum (at site 4) [44]. A dosage of 50 µg/L (in pond water at pH 8.2) natural pyrethrum was the minimum to achieve 100% mortality of crayfish within 24 h. Subsequent tests ([10] and unpublished data) showed that the presence of clay or silt in the water led to reduced toxicity and slower mortality, necessitating higher dosages in field conditions.
Synthetic pyrethroid insecticides are more toxic to crayfish [30] and are more stable than natural pyrethrum in field conditions [45]. Synthetic pyrethroids were not authorised for the projects in the U.K. because of previous pollution incidents with synthetic pyrethroid insecticides from careless use of sheep-dips. A precedent for use of a biocide treatment in Norwegian waterbodies had already been established [46,47]. The cypermethrin-based BETAMAX VET® was used because this pharmaceutical product was already widely used as a chemo-therapeutant in Norway to treat infestations of the salmon louse Lepeophtherius salmonis (Kroyer, 1837), a crustacean parasite on farmed Atlantic salmon (Salmo salar (Linnaeus, 1758)). The projects on signal crayfish were an experimental extension of its use against another crustacean. In Sweden, deltamethrin was chosen, after some discussion with the environmental authorities, as a less expensive biocide than natural pyrethrum for use in large volumes of water, which would still break down fairly rapidly.
Stage 2 (or in some cases stage 3), included on-site toxicity tests using the water and substrate to be treated and signal crayfish obtained from the site. Relevant approvals or consents were obtained for experimental tests and for treatments according to national requirements. The target dosage for the waterbodies had to be high enough to make it likely that the water would achieve a concentration that would produce 100% mortality in crayfish, in field conditions (sun, vegetation and other organic matter, turbidity, temperature, etc.), in the entire treated population. At the dosage required in field conditions, the biocides were toxic to other aquatic invertebrates and fish (i.e., likely impacts on non-target species). It was desirable to minimize the quantity of biocide used to reduce the recovery time and minimize the cost of the chemical, but it was essential to avoid under-dosing. The aim of each of these projects was to achieve complete eradication. The decision-makers for the individual projects considered it important to minimize the likelihood of needing an unplanned retreatment, which would require additional resources of people, materials, time and funding. Table 3 summarises the physical characteristics of the sites treated, the target dosages of biocides and whether hydraulic control of inflows or outflows was required.
2.2.3. Stage 3: Preparation for Treatment
On-site preparation for treatment included advance works, such as the installation of any required fencing or notices; setting up a site base or other local facilities, e.g., for storage of the biocide, other materials and equipment, and for carrying out bioassays or other biomonitoring during treatment; and temporary or permanent controls on inflows or outflows, if needed, and reduction of water level if used. It also included management of vegetation at some sites to facilitate access for the application of biocide (sites 2 and 5). Fish were removed in advance as required. Natural pyrethrum was also toxic to amphibian larvae at the dosages used, but the short-term impact of biocide treatment was mitigated by carrying out treatments after the amphibian breeding season, when most of the population would be in terrestrial habitats. Materials were also obtained in advance, including crayfish for use in monitoring cages during treatment (see stage 4 below).
Because of the difficulties of observing crayfish in a waterbody during the biocide treatment, the projects generally used caged sentinel crayfish to monitor the effectiveness of treatment during the operation. Before the start of application of biocide in stage 4, cages of crayfish were lowered to the bed of the waterbodies. Crayfish in artificial burrows were also used to monitor treatment (e.g., at site 5).
2.2.4. Stage 4: Treatment
The treatment at each site comprised application of pre-diluted biocide to the surface of each waterbody from a boat, the banks or a combination of both. Care was taken to ensure complete coverage of all areas, including the margins and any small inflows or outflows. Each waterbody was treated in its entirety on one day. There were some differences between projects in the way the biocide was applied, with methods used being mainly low-volume application of the biocide with a sprayer, or high-volume application by pump, plus a drip-feed of biocide to small inflows in some cases. Supplementary Tables S2 and S3 provide additional details on application methods at individual sites. Table 3 shows the target dosages and whether hydraulic control was used to prevent leakage of treated water. Sites where hydraulic control was used had biomonitoring downstream to check that there was no pollution outside the treated area. Mesh bags containing aquatic invertebrates were deployed downstream and were inspected at intervals during treatment.
The most complex treatment was 680 m of a small watercourse (site 5). This required continuous hydraulic control to prevent any leakage of treated water during and after treatment. Treatment was carried out in each of five sections in succession downstream. Each section was dammed at both ends and clean flow was diverted around it. Additional sandbags were used to create small steps in the section to ensure undercut banks were fully inundated. The section under treatment was sprayed with biocide, while flow was recirculated continuously by pumping for about four hours. Treated water was then pumped out and the section of stream was then continuously flushed with clean water and dewatered by pumping onto adjacent pasture until bioassays confirmed it was no longer toxic. Treatment progressed to subsequent sections during the flushing phase. The dosage rate in the stream sections was increased to 1 mg/L because treatment was for only four hours in recirculation. In one of the preliminary tests, adult crayfish were given a short exposure time of 15 min or two hours before being rinsed and put in clean water. A few crayfish survived 15 min exposure to 1 mg/L natural pyrethrum (affected by it, but recovered), whereas two hours exposure was lethal (Peay, personal observation).
In all of the projects, monitoring progress of the treatment using caged crayfish enabled a supplementary application to be carried out on the day after the initial application if necessary (site 6). Cages were lifted for inspection after completion of the biocide application and inspected at intervals to check for mortality. If any cages had crayfish that were unaffected on the day after treatment, it indicated that an area required better coverage with biocide (e.g., as at site 6 where active crayfish were seen in one of the cages in the deepest part of the quarry a few hours after the application).
In five of the projects, there was a second treatment with biocide after the first treatment had finished (U.K.: sites 1 and 2; Sweden: Smöjen, and Norway: both sites). At two sites, this was because survivors were detected (sites 1 and 2). Those two sites had a pre-treatment with sodium sulphite to chemically deoxygenate the water. The pre-treatment was to encourage crayfish to leave refuges and so be more readily exposed to the natural pyrethrum. In practice, residual sodium sulphite partially degraded the natural pyrethrum, reducing its effectiveness. Retreatment of the sites and subsequent treatments were carried out without preliminary deoxygenation [14]. In Norway, the sites were given two biocide treatments, with a two-week interval, as a precaution. Those ponds were then partially drained for one winter before they were allowed to refill [38,39,48]. In Sweden, the site Smöjen was given two treatments with a three-month interval, as a precaution, since the limestone bedrock had many crevices as potential refuges for crayfish.
2.2.5. Stage 5: Post-Treatment Management
Stage 5 was the management during the recovery period (i.e., the period until the treated water was no longer toxic to aquatic invertebrates). In the U.K. projects, the reduction in toxicity after completion of treatment was recorded using bioassays with freshwater shrimps Gammarus pulex (Linnaeus, 1758). In Sweden, the decrease in concentration of the biocide over time was determined by chemical analysis and by testing with sentinel cages of noble crayfish in the waterbody. In Norway, the recovery of the water was not recorded in any detail, because the ponds were partially drained before winter. At ponds with an outflow, it was necessary to prevent any discharge from the treated area until the biocide had degraded sufficiently to avoid adverse effects in untreated areas downstream. Where ponds were dewatered (site 5), there was the opportunity to search the exposed bed for any live crayfish. In one of the projects (site 5), a previously channelised 200-m length of watercourse was excavated after the treatment and the material removed was thoroughly searched for any live or dead crayfish.
The projects were carried out in accordance with risk assessments for health, safety and environment. Mitigation measures were included to avoid or minimise the risks of impacts outside the areas intended for treatment; for example, at sites with hydraulic control, contingency measures were put in place, such as additional pumps on stand-by, in case there was an increase in flow due to rainfall. Treatments and recovery periods were completed without causing pollution in untreated areas.
The three sites in Sweden were stocked with native crayfish two months to one year after treatment. One of the sites in Norway (Ostøya) was stocked with fish, as were two of the U.K. sites (sites 3 and 5).
2.2.6. Stage 6: Post-Treatment Monitoring to Determine the Outcome
The outcome of each biocide treatment was determined by a monitoring programme. The minimum was five years of annual post-treatment monitoring using extensive coverage of the site with baited crayfish traps, in some cases supplemented by additional methods. Five years without any detected presence of signal crayfish was selected as the threshold for successful eradication of a population of signal crayfish. As described in stage 1 above, it is likely that a recovering population could be detected within this time. Post-treatment monitoring was extended in Sweden and Norway to seven and six years, respectively. This was to increase the confidence in using five years without any crayfish detected as the criterion for successful eradication and to take account of the slower maturation rates in Scandinavia.
Monitoring annually after treatment enabled any failure to eradicate the population to be detected as soon as possible. If any crayfish were caught, a decision could be made quickly as to whether to carry out another session of treatment.
An intensive survey effort increased the probability of detecting of a population at a low abundance. The effective range of individual baited crayfish traps in ponds and lakes has been estimated at approximately 12.5 m2 by Abrahamsson and Goldman [49] and at 56.3 m2 by Acosta and Perry [50] in a mark recapture trial in a controlled grid. In the first five U.K. projects, the approach was to use many traps in a single trapping session. Trap coverage of sites across all post-treatment years averaged one trap per 64 m2 (standard error (SE) ± 4.8, n = 32). This is less than 100% coverage in individual trapping sessions, but likely to detect crayfish in repeated annual monitoring of an increasing population. Any crayfish recorded would show that eradication had not been achieved. Traps were not deployed on an entirely uniform grid at each site. Distribution was skewed where necessary to ensure good coverage of areas with potential refuges for crayfish, especially near steep banks, with reduced cover in areas with a flat, fine substrate. At site 6, 15 traps were set and lifted daily at locations around the site for a total of 200 trap nights per year. The trapping effort used in the Norwegian ponds was similarly intensive, at one trap per 16 m2. During trapping at site 1, a note was made of the by-catch of amphibians, and other ad hoc observations of changes in conditions were recorded.
As well as the absence of signal crayfish in trap catches, an additional requirement in Norway was that no crayfish plague should be detected in sentinel native noble crayfish during the last three years of monitoring. Water samples were also taken annually using the filtration method of Strand et al. [51] and tested for the presence of spores of crayfish plague as a potential indicator of signal crayfish, the carriers of the disease. In Sweden, trapping to confirm the presence of restocked noble crayfish was taken to be a likely indicator of absence of crayfish plague and hence absence of signal crayfish.
2.3. Assessment of Constraints on Projects
The 13 biocide projects in the U.K. (six of them treated, seven not treated) had a range of constraints that were encountered by the project teams during the appraisal and feasibility assessments (stages 1 and 2). The constraints were discussed and noted during the decision-making process in each project. After the projects had either progressed to treatment or were abandoned, the constraints for all the projects were classified by type and their relative influence on the decision-making was given a qualitative rating. There were not enough details available for this study to include constraints on the projects in Norway and Sweden in the same analysis.
Eleven types of constraint were identified, related to the environment, the benefits to be achieved from the project and issues of stakeholder acceptance and project resourcing (Table 4). There were interactions between constraints, because the environmental categories in combination affected the likelihood of being able to treat the whole population and this influenced the views of stakeholders about the project and its benefits, but interactions were not analysed separately. Constraints were given a qualitative ranking according to their importance, or impact, on the project, on the following scale:

Table 4.
The categories of constraints on biocide treatment projects.
- Minor, a matter for project planning and action, but not likely to prevent or significantly constrain the project;
- Moderate, a significant constraint causing increased technical difficulty, cost and/or delay, and
- Major, sufficient to stop a project on its own.
Table 5 shows the constraints on the individual projects. The constraint scores for each project were summed and the projects were ranked. The progress achieved on each project was scored according to the stage reached (1 = rapid appraisal, 2 = detailed feasibility, 3 = preparations for treatment, 4 = biocide treatment). A Spearman rank correlation test was used to determine whether there was a correlation between the total constraint score and the stage a project reached. In addition, a Spearman rank correlation test was used to test for a correlation between the time elapsed in years between first detection of signal crayfish and an appraisal (stage 1) being carried out and the time until a project either progressed to biocide treatment (stage 4) or was abandoned at an earlier stage.

Table 5.
The U.K. sites with constraints and the stage reached by the project.
3. Results and Discussion
3.1. Biocide Treatment Outcomes
The results of the biocide treatments are summarised in Table 6, with initial results and long-term outcomes determined by monitoring. At eight of the eleven sites, annual monitoring for five years or more did not record any signal crayfish, indicating that eradication was successful at those sites. At both sites in Norway, absence of any crayfish plague spores in water samples gave additional confidence that there were no crayfish surviving below the limits of detection by trapping. Similarly, at the three sites in Sweden, there were no signal crayfish recorded (in seven years) and the re-establishment of populations of noble crayfish was a further indication of absence of crayfish plague and the signal crayfish that carry it.

Table 6.
The results of biocide treatments against signal crayfish, initial and final outcomes.
At the three sites where eradication was not achieved with natural pyrethrum, IAC were caught two or three years after treatment (see Supplementary Table S5 for details of trap catches). This increased confidence that, at sites where crayfish were not recorded for five or more years, it was due to absence, i.e., successful eradication, rather than a failure to detect a recovering population.
To date (autumn 2018), none of the successfully treated sites has been reinvaded or subject to further illegal introduction of IAC.
The treatments with synthetic pyrethroid insecticides (in Norway and Sweden) killed all of the caged crayfish within 24 h, more quickly than the U.K. sites where natural pythethrum was used (together with larger numbers of sentinel crayfish). Mortality of wild crayfish was also observed during treatment at most of the sites where crayfish had been recorded during prior surveys.
Bioassays showed that the toxicity of treated water reduced over a few weeks, the longest being at site 4, where the deep calcareous water became cold through the winter and remained so for weeks.
Comparative surveys of aquatic invertebrates were carried out in Sweden [52]. As expected, there was a temporary loss of sensitive taxa, especially insects and crustaceans, although rotifers, oligochaetes and molluscs showed greater tolerance. Recovery of the invertebrate fauna started within a month of treatment and continued during the three months of monitoring. There were no comparative surveys of aquatic invertebrates before and after the biocide treatments in the U.K. projects. Recolonization by aquatic invertebrates occurred by natural spread from connected waterbodies or others nearby and was noted during post-treatment monitoring for crayfish.
3.2. Additional Post-Treatment Observations
At several treated sites, a post-treatment increase in the abundance of aquatic macrophytes was seen (Table 6), likely due to removal of crayfish and herbivorous invertebrates. For example, at site 1, (native) alternate water-milfoil Myriophyllum alternifolium (DC.) increased markedly in the margins and there was some increase in the predominant (alien) leafy elodea Egeria densa (Planchon, 1849). The submerged vegetation cover increased after treatment from approximately 5% to 90% at site 2 and remained abundant until year 3, when intensive grazing by captive-bred ducks removed almost all of it.
Predation of amphibians by signal crayfish was reported by Axelsson et al. [53], and, similarly, predation of amphibians by red swamp crayfish [54,55]. Low numbers of larvae of common toad Bufo bufo (Linnaeus, 1758) and palmate newt Lissotrichon helveticus (Razoumowsky, 1789) were observed during the summer before biocide treatment at site 1; however, there was insufficient time for a quantitative survey. During post-treatment monitoring for crayfish, a by-catch of larvae was noted from traps with a 4-mm mesh (those with a larger mesh did not retain amphibian larvae, Table 7). Catches of newts increased significantly over time (Kruskall–Wallis test of ranked counts in years; n = 116, df = 2, K = 42.485, p < 0.001). The observations here give a circumstantial indication of potential benefits to native amphibians, which could be investigated in more detail in future projects.

Table 7.
The recorded bycatch of amphibian larvae in fine-mesh (4 mm) crayfish traps in years following biocide treatment at the gravel pit (U.K. site 1).
3.3. Factors that Influenced the Success of Biocide Treatments
3.3.1. Identifying the Maximum Extent of the Population
The ease with which the geographical extent of signal crayfish within a site could be assessed was influenced by a range of factors. These include the nature of the waterbody itself, size, isolation from other waterbodies (ponds, lakes, ditches streams, rivers and flooded terrestrial habitats), habitat complexity (e.g., presence of aquatic and fringing vegetation) and waterbody bathymetry.
The deployment of baited traps of various design and mesh size was the most commonly used method for determining crayfish presence in large standing waterbodies, such as ponds and lakes, as well as deeper running waters. All crayfish survey methods have limitations [43]. Baited traps are dependent on the proximity of active crayfish and the accessibility and retention of the type of trap used. Where it was practicable, additional survey methods were used, in addition to trapping effort, to detect the limits of populations. Manual searching was also used, particularly in shallow littoral habitats and riparian areas. Surveys that confirmed presence gave the minimum extent, but the likely maximum extent of population had to be estimated. For example, where ponds were in close proximity, with connection by water between them, it was necessary to treat them all together, unless they were effectively isolated by barriers. Determining the limits in a watercourse was difficult. For example, in an invaded headwater stream (site 9), the downstream limit detected by intensive manual survey was 400 m further than that detected by trapping [56].
3.3.2. Achieving an Effective Dosage in the Waterbody
Delivering an effective dosage of biocide to all habitats where signal crayfish are present, or are likely to be so, is the key component of the treatment process. Such features as submerged vegetation, a complex bank structure and deep water were factors affecting the dosage required and the application method. Submerged and emergent vegetation reduced the effectiveness of application by interception and by increasing degradation of the biocide [57,58]. A high-volume application using a pump improved treatment of the margins in the treatments (e.g., in Norway and Sweden, and U.K. sites 5 and 6). Prior management of vegetation helped to improve access for treatment (site 5 stream banks).
Deep water affected the application by increasing the time required for complete mixing. With the half-life of the biocide being potentially less than 24 h, this risked incomplete dosage at the bed, especially where the water was stratified (site 6). Cages of sentinel crayfish identified this problem during treatment and allowed for supplementary treatment via a vertical drop-pipe, plus additional mixing by boat and pumps. A drop-pipe could be used at other sites to put biocide below floating-leaved vegetation to avoid losses at the surface. On sites where stratification occurs, treatment in autumn after over-turn would also mitigate this problem, in conjunction with the use of a vertical pipe.
3.3.3. Treatment of Crayfish in Banks and Margins
A complex habitat in the margins reduced the effectiveness of treatment if the treated water could not adequately penetrate areas with refuges for crayfish, or refuges were not submerged, or undetected inflows caused local dilution of the biocide. For example, at site 6, there was a stone-revetted bank, overhung by dense vegetation, with spring seepage, and a surviving crayfish was caught there post-treatment. To adequately treat the margins of quarry ponds, which had loose rock or crevices, the margins were thoroughly drenched with treated pond water to dose all of the potential refuges.
A reduction in water level had benefits in reducing the volume to treat and by bringing the water level away from complex, vegetated habitat in the margins (such as the fibrous tree roots at site 4, which were full of shallow burrows). Some signal crayfish remained in exposed burrows for hours to a few days after exposure (in a trial at site 10 [59]). After several weeks of drying, test excavations (site 4) showed that exposed burrows were unoccupied. By contrast, an unplanned reduction in water level just before treatment (site 3 [14]) risked incomplete treatment, especially if cold conditions discouraged crayfish from leaving exposed refuges.
Attention to the details of sites and operations is critical both before and during biocide treatment. A good understanding of the technical issues involved and how problems can be overcome is important in the appraisal and detailed feasibility stages of any eradication treatment.
3.3.4. Interaction of Cumulative Constraints and Time
Any large field-scale project is likely to encounter problems or constraints, which need to be overcome if the project is to progress from planning to action and successful outcome.
Table 5 above shows that the environmental constraints that occurred most frequently as moderate or major were the often inter-related environmental factors of site size and habitat complexity (at seven and four sites, respectively), and the need to avoid or minimize environmental risks of treatment.
Of the constraints from stakeholders, funding was a major constraint on four projects. Landowner consent for works was a moderate or major constraint on five projects, especially where there were multiple owners or occupiers involved, the type and scale of work was not fully understood by the landowner or occupiers, or they had concerns about impacts on their interests.
As the number and magnitude of constraints increased, the projects were significantly less likely to proceed (Spearman Rank correlation: n = 13, r = −0.633, P = 0.02). The projects that had longer times between detection and a feasibility study also had longer times between feasibility and the treatment or the decision not to proceed further (Spearman rank correlation: n = 13, r = 0.846, P = 0.01).
Projects that were clearly too difficult or not feasible were dismissed at an early stage (stage 1 appraisal) (Figure 4). Projects with only minor constraints, or moderate ones that could be overcome during planning, were able to proceed relatively quickly to stage 4 (treatment). For three projects, there was less than a year between detection and treatment, and for the other three it was less than three years. Four projects had the potential to be treated, but they were beset by problems. They incurred the longest delays, with problems increasing over time, until eventually the project was ended without treatment being undertaken (terminated at stage 2 or 3, Figure 4).
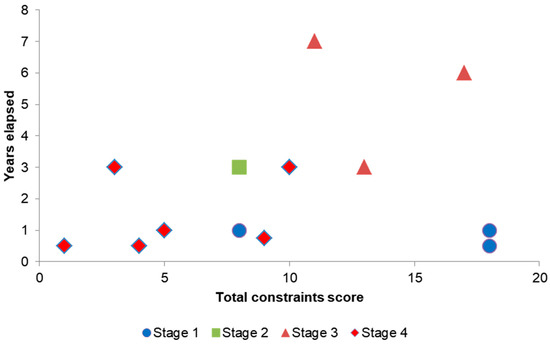
Figure 4.
The effect of constraints on the total time in years between detection and the stage reached in the biocide treatment projects. Stage 1: appraisal, stage 2: detailed feasibility and planning, stage 3: preparatory works on site, stage 4 biocide treatment. Constraints in 11 categories scored as 1 minor, 2 moderate, 3 major. For constraints see Table 4 and Table 5.
3.3.5. Environmental Constraints
Determining feasibility of treatment involved assessing interacting factors. In some cases, by the time the population of IAC was detected, the invaded areas were just too large, complex or had unacceptably high environmental risks. For example, at site 12, an ornamental pond and outfall ditch would have been feasible to treat with biocide had the invasion been detected early. However, invasion was only detected during a routine fisheries survey of a much larger watercourse downstream and traced back to the pond.
The greater and more complex the area to be treated, the greater the risk of incomplete treatment or high costs. Measures were taken in some of the projects to try to prevent further spread of crayfish prior to treatment. At site 7, the mill lade was fitted with traps set as a barrier across the channel and large plastic boxes were sunk into the bed to act as catch-pits to monitor and remove crayfish moving downstream. Because signal crayfish will emerge onto the margins for short spells (Peay, personal observation at site 1 and shown experimentally [60]), barrier fencing was used at site 8 to prevent crayfish moving over wet grassland to a river. Vertical plastic barrier fencing, with the lower edge secured below ground (as used by Peay and Dunn [59]) is routinely used to exclude amphibians and reptiles from construction sites in the U.K. Its use at site 8 would have prevented amphibians accessing breeding ponds prior to a biocide treatment. Barrier fencing has also been used to prevent overland escape of IAC from substrate dredged from the River Wharfe, England (Peay, personal observation) during maintenance of a weir.
3.3.6. Securing Resources
Stakeholder support for projects was highest if there was considered to be a high likelihood of success and confidence that environmental risks were low, or could be mitigated. This was important in securing approvals and funding.
Obtaining funding was necessary for all the projects and was a major constraint on several of them, but generally in combination with the scale or complexity of the projects. In Scotland, the agency responsible for advising government on biodiversity and nature conservation, Scottish Natural Heritage, secured consents and funding very quickly. This allowed for the first two sites (sites 1 and 2) to be treated within six months of detection and within three months of the feasibility study, a commendably rapid response.
In England, funding had to be obtained regionally, or from several sources, which incurred delays of more than two years in some cases. Delays affected the likelihood of achieving eradication. For example, at site 9 (Table 2), a headwater stream, biocide treatment was assessed as feasible in 2005, three years after detection, but the funding required was not secured until 2007. There were further delays due to the need to obtain the cooperation of approximately 20 landowners and occupiers. Hydrological investigations were required to show that a local water supply would not be affected. After further delay due to wet weather, by late summer 2008 signal crayfish had reached the confluence of a watercourse too large to treat (due to the scale, cost and environmental risks) and the project was terminated.
In Sweden, it took up to two years to progress from the appraisal to treatment (Table 1). In Norway, the first project (Dammane) had its feasibility appraisal within a year of detection [61] and proceeded to treatment the following year. With the experience gained, in the second project (Ostøya golf course ponds) treatment was carried out rapidly in the same year as the population was detected, funded by government. The rapid action in Norway and Scotland may have been because the government agencies recognised the strategic importance of dealing promptly with invasive alien species while there were few localities.
The project leader or ‘champion’ had a key role in obtaining funding, other support and approvals. Having a proactive person for this role was important for all of the projects. A lack of staff experience of biocide treatment of crayfish was a common issue, because the treatment was new and there were different teams for each project. After the first two treatments, the experience gained was shared with each of the subsequent projects.
3.3.7. Public Acceptance
In the projects that proceeded to biocide treatment, there were no public objections raised. The first few treatments (in Scotland) were carried out without advance publicity, as all were on private land, but the active involvement and support of the rivers trusts ensured that local anglers were well-informed. Site 6 had public access, so there was prior involvement with the local community and local press, plus national television news coverage during the treatment. Once the need was explained and any questions about public safety had been addressed, there was either general acceptance or active support locally.
In the first two Swedish projects (Smöjen and Stenkyrka), the treatments were advertised before being performed and local press and television covered all of the action. The reactions from the general public were generally very positive and supportive. In addition, the attitudes of fishing associations and owners of the ponds were also positive, since the County Administration had secured money for restocking the ponds with native crayfish after the treatment. In the third project (Hangvar), there were initially some concerns from people living nearby that the biocide would be a health hazard in water from their private wells. This was despite assurance that, even in the treated pond, the biocide would be within safe limits for human consumption. After trials using tracer dye confirmed that wells would be unaffected, local people accepted the project. The Norwegian projects were carried out on both public and private land. Stakeholders and the media were well-informed before and during the treatments. The media attended during treatments. No public objections were raised. Both local and national media focused on solving the problem of alien crayfish carrying crayfish-plague and not on the use of the biocide.
3.4. Subsequent Projects
Experience gained from all of the projects in this study has already informed subsequent projects. These have used partial dewatering as a pre-treatment [62,63], which reduces the volume to be treated and, by increasing storage capacity, it minimizes the risk of any overspill of treated water during the post-treatment recovery period. Physical barrier fencing has been used to restrict further invasion prior to treatment [63]. Physical controls have been installed at outlets before treatment [63], during dewatering [62], and to contain crayfish by installing catch-pits on outfalls [64] (as per [65] (pp. 64–67)), where biocide treatment could not be carried out.
Biocide treatments have been tried on red swamp crayfish, a sub-tropical species of seasonal wetlands, which can construct extensive burrows above and below the water level. Natural pyrethrum treatment of ditches with red swamp crayfish [66] did not achieve 100% mortality, likely due to survival of individuals in burrows. This problem of survival of red swamp crayfish in exposed burrows was encountered in a preliminary biocide treatment at two sites in Wisconsin, U.S. [63,67]. This highlighted the need for pre-treatment habitat modification, namely, covering all burrowed areas with a surfacing material that is impermeable to burrowing. An alternative to biocide treatment, eradication by physical habitat destruction, was carried out by infilling an urban pond (Sam Poerio Park Pond in Kenosha, Wisconsin) [63].
4. Recommendations
Using experience gained from the projects, recommendations are given below to help those considering carrying out a biocide treatment against IAC, especially for initial appraisal and feasibility studies. Details of sites and methods of work need to be considered as early as possible in order to determine the benefits, risks, feasibility and costs of work.
4.1. Is It Worth Considering Carrying Out a Biocide Treatment Against Invasive Crayfish?
Before considering whether to make any intervention, it is worthwhile considering the benefits of doing so. The E.U. Regulation on Invasive Alien Species (Regulation No. 1143/2014) is a potential driver for future rapid eradication treatments in Europe. Prevention is the highest priority, but rapid eradication is the next intervention to be considered in the hierarchy of action. This does not mean that member states are required to eradicate IAC, and, in many states in Europe, including the U.K. and Sweden, populations are already too extensive for eradication from the state as a whole. There are still benefits in considering the issue at other scales, especially at catchment scale.
Table 8 recommends priorities for undertaking eradication projects based on the existing status of IAC. It is worth reiterating that the biocides available are not selective for crayfish, which means that there will be a temporary loss of invertebrates within the treated areas, plus a loss of amphibians and fish if any are present at the start of treatment. Where the benefits of successful eradication are high, it is more likely that the cost of treatment and its localised, recoverable environmental impacts will be accepted by stakeholders than if the benefits will be lost soon due to invasion from other sites.

Table 8.
Recommendations on how to prioritise possible biocide treatment of invasive alien crayfish (IAC).
For each year that a successful biocide treatment (or other intervention) prevents invasion, an annual increment of environmental impact is avoided. A cost–benefit model of biocide treatment, developed for the North Esk (sites 1–3) [68] (pp. 179–219), used the fish restocking cost as a surrogate environmental cost to assign a value to the potential environmental impact of IAC per kilometre of invaded watercourse, annually and cumulatively. The cost of uncontrolled invasion over time was compared with the outlay cost of biocide treatment. Even when modelled with a low unit cost of environmental impact, the cost of a successful rapid eradication was calculated as being less than the impact cost within seven years.
It is recommended that regulatory authorities, or others responsible for strategy on IAS, consider priorities for a rapid eradication response, nationally and regionally, to help secure authorisations and funding quickly for priority cases when they arise.
4.2. Is There An Alternative to Use of Biocide for IAC?
Biocide treatment of IAC is intended for localised, rapid eradication. Current biocides have too much impact on non-target fauna (invertebrates, amphibians and fish) to be suitable as a recurrent control measure, or at sites where there is no reasonable likelihood of achieving eradication. Few of the projects in this study had detailed surveys of fauna and flora before and after treatment and not over the full period of monitoring for outcome. This would be worth doing in any future projects, provided that collection of baseline data did not delay treatment.
It is to be hoped that future research will find low-cost, effective eradication or control measures, with acceptably low environmental impacts, which are suitable for extensively invaded areas. In the meantime, the only confirmed successful eradications of IAC have been with biocides, or complete habitat destruction (infilling). Infilling has greater environmental impacts within sites than biocide treatment, due to the habitat loss, but the methods required are used throughout the construction industry and could be considered for small urban ponds. The cost relative to biocide treatment would depend on the volume and the cost of materials.
4.3. Is Eradication with Biocide Feasible?
The key questions are firstly, what is the extent of the population and secondly, could all of that population be treated? After the first report of a population of an IAC, some assumptions will need to be made about the likely limits of population. Recent progress in eDNA sampling [69,70,71] means that this is a potentially useful tool for rapid appraisal, and specific assays for signal crayfish have already been developed (e.g., [72]). There is uncertainty about thresholds for detection at a low abundance in different habitats, but quickly determining the detectable range of population using eDNA sampling would be a useful addition to existing survey methods.
Whether to treat additional areas where it is uncertain whether crayfish are present will have to be decided on a case-by-case basis. The maximum range upstream in any inflows to a pond is critical, because if any crayfish are untreated they will readily reinvade treated areas downstream. There is also potential for untreated crayfish downstream to reinvade. There is a much greater likelihood of being able to install temporary or permanent physical barriers to the upstream movement of crayfish than downstream. In the event that treatment has to be done in two phases, it should be done from upstream to downstream. Such factors as time since introduction, population density and habitat will have to be taken into account. Expansion of range is usually low during establishment, but more rapid thereafter. If crayfish have already reached a watercourse too large to treat, eradication is no longer feasible.
To help assess possible future projects, a decision tree (Figure 5) has been developed for a preliminary appraisal of the feasibility of treatment of signal crayfish at a site where a population has been newly detected. It takes into account such factors as site size and habitat complexity. From the Start in the Figure 5, making choices (y = yes, n = no) leads to summaries of risks and the potential for biocide treatment. Types a to c would be relatively straightforward. Types d to e would be more complex, but could still be successful, depending on the site. Types f and g would have greater risks of failure to achieve eradication and/or have high costs associated with them. Type h would not achieve eradication.
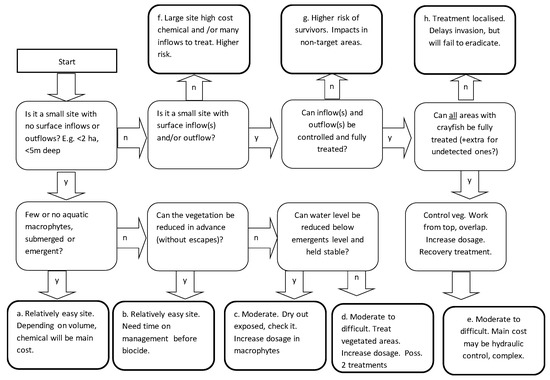
Figure 5.
The decision tree for initial appraisal of a potential biocide treatment on a site with a population of invasive signal crayfish.
Securing the cooperation of all of the relevant stakeholders is essential, and if not achieved in time may prevent timely treatment. Agreement in principle should be sought from landowners and other stakeholders during the appraisal stage and reinforced as necessary during the feasibility stage, so that everyone is clear about the programme, the operations involved and the risks (environmental and operational), including any contingency measures.
To help secure cooperation of landowners in the eradication or control of priority invasive alien species, regulations are being introduced as part of the implementation of the E.U. Regulation on Invasive Alien Species (EEC/1143/2014). An example of this is the Wildlife and Natural Environment (Scotland) Act 2011, which gives statutory authorities the option of legal enforcement, if voluntary cooperation is not forthcoming.
4.4. Which Biocide and How Much to Use?
When considering which biocide to use, recommendations based on this study are as follows:
1. for a pond where outflow can be controlled without pumping: synthetic pyrethroid cypermethrin, or deltamethrin, on grounds of higher toxicity to crayfish and hence a low cost of biocide per unit volume treated (although natural pyrethrum is more readily degraded and the recovery time is shorter, hence it may be preferable for some sites).
2. for a pond with leakage: natural pyrethrum (a recovery period of one week to four months), or possibly a synthetic pyrethroid (if the risks of pollution outside the target area are manageable).
3. for a small watercourse controlled by recirculation: natural pyrethrum (a recovery period of 4–11 days with flushing). The use of a synthetic pyrethroid may be a suitable option in some cases (e.g., on a muddy site, or with post-treatment management to accelerate recovery, e.g., dewatering, or offsite disposal of treated water).
When deciding on the quantity of biocide to be applied, toxicity tests under site conditions are recommended prior to field-scale treatment, because the minimum dosage for 100% mortality may be significantly greater than in laboratory acute toxicity tests. Further allowance needs to be made for variable rates of environmental degradation of the biocide during treatment (influenced by water chemistry, temperature, sunlight, substrate, aquatic plants, organic matter, including plankton, and other suspended solids in the water). The half-life of both natural pyrethrum and synthetic pyrethroids can be less than 24 h and may be reduced to only a few hours [29].
Natural pyrethrum may have a reduced likelihood of success if the site has a lot of burrows, which need to be fully submerged to be treated. Based on the limited number of studies so far, it is unlikely that eradication of red swamp crayfish would be achieved by natural pyrethrum alone. Destruction of burrows would also be needed.
The approach described for signal crayfish may be suitable for other IAC species with similar habitats and behaviour. There are likely to be some differences in the susceptibility of different crayfish species, as well as between biocides. In all cases, however, there will be a need for field target doses to take account of the variations in environmental conditions on the site, the method of product application, its rate of degradation and the need to fully expose all IAC to treatment.
4.5. Case for Action
When attempting to control a biological invasion, other authors have recommended considering the ease with which the species can be detected and targeted, the risks associated with management action, the likelihood of success and the extent of public concern and stakeholder involvement [73]. Other factors mentioned include funding for the whole programme, prevention of reinvasion and awareness of possible management requirements after treatment [74]. This study shows how such factors operate in combination when attempting to eradicate populations of invasive alien crayfish.
In this study, a delay of three or more years meant a project probably had too many difficulties to proceed. Biocide treatment within two years from detection is recommended and within one year if possible, with rapid treatment being most critical in sites with inflows or outflows.
The successful biocide treatments against signal crayfish at site-scale are encouraging, with success in eight of eleven treatments (c. 70%). There is always some uncertainty about the future success of an eradication treatment and the benefits are not guaranteed. In many cases, perhaps the majority of cases, by the time a population is detected it is already too widespread for effective eradication treatment.
Where a rapid eradication treatment is potentially feasible, the precautionary approach is to make use of the limited ‘window of opportunity’ for rapid eradication. Postponing a decision, or starting by using methods that are only likely to partially reduce the population, is likely to have irreversible consequences. It can be predicted with confidence that once a population of invasive alien crayfish is established and beyond the feasible range of rapid eradication treatment, the population will expand until it occupies all accessible areas of suitable habitat in the catchment, bringing with it the associated impacts on aquatic ecosystems. Alas, this is an all too common theme with aquatic invaders.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/11/3/29/s1. Table S1: Outline of stages of work likely to be required for a biocide treatment against invasive crayfish. Table S2: Details of projects in the U.K. where biocide treatment against signal crayfish was carried out. Table S3: Details of projects in Norway and Sweden where treatment against signal crayfish was carried out using synthetic pyrethroids. Table S4: Summary of projects in the U.K. in which feasibility of biocide treatment against signal crayfish was assessed, but treatment was not carried out. Table S5: Annual monitoring results of crayfish trapping at sites treated with natural pyrethrum.
Author Contributions
Conceptualization, S.P.; Methodology and investigation, S.P., R.S., S.I.J., C.W.B.; Writing (original draft preparation), S.P.; Writing (review and editing), R.S., S.I.J., A.M.D., C.W.B., L.E.
Funding
The projects in Sweden were funded by the Swedish National Board of Fisheries and the Swedish Agency for Marine and Water Management, treatments were undertaken by the County Administrative Board on Gotland and additional information was provided by Rolf Gydemo (County Administration). The projects in Norway were financed by the County Governor of Telemark, the County Governor of Oslo and Akershus, the Norwegian Food Safety Authority and the Directorate for Nature Management. Contributions to funding of the projects in the U.K. were made by Scottish Natural Heritage (SNH), the Scottish Executive, Environment Agency (EA), the Tay Foundation, the Scottish Environment Protection Agency (SEPA), Highland Council, English Nature (now Natural England) and also the Yorkshire Dales National Park, Esme Fairburn Foundation and Countryside Council for Wales.
Acknowledgments
Contributions in kind were provided by individuals and agencies on all the projects. The biocide projects were only accomplished with the efforts of many people, especially, in the U.K., the individual project managers Isla Martin (SNH), Roger Martin (EA), David Summers (Tay District Salmon Fisheries Board), Diane Baum (Lochaber Fisheries Trust), Neil Guthrie (EA), Julia Stansfield (EA), Chris Dyson (Countryside Council for Wales), and Matt Brazier (EA); plus Peter Collen (Marine Scotland Science) for crayfish surveys and treatments in Scotland and Colin Bean (SNH), adviser and facilitator on the Scottish projects; also Paul Bryden who helped on all of the projects that progressed beyond stage 1. The help of many other individual contributors to projects is also gratefully acknowledged, including the landowners and all those who assisted with site work on one or more projects. The contributions of the anonymous referees to the development of the paper into its current form are most gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lodge, D.M.; Lewis, M.; Shogren, J.F.; Keller, R.P. Introduction to biological invasions: Biological, economic and social perspectives. In Bioeconomics of Invasive Species: Integrating Ecology, Economics, Policy, and Management; Keller, R.P., Ed.; Oxford University Press: Oxford, UK, 2009; pp. 1–24. [Google Scholar]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Change Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, F.; Aquiloni, L.; Dieguez-Uribeondo, J.; Tricarico, E. Managing invasive crayfish: Is there a hope? Aquat. Sci. 2011, 73, 185–200. [Google Scholar] [CrossRef]
- European Commission. List of Invasive Alien Species of Union concern. Available online: http://ec.europa.eu/environment/nature/invasivealien/list/index_en.htm (accessed on 20 December 2018).
- Diéguez-Uribeondo, J.; Cerenius, L.; Dykova, I.; Gelder, S.R.; Henttonen, P.; Jiravanichpaisal, P.; Lom, J.; Söderhall, K. Pathogens, parasites and ectocommensals. In Atlas of Crayfish in Europe; Souty-Grosset, C., Holdich, D.M., Nöel, P.Y., Reynolds, J.D., Haffner, P., Eds.; Museum National d’Histoire naturelle: Paris, France, 2006; pp. 133–149. [Google Scholar]
- Kouba, A.; Petrusek, A.; Kozák, P. Continental-wide distribution of crayfish species in Europe: Update and maps. Knowl. Manag. Aquat. Ecosyst. 2014, 413. [Google Scholar] [CrossRef]
- Holdich, D.M.; James, J.; Jackson, C.; Peay, S. The North American signal crayfish, with particular reference to its success as an invasive species in Great Britain. Ethol. Ecol. Evol. 2014, 26, 232–262. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. We can eliminate invasions or live with them. Successful management projects. Biol. Invasions 2009, 11, 149–157. [Google Scholar] [CrossRef]
- Kean, J.; Suckling, D.; Sullivan, N.; Tobin, P.; Stringer, L.; Smith, G.; Kimber, B.; Lee, D.; Flores Vargas, R.; Fletcher, J.; et al. Global eradication and response database. Available online: http://b3.net.nz/gerda (accessed on 27 January 2019).
- Pluess, T.; Jarosik, V.; Pysek, P.; Cannon, R.; Pergl, J.; Breukers, A.; Bacher, S. Which factors affect the success or failure of eradication campaigns against alien species? PLoS ONE 2012, 7, e48157. [Google Scholar] [CrossRef] [PubMed]
- Beric, B.; MacIsaac, H.J. Determinants of rapid response success for alien invasive species in aquatic ecosystems. Biol. Invasions 2015, 17, 3327–3335. [Google Scholar] [CrossRef]
- Peay, S.; Hiley, P.D.; Collen, P.; Martin, I. Biocide treatment of ponds in Scotland to eradicate signal crayfish. Bulletin Français de la Pêche et de la Pisciculture 2006, 380–381, 1363–1379. [Google Scholar] [CrossRef]
- Freeman, M.A.; Turnbull, J.F.; Yeomans, W.E.; Bean, C.W. Prospects for management strategies of invasive crayfish populations with an emphasis on biological control. Aquat. Conserv. Mar. Freshwater Ecosyst. 2010, 20, 211–223. [Google Scholar] [CrossRef]
- Stebbing, P.; Longshaw, M.; Scott, A. Review of methods for the management of non-indigenous crayfish, with particular reference to Great Britain. Ethol. Ecol. Evol. 2014, 26, 204–231. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Macdonald, D.W. The effect of manual removal on movement distances in populations of signal crayfish (Pacifastacus leniusculus). Freshw. Biol. 2011, 56, 2370–2377. [Google Scholar] [CrossRef]
- Aquiloni, L.; Becciolini, A.; Berti, R.; Porciani, S.; Trunfio, C.; Gherardi, F. Managing invasive crayfish: Use of X-ray sterilisation of males. Freshw. Biol. 2009, 54, 1510–1519. [Google Scholar] [CrossRef]
- Aquiloni, L.; Gherardi, F. The use of sex pheromones for the control of invasive populations of the crayfish Procambarus clarkii: A field study. Hydrobiologia 2010, 649, 249–254. [Google Scholar] [CrossRef]
- Aquiloni, L.; Brusconi, S.; Cecchinelli, E.; Tricarico, E.; Mazza, G.; Paglianti, A.; Gherardi, F. Biological control of invasive populations of crayfish: The European eel (Anguilla anguilla) as a predator of Procambarus clarkii. Biol. Invasions 2010, 12, 3817–3824. [Google Scholar] [CrossRef]
- Vennie-Vollrath, E.; Van Egeren, S. Aquatic Invasive Species Highlight: Red swamp crayfish. Available online: https://lakes-l.blogs.govdelivery.com/2012/06/aquatic-invasive-species-highlight-red-swamp-crayfish/ (accessed on 20 December 2018).
- Hein, C.L.; Vander Zanden, M.J.; Magnuson, J.J. Intensive trapping and increased fish predation cause massive population decline of an invasive crayfish. Freshw. Biol. 2007, 52, 1134–1146. [Google Scholar] [CrossRef]
- Ray, J.; Stevens, V. Using Baytex to Control Crayfish in Ponds. Progress. Fish Cult. 1970, 32, 58–60. [Google Scholar] [CrossRef]
- Chang, V.C.S.; Lange, W.H. Laboratory and Field Evaluation of Selected Pesticides for Control of the Red Crayfish in California Rice Fields. J. Econ. Entomol. 1967, 60, 473–477. [Google Scholar] [CrossRef]
- Cheah, M.-L.; Avault, J.W.; Graves, G.B. Some effects of thirteen rice pesticides on crawfish Procambarus clarkii and P. acutus acutus. Freshw. Crayfish 1979, 4, 350–361. [Google Scholar]
- Laurent, P.J. Eradication of unwanted crayfish species of astacological management purposes. Freshw. Crayfish 1995, 8, 121–133. [Google Scholar]
- Jaga, K.; Dharmani, C. Sources of exposure to and public health implications of organophosphate pesticides. Pan Am. J. Public Health 2003, 14, 171–185. [Google Scholar] [CrossRef]
- Leahey, J.P. The metabolism and environmental degradation of the pyrethroid insecticides. Outlook Agric. 1979, 10, 135–142. [Google Scholar] [CrossRef]
- Palmquist, K.; Salatas, J.; Fairbrother, A. Pyrethroid insecticides: Use, environmental fate, and ecotoxicology. In Insecticides–Advances in Integrated Pest Management; Perveen, F., Ed.; InTech: London, UK, 2012; pp. 251–278. [Google Scholar]
- Eversole, A.G.; Seller, B.C. Comparison of relative crayfish toxicity values. Freshw. Crayfish 1997, 11, 274–285. [Google Scholar]
- Bills, T.D.; Marking, L. Control of nuisance populations of crayfish with traps and toxicants. Progress. Fish Cult. 1988, 50, 103–106. [Google Scholar] [CrossRef]
- Kozak, P.; Policar, T. Practical elimination of signal crayfish, Pacifastacus leniusculus (Dana), from a pond. In Proceedings of the Management & Conservation of Crayfish, Bristol, UK, 7 November 2002; pp. 200–208. [Google Scholar]
- Bohman, P.; Nordwall, F.; Edsman, L. The effect of the large-scale introduction of signal crayfish on the spread of crayfish plague in Sweden. Bulletin Français de la Pêche et de la Pisciculture 2006, 380–381, 1291–1302. [Google Scholar] [CrossRef]
- Bohman, P.; Degerman, E.; Edsman, L.; Sers, B. Exponential increase of signal crayfish in running waters in Sweden—Due to illegal introductions? Knowl. Manag. Aquat. Ecosyst. 2011, 401, 23. [Google Scholar] [CrossRef]
- Johnsen, S.; Taugbøl, T.; Andersen, O.; Museth, J.; Vrålstad, T. The first record of the non-indigenous signal crayfish Pasifastacus leniusculus in Norway. Biol. Invasions 2007, 9, 939–941. [Google Scholar] [CrossRef]
- Gederaas, L.; Salvesen, I.; og Viken, Å. Norsk svarteliste 2007—Økologiske risikovurderinger av fremmede arter. Available online: https://www.artsdatabanken.no/fremmedearter/svartelista2007 (accessed on 30 October 2018).
- Ljunggren, N.; Sundin, J. Eliminering av signalkräfta på Gotland: En redovisning av utförda åtgärder inom åtgärdsprogrammet för bevarande av flodkräfta under 2007-2009; Länsstyrelsen i Gotlands län: Visby, Sweden, 2010; ISSN 1653-7041.
- Sandodden, R.; Johnsen, S.I. Eradication of introduced signal crayfish Pacifastacus leniusculus using the pharmaceutical BETAMAX VET®. Aquat. Invasions 2010, 5, 75–81. [Google Scholar] [CrossRef]
- Sandodden, R.; Bardal, H. Bekjempelse av signalkreps (Pasifastacus leniusculus) på Ostøya i Bærum kommune. In Veterinærinstituttets Rapportserie; Veterinærinstituttet: Oslo, Norway, 2010; Volume 1–2010. [Google Scholar]
- Lewis, S.D. Pacifastacus. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2002; pp. 511–540. [Google Scholar]
- Havs-och vattenmyndigheten. Fisk-och skaldjursbestånd i hav och sötvatten 2017 Resursöversikt; Havs-och vattenmyndigheten: Göteborg, Sweden, 2018; p. 273. [Google Scholar]
- Green, N.; Bentley, M.; Stebbing, P.; Andreou, D.; Britton, R. Trapping for invasive crayfish: Comparisons of efficacy and selectivity of baited traps versus novel artificial refuge traps. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 15. [Google Scholar] [CrossRef]
- Peay, S. A cost-led evaluation of survey methods and monitoring for white-clawed crayfish—Lessons from the UK. Bulletin Français de la Pêche et de la Pisciculture 2004, 372–373, 335–352. [Google Scholar] [CrossRef]
- Hiley, P.D.; Peay, S. Signal crayfish eradication—Preliminary biocides trial. Freshw. Crayfish 2006, 15, 261–270. [Google Scholar]
- O’Reilly, S. Assessing the Toxicity of Biocides on the North American Signal Crayfish Pacifastacus leniusculus (Dana) to Aid Eradication. Master’s Thesis, Glasgow University, Glasgow, UK, 2015. [Google Scholar]
- Johnsen, B.O.; Jensen, A.J. The Gyrodactylus story in Norway. Aquaculture 1991, 98, 289–302. [Google Scholar] [CrossRef]
- Sandodden, R. Eradication of Gyrodactylus salaris infested Atlantic salmon (Salmo salar) in the Rauma River, Norway, using rotenone. Manag. Biol. Invasions 2018, 9. [Google Scholar] [CrossRef]
- Sandodden, R.; Bardal, H. Bekjempelse av Signalkreps (Pasifastacus leniusculus) og sørv (Scardinius erythrophthalmus) i Dammane landskapsvernområde. In Veterinærinstituttets rapportserie; Veterinærinstituttet: Oslo, Norway, 2008; Volume 15–2008, pp. 1–23. [Google Scholar]
- Abrahamsson, S.A.A.; Goldman, C.R. Distribution, density and production of the crayfish Pacifastacus leniusculus Dana in Lake Tahoe, California—Nevada. Oikos 1970, 21, 83–91. [Google Scholar] [CrossRef]
- Acosta, C.A.; Perry, S.A. Effective sampling area: A quantitative method for sampling crayfish populations in freshwater marshes. Crustaceana 2000, 73, 425–431. [Google Scholar]
- Strand, D.A.; Jussila, J.; Johnsen, S.I.; Viljamaa-Dirks, S.; Edsman, L.; Wiik-Nielsen, J.; Viljugrein, H.; Engdahl, F.; Vrålstad, T. Detection of crayfish plague spores in large freshwater systems. J. Appl. Ecol. 2014, 51, 544–553. [Google Scholar] [CrossRef]
- Nygren, D. Direkta effekter av insekticiden deltametrin på zooplankton och bottenfauna: En fältstudie av bieffekter av insekticidinducerad eliminering av signalkräfta på Gotland. Master’s Thesis, Gotland University, Gotland, Sweden, 2009. [Google Scholar]
- Axelsson, E.; Nyström, P.; Sidenmark, J.; Brönmark, C. Crayfish predation on amphibian eggs and larvae. Amphibia-Reptilia 1997, 18, 217–228. [Google Scholar] [CrossRef]
- Cruz, M.J.; Segurado, P.; Sousa, M.; Rebelo, R. Collapse of the amphibian community of the Paul do Boquilobo Natural Reserve (central Portugal) after the arrival of the exotic American crayfish Procambarus clarkii. Herpetol. J. 2008, 18, 197–204. [Google Scholar]
- Ficetola, G.F.; Siesa, M.E.; De Bernardi, F.; Padoa-Schioppa, E. Complex impact of an invasive crayfish on freshwater food webs. Biodivers. Conserv. 2012, 21, 2641–2651. [Google Scholar] [CrossRef]
- Peay, S.; Guthrie, N.; Spees, J.; Nilsson, E.; Bradley, P. The impact of signal crayfish (Pacifastacus leniusculus) on the recruitment of salmonid fish in a headwater stream in Yorkshire, England. Knowl. Manag. Aquat. Ecosyst. 2009, 394–395. [Google Scholar] [CrossRef]
- Gunasekara, A.S. Environmental Fate of Pyrethrins; Environmental Monitoring Branch, Department of Pesticide Regulation: Sacramento, CA, USA, 2005; p. 22.
- Hand, L.H.; Kuet, S.F.; Lane, M.C.G.; Maund, S.J.; Warinton, J.S.; Hill, I.R. Influences of aquatic plants on the fate of the pyrethroid insecticide Lambida-cyhalothrin in aquatic environments. Environ. Toxicol. Chem. 2001, 20, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Peay, S.; Dunn, A.M. The behavioural response of the invasive signal crayfish Pacifastacus leniusculus to experimental dewatering of burrows and its implications for eradication treatment and management of ponds with crayfish. Ethol. Ecol. Evol. 2014, 26, 277–298. [Google Scholar] [CrossRef]
- Thomas, J.R.; Fisher, J.; Cable, J.; Griffiths, S.W. Terrestrial dispersal of invasive signal crayfish during vulnerable life stages. Behav. Process. 2018, 157, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Sandodden, R.; Bjøru, B. Bekjempelse av Signalkreps (Pasifastacus leniusculus) i Dammane landskapsvernområde. In Veterinærinstituttets rapportserie; Veterinærinstituttet: Oslo, Norway, 2007; Volume 3–2007, pp. 1–29. [Google Scholar]
- APEM. Eradicating signal crayfish at Dalbeattie Reservoir. Available online: http://www.apemltd.co.uk/eradicating-signal-crayfish-dalbeattie-reservoir/ (accessed on 30 October 2018).
- Bunk, H.; Van Egeren, S. Containment, Control and Eradication of Ambitious Architects: Procambarus clarkii, The Red Swamp Crayfish. Available online: https://www.uwsp.edu/cnr-ap/UWEXLakes/Documents/programs/convention/2013/HeidiBunk-ContainmentControlAndEradicationofRedSwampCrayfish.pdf (accessed on 30 October 2018).
- URS Environment and Infrastructure UK. Design of Catchpit Barriers for Signal Crayfish at None-Go-Bye Farm; Report to Environment Agency; URS Environment and Infrastructure UK: Leeds, UK, 2012. [Google Scholar]
- Peay, S. Eradication of Alien Crayfish Populations, Environment Agency Technical R&D Report W1-037/TR. Available online: http://www.freshwaterlife.org/projects/media/projects/images/2/52902_ca_object_representations_media_226_original.pdf (accessed on 30 October 2018).
- Cecchinelli, E.; Aquiloni, L.; Maltagliati, G.; Orioli, G.; Tricarico, E.; Gherardi, F. Use of natural pyrethrum to control the red swamp crayfish Procambarus clarkii in a rural district of Italy. Pest Manag. Sci. 2012, 68, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Steckart, B. Aquatic Invasive Species Update, Waukesha County September 2017. Available online: https://www.waukeshacounty.gov/globalassets/parks--land-use/land-conservation/aquatic-invasive-species/copy-of-aquatic-invasive-species-update-sept2017.pdf (accessed on 30 October 2018).
- Peay, S. Developing tools for the management of freshwater crayfish. Ph.D. Thesis, University of Leeds, Leeds, UK, 2013. [Google Scholar]
- Dougherty, M.M.; Larson, E.R.; Renshaw, M.A.; Gantz, C.A.; Egan, S.P.; Erickson, D.M.; Lodge, D.M. Environmental DNA (eDNA) detects the invasive rusty crayfish Orconectes rusticus at low abundances. J. Appl. Ecol. 2016, 53, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Cowart, D.A.; Breedveld, K.G.H.; Ellis, M.J.; Hull, J.M.; Larson, E.R. Environmental DNA (eDNA) applications for the conservation of imperiled crayfish (Decapoda: Astacidea) through monitoring of invasive species barriers and relocated populations. J. Crustac. Biol. 2018, 38, 257–266. [Google Scholar] [CrossRef]
- Geerts, A.N.; Boets, P.; Van den Heede, S.; Goethals, P.; Van der heyden, C. A search for standardized protocols to detect alien invasive crayfish based on environmental DNA (eDNA): A lab and field evaluation. Ecol. Indic. 2018, 84, 564–572. [Google Scholar] [CrossRef]
- Harper, K.; Anucha, N.P.; Turnbull, J.F.; Bean, C.W.; Leaver, M.J. Searching for a signal: Environmental DNA (eDNA) for the detection of invasive signal crayfish, Pacifastacus leniusculus (Dana, 1852). Manag. Biol. Invasions 2018, 9, 137–148. [Google Scholar] [CrossRef]
- Hulme, P.E. Beyond control: Wider implications for the management of biological invasions. Journal of Appl. Ecol. 2006, 43, 835–847. [Google Scholar] [CrossRef]
- Myers, J.H.; Simberloff, D.; Kuris, A.M.; Carey, J.R. Eradication revisited: Dealing with exotic species. Trends Ecol. Evol. 2000, 15, 316–320. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).