3. Taxonomy
Tribe Anchonini Imhoff 1856
Anchonidae Imhoff 1856-XX [
17] (
Anchonus Schoenherr)
Anchoninae; Faust 1892-19 [
18]
Anchonina; Champion, 1902-66 [
19]
Anchonini; Blatchley & Leng 1916-518,k [
20]
Species of the tribe Anchonini are flightless, apterous, and easily distinguishable from other Molytinae by the following characters: basal club antennomere separate from rest of club and similar in pubescence to other funicle antennomeres; eyes flattened; ommatidia lightly domed to flattened; penis sub-cylindrical to cylindrical, penis apodemes not fused to penis, tegminal ring longitudinally expanded and mostly membranous, tendons generally densely present on tegmen; females with bursa absent, vestigial, or elongate, and with ridges running longitudinally and teeth present within; bursal atrium present, well-developed, and folded within, more so if bursa absent or vestigial. Some of these characters are discussed above; a more comprehensive description of the Anchonini genitalic characters and synapomorphies is being developed for publication by Lyal & Cristóvão [
21].
These apomorphic character states are shared by the two African genera described below, placing them unequivocally in the tribe.
Genus Aethiopacorep Voisin, 1992
Acorep (
Aethiopacorep) Voisin, 1992: 265 [
22]. Type species
Anchonus africanus Hustache, 1932, by monotypy.
Aethiopacorep; Poinar & Voisin, 2002: 381 [
2] stat. nov.
Aethiopacorep Voisin, 1992 was elevated from a subgenus of
Acorep Voisin, 1992 to genus by Poinar & Voisin [
2], together with others that are described by the same author in 1992 and 1994 [
22,
23]. However, the reasons for this decision were not specified and a clear description of this genus was lacking.
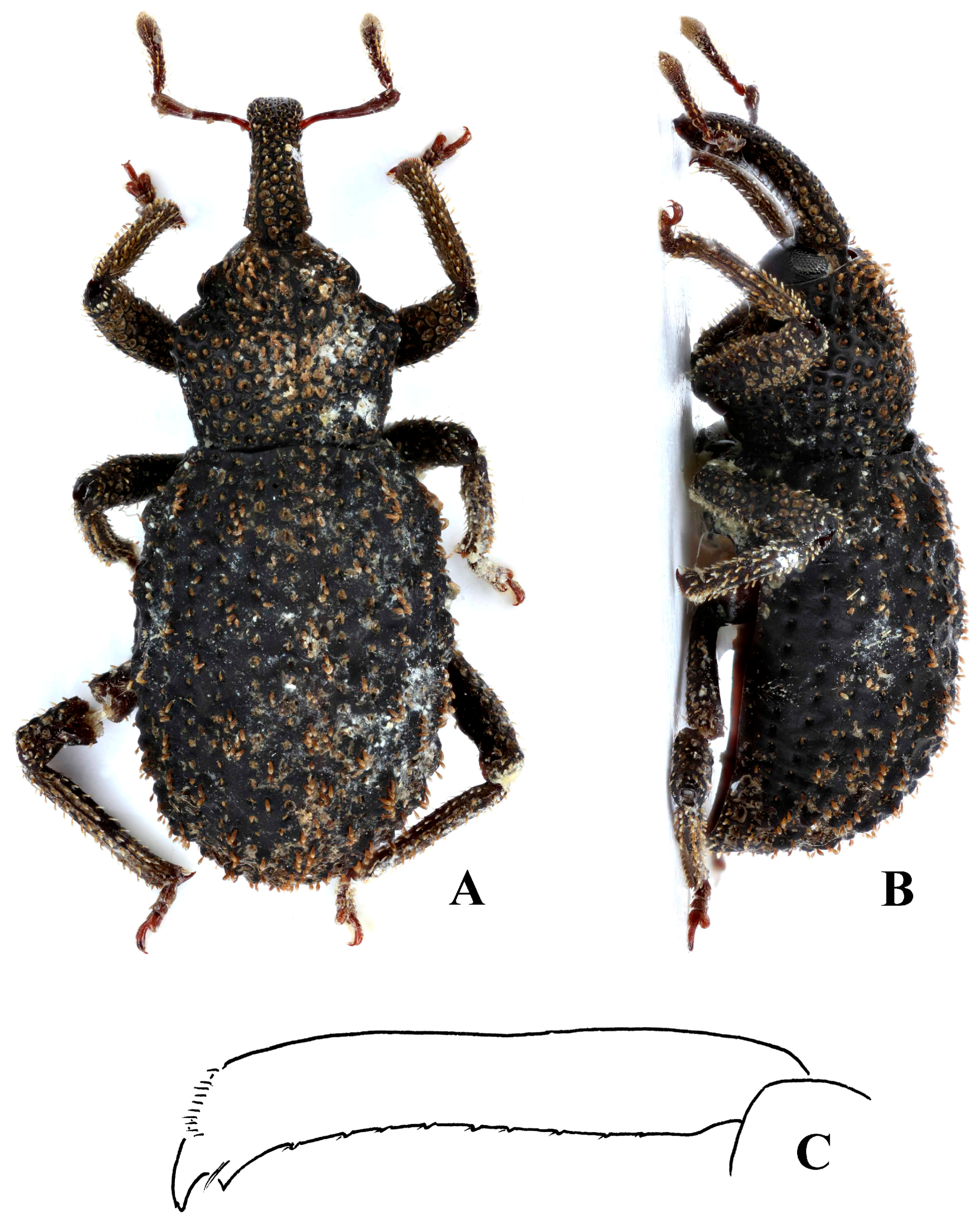
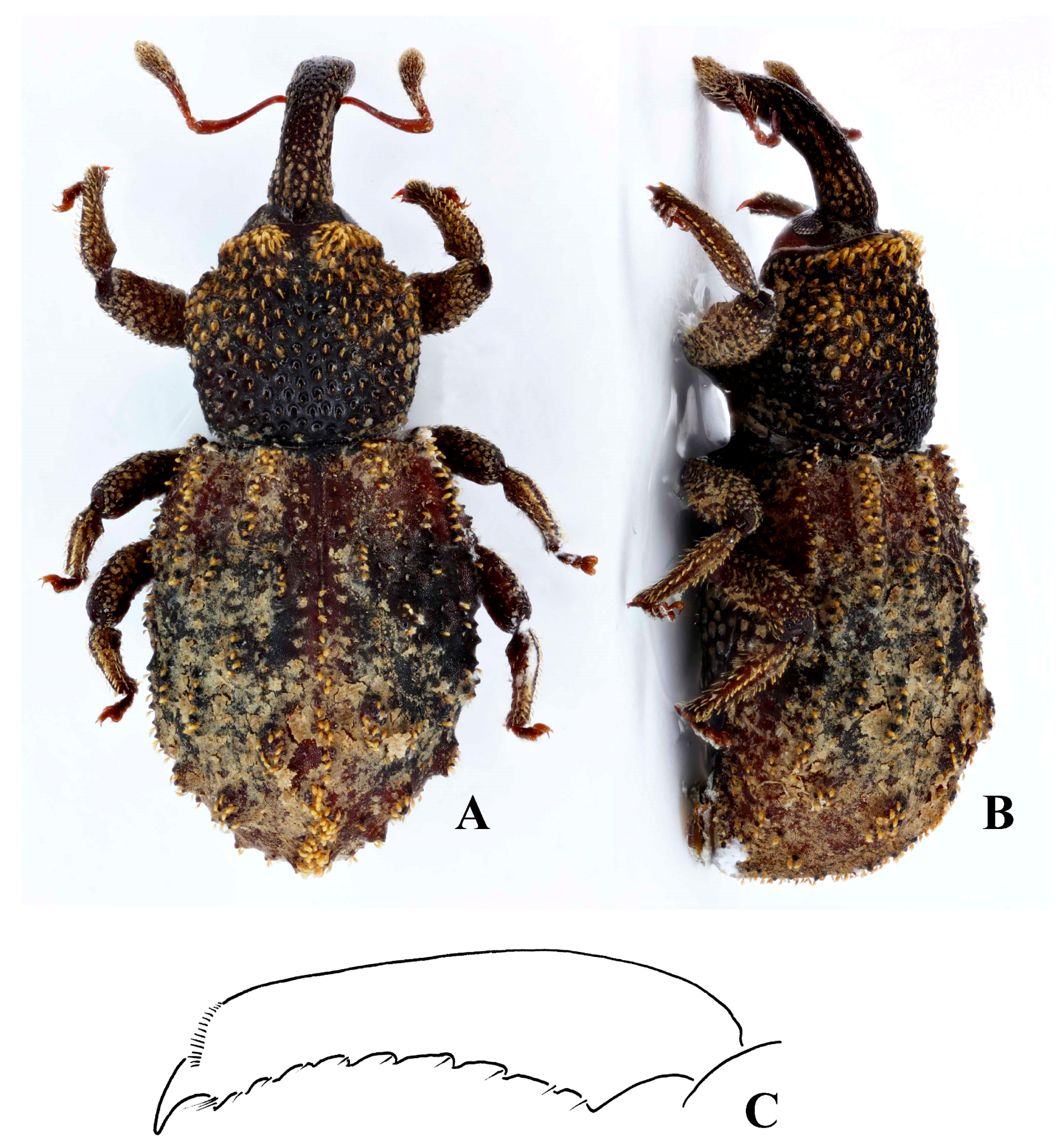
Redescription. Length 3.8–4.2 mm; apterous; rufous.
Head. Head capsule dorsally with fine reticulation, not glossy. Eyes flat, oval; ommatidia separately convex. Scrobes diagonal, opening ventrally for most of length; not punctate internally. Rostrum punctate dorsally, laterally and ventrally, with weak irregular longitudinal carinae; each punctures with short erect scale. Club with basal antennomere separate from the rest of club, its pilosity being similar to that of funicle antennomeres.
Thorax. Pronotum with punctation deep; widest in basal half; anteriorly constricted laterally so collar formed; pair of weak submarginal prominences anteriorly. Scutellum concealed.
Elytra. Broader than the pronotum, longer than wide; weakly convex dorsally, apical declivity steep. Tubercles at base of interstriae 3 and 5 elevated and elongate, extending anterior to base on interstria 5; interstriae otherwise with scattered elongate tubercles of similar height, somewhat asymmetrical.
Legs. Femora weakly setose, pair of longitudinal rows of pale erect scales ventrally; tibiae with pale erect scales in longitudinal rows. Tibiae weakly curving ventrad apically. Tarsomere 3 symmetrical.
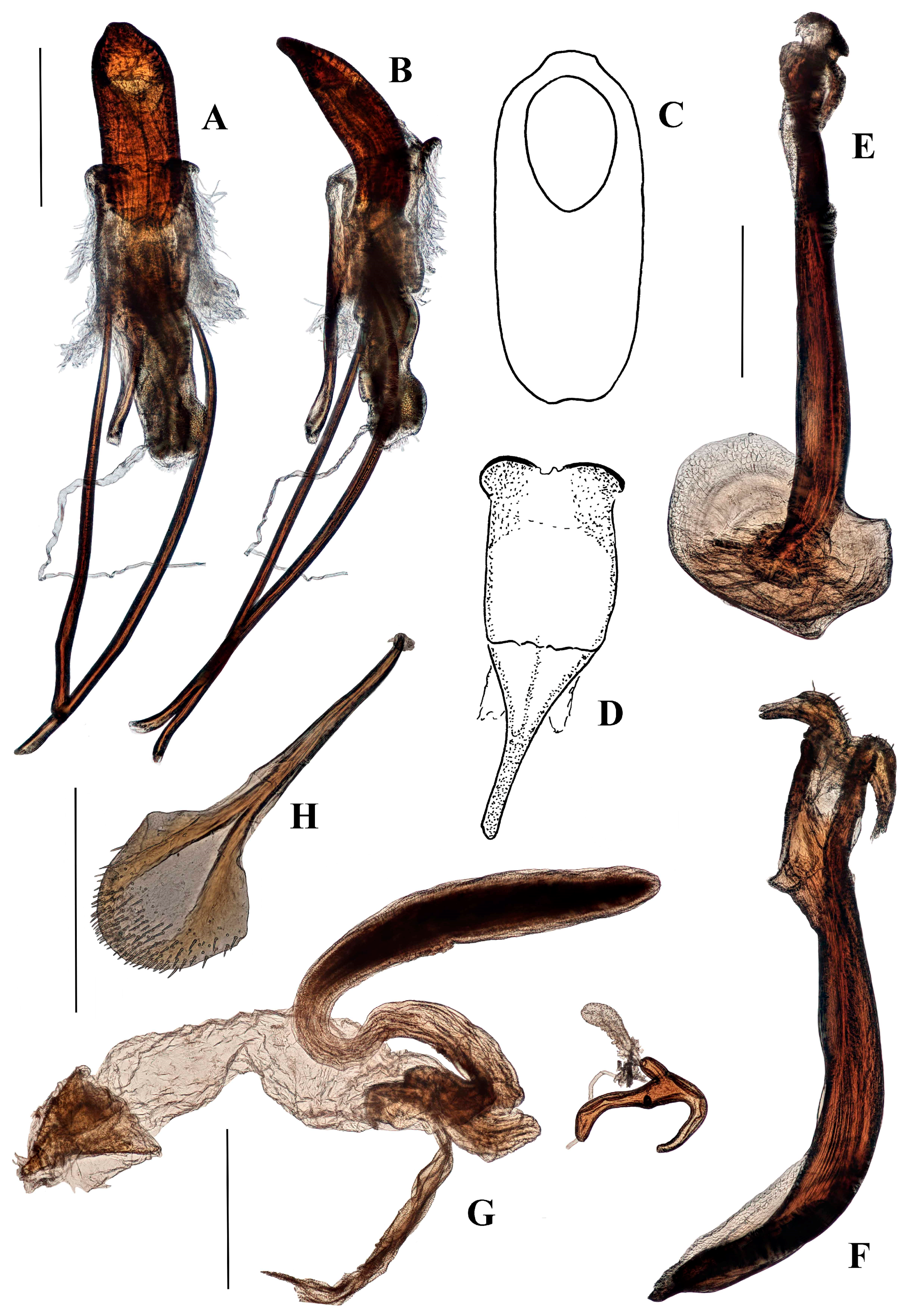
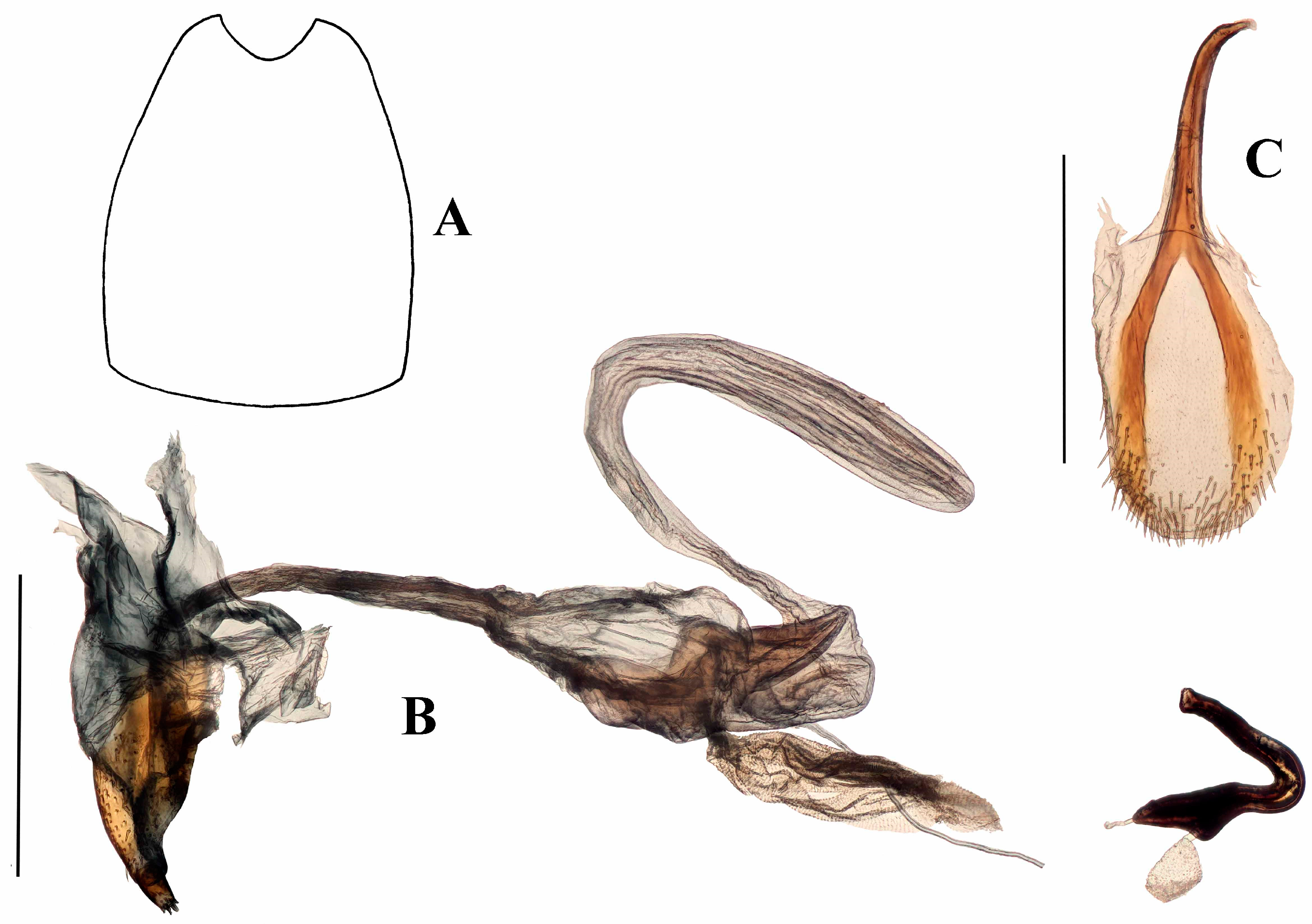
Terminalia. Male. Penis cylindrical, curved ventrad basally and apically, well sclerotised and pigmented. Pigmented quadrate patterns present dorso-apically within the ostium. Penis apodemes pigmented, connected to tegminal membrane instead of the base of penis. Tegminal ring expanded, unpigmented, mostly membranous, lacking parameroid lobes or tegminal plates dorsally; tegminal apodeme pigmented; tegmen lacking tendons. Spiculum gastrale slender, with basal arms elongate, sub-parallel for most of length; lateral flange anterior to basal arms absent; apex spatulate, not circular.
Female. Tergite VII with posterior margin broadly emarginate (Figure 4F). Spiculum ventrale with posterior margin convex. Vagina with membrane thin, weakly pigmented. Bursal atrium pigmented with concavity from which common oviduct and spermathecal duct arise. Bursa elongate, constricted at base. Bursal membrane thick, pigmented, lacking longitudinal folds; teeth present within bursa. Spermatheca slender, tubular, curved, with duct lobe weak, lateral.
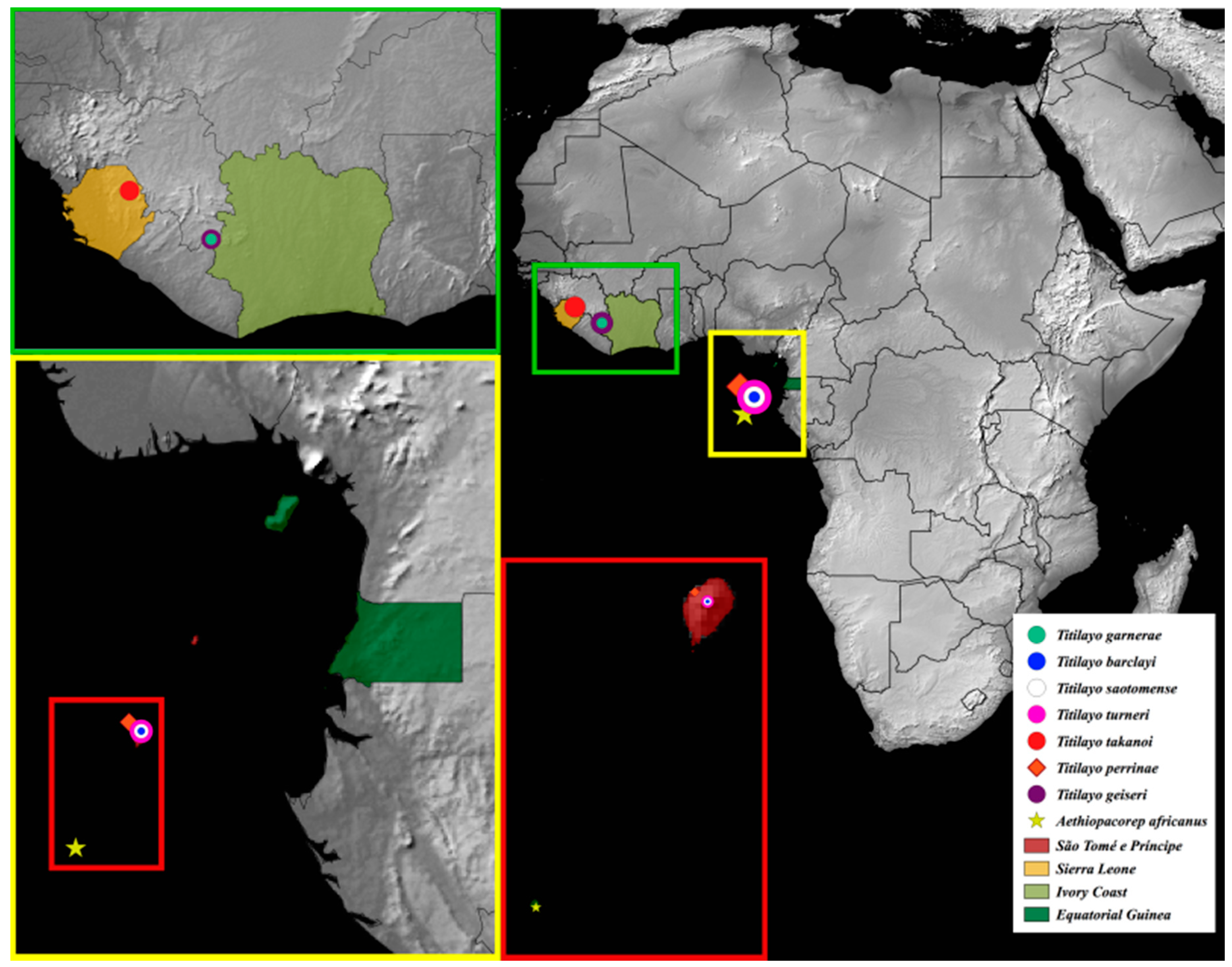
Distribution.Aethiopacorep is known only from the type locality, Annobón Island of Equatorial Guinea (Figure 20).
Remarks.Aethiopacorep lacks any clear synapomorphies with the other African genus, Titilayo gen. nov. It can be distinguished from almost all other genera of Anchonini by the combination of weakly expanded apex of the male spiculum gastrale and the lack of tendons on the tegmen. The species of the American genus Gonianchonus share these characters but the females lack a bursa, while Aethiopacorep has a very large, elongate bursa. Rhyparonotus impar Voss, from St Helena, also has a very weak apex to the spiculum gastrale and lacks tendons on the tegmen, but in this species the bursa is far more membranous and the ommatidia are much less concave and more united. Relationships of Aethiopacorep are unclear but they may lie with Acorep and related genera, based on the form of the bursa. Although the genus was originally described as a subgenus of Acorep, no synapomorphies were given to justify this, a situation characteristic of most if not all Anchonine genera.
Anchonus africanus Hustache, 1932: 50 [
24].
Acorep (
Aethiopacorep)
africanus; Voisin, 1992: 266 [
22].
Aethiopacorep africanus; Poinar & Voisin, 2002: 381 [
2].
Diagnostic characters.
Aethiopacorep africanus can be distinguished from other known African species of Anchonini by the lack of tegminal plates on the male genitalia. It can be distinguished from other known species of Anchonini both in and outside Africa by the combination of: spiculum gastrale with apex weakly expanded; tegmen lacking tendons (
Figure 4B); bursa very large, elongate, membrane thick and pigmented (
Figure 4D). While there are currently no other known species of
Aethiopacorep it is expected that the detail of the male and female genitalia, as figured here, will be sufficient to distinguish
A. africanus.
Redescription. Length 4.1–4.6 mm. Apterous. Completely rufous.
Head. Rufous. Head capsule dorsally with fine reticulation, not glossy. Eyes medium-sized, approximately 0.83 × depth of rostrum where it meets head capsule; dorsal margin lower than top of rostrum basally; flat, oval, just less than twice as long as deep; ommatidia hemispherical and well-defined, lacking dips at the centre of each ommatidium. Rostrum arising abruptly from head capsule with dorsal margin and head capsule forming an obtuse angle; no notch dorsally between head capsule and rostrum, weak notch laterally at base of scrobe. Rostrum curved ventrad, more strongly so distal to antennal insertions; strongly punctate dorsally, laterally and ventrally, punctures sometimes confluent along rostrum, especially laterally, causing irregular longitudinal carinae dorsally; punctation in females shallower and rostrum smoother, especially from antennal insertions to apex; each puncture with short (1/2 puncture length) golden scale. Antennae rufous. First funicle antennomere shorter than second; club with basal antennomere separate from rest of club but broadened to meet rest of club, less setose and more glossy than other club antennomeres, which are obscured by dense golden setae; club oval.
Thorax. Pronotum approximately 0.4 times the length of elytra; pronotal breadth: length ratio 1.22; broadest before middle; collar apparent laterally; dorsally convex in lateral aspect. Punctation coarse, deep; anteriorly submarginally with weak prominence on each side of midline punctures at these protuberances, as well as dorsally across broadest part of pronotum, with scales that are up to 3.5 times longer than those found over rest of pronotum.
Elytra. Elytra broader than pronotum; approximately 1.4 times longer than broad; lightly convex dorsally with steep apical declivity. Interstria 1 lacking tubercles; other interstriae with elevated, elongate tubercles weakly or not symmetrical between elytra, basal tubercle on interstriae 3 and 5, that on 5 projecting anterior to basal elytral margin. Each tubercle with several long golden scales that are identical to those on pronotal protuberances.
Legs. Tibia curving ventrad apically, more strongly in fore tibia than others. Neat rows of sparse setae running longitudinally between base and apex. Ventral longitudinal row of teeth present at apical 1/3 of all tibiae. Single anterior apical setal comb and double posterior apical setal comb on fore tibia. Tarsomere 3 symmetrical, lobes separate for 1/3 length of tarsomere.
Genitalia. Male (
Figure 4A–C). Penis cylindrical, curved ventrad basally and apically; thoroughly sclerotised and pigmented brown, including dorsally; pigmented quadrate patterns apically. Endophallus with longitudinal dense bands of very small teeth. Ductus seminalis arising subapically on endophallus. Penis apodemes connected to an unpigmented membrane instead of the base of penis, pigmented dark brown. Tegmen unpigmented with exception of its apodeme, which is fully pigmented; tegminal ring membranous, expanded, apodeme extending beyond rest of tegmen by about 0.2 of total tegmen length; tendons absent. Spiculum gastrale with apex spatulate. Apodemes of spiculum gastrale fused medially.
Female (
Figure 4D–F). Tergite VII with posterior margin broadly emarginate. Spiculum ventrale with posterior margin convex. Vagina with membrane thin, unpigmented. Bursal atrium pigmented with concavity from which common oviduct and spermathecal duct arise, slightly separate. Bursa elongate, constricted at base, lacking longitudinal folds; bursal membrane thick, pigmented; teeth present within bursa. Spermatheca slender, curved, of similar diameter throughout.
Type locality: Equatorial Guinea, Annobón Island.
Type material: NEOTYPE, here designated, ♂, with labels: “Neo- / type” (purple-bordered disk) and “ANNOBON IS: / 9.vii.1959-22.vii.1959. / Cambridge Univ. Exped. / B.M.1960-51” and “NEOTYPE / Anchonus africanus / Hustache, 1932 / Cristóvão & Lyal 2018”. PARANEOTYPES: 2♀♀ with same data as Neotype and with labels: “Paraneo- / type” (yellow-bordered disk) and “PARANEOTYPE / Anchonus africanus / Hustache, 1932 / Cristóvão & Lyal 2018”.
Depository: BMNH.
Neotype designation. A neotype is designated for
Anchonus africanus Hustache, 1932 in accordance with Article 75.3 of the Code of Zoological Nomenclature. This was the first species of Anchonini to be described from an African locality, Annobón island. Three syntypes were deposited in Hamburg Museum. In 1943, during World War II, Hamburg was bombed; Weidner [
10] states that the Museum’s Coleoptera collection was burnt, with the exception of the larval spirit collection. The account of some of the lost material [
9,
10], is partial and it mentions only one Anchonini species (
Anchonus assimilis Voss, 1954). Professor Thure Dalsgaard has confirmed [
25] that no specimens of the species could be found in the collection in Hamburg. Hustache is known to have sometimes kept syntype specimens as desiderata for his collection, currently found in the MNHN [
26]. However, Hélène Perrin confirms [
27] that no specimen(s) could be found in Hustache’s collection or the main collection of Anchonini in the MNHN.
The designation of the Neotype is performed here with the express purpose of clarifying the taxonomic status of the species. Anchonini are a very speciose group, and morphological differences between species are often very subtle. Very few of the published descriptions are adequate to distinguish species, and virtually none have images of the male or female genitalia, which appear to hold good diagnostic characters. Hustache’s description of A. africanus is adequate but it contains no characters that appear at first sight to be obvious apomorphies that might serve to distinguish a species. While currently we have seen only one species from Annobón, the presence of multiple sympatric species of Titilayo in Africa, and similar sympatry in congeneric Anchonini species in Central and South America, suggests the likelihood of further species of Aethiopacorep being discovered in time. Fixing the identity of the species (and consequently the genus) at this point allows for a description of the new genus Titilayo, which might otherwise be confused with Aethiopacorep, and will also support current revisionary work on the tribe by Cristóvão & Lyal.
A statement of distinguishing characters is given above (Art. 75.3.2).
The specimens listed above, which form the neotype series, are from the type locality of Annobón Island and fit the description given by Hustache [
24].
Genus Titilayo Cristóvão and Lyal gen nov.
Type species. Titilayo geiseri sp. nov.
Diagnostic characters. Despite its external similarity to
Spinanchonus Voisin 1992 [
22,
23], especially
S. galapagoensis,
Titilayo gen. nov. can be separated from all other genera of Anchonini by the presence of tegminal plates in the male terminalia (Figures 6E, 12D, 13, 15C and 17C), which is an apomorphy within this tribe (see “remarks”).
Description. Length 3.5–5 mm; apterous; black and rufous to completely black. Often with gummy substance coating part of body.
Head. Black to black and rufous; capsule dorsally with fine reticulation, not glossy. Eyes not protruding, oval, ommatidia separately weakly convex. Scrobes lateral for at least first quarter, then running ventrally until base; not punctate internally; antennal insertion closer to apex of rostrum in male than in female. Rostrum punctate dorsally, laterally, and ventrally sometimes shallowly, so, each puncture bearing a short scale that is no longer than the radius of each puncture; weak and generally irregular longitudinal carinae present latero-dorsally; often a dorsal notch present at base of rostrum where it meets head capsule. Club with basal antennomere separate from the rest of the club; other club antennomeres obscured by dense golden setae.
Thorax. Pronotum with punctation deep, strong, sometimes punctures confluent and broad glossy irregular ridges formed between them; widest in basal half, generally fairly abruptly widened behind collar; collar constriction strong laterally and weaker dorsally; pair of sometimes very weak submarginal prominences anteriorly, bearing erect scales. Scutellum concealed.
Elytra. Black, sometimes with rufous patches. Broader than the pronotum, longer than wide; weakly convex dorsally, apical declivity steep. Tubercles at the base of interstria 3 and 5 elevated and elongate, beginning at or just past the base of the elytra. Tubercles small to large and elliptical with some merging together, sometimes extending anterior to base on interstria 5; interstriae otherwise with scattered elongate tubercles that are generally of similar height, being somewhat asymmetrical.
Legs. Black, rufous, or both. Femora with disorganised deep punctation, each puncture bearing a golden seta of approximately the same diameter of the puncture. Tibiae curving apically, with single anterior and double posterior apical setal comb. Tarsi rufous.
Abdomen. Tergites I-VI weakly or not pigmented. Rectal ring present, with posteriorly convex loops between six nodes.
Terminalia. Male. Penis sub-cylindrical to cylindrical, weakly curving basally and apically, well sclerotised and pigmented. Pigmented quadrate patterns present dorso-apically inside ostium. Penis apodemes pigmented dark-brown, connected to tegminal membrane instead of base of penis. Tegminal ring expanded, unpigmented, mostly membranous, with two asymmetrical lobes ventrally and two tegminal plates dorsally (see remarks); tegminal ring and plates bearing tendons. Spiculum gastrale with apex large, concave, laminate; basal arms of similar size, right arm (in dorsal aspect) with crest-like lobe anteriorly.
Female. Vaginal membrane weakly pigmented. Bursal atrium expanded and folded internally, creating a concavity from which common oviduct arises, generally expanded, and membranous between the folded area and the junction with the bursal duct (Figures 10B, 12G, 15F and 19B), the only exception being found in T. garnerae (Figure 8F). Bursa elongate with narrow or broad bursal duct; minute teeth present in bursa. Collum of spermatheca expanded or more cylindrical.
Composition. Other than the type species, this genus currently includes Titilayo takanoi sp. nov., T. garnerae sp. nov., T. perrinae sp. nov., T. saotomense sp. nov., T. turneri sp. nov. and T. barclayi sp. nov.
Distribution. Ivory Coast, Mount Nimba; Sierra Leone, Loma Mountains; and São Tomé e Príncipe, Isle of São Tomé (Figure 20).
Etymology. The name “Titilayo” comes from the Yoruba language which is spoken in many countries in Western Africa and means “everlasting joy”. Despite it being a name that is given to females, it is here considered as neuter.
Remarks. Members of the Anchonini generally lack parameroid lobes, the known exceptions being Capsonotus smilodon (Voisin, 2006), Rhyparonotus libertinus (Faust, 1892) and Sulconotus scapha (Faust, 1893). In Titilayo, the tegmen bears a pair of transverse sclerotised, pigmented plates dorsally on the ring, extending weakly posteriad (Figure 13). These “tegminal plates” may join dorsally, as seen in Titilayo geiseri (Figure 6A,B,E) or are separated, to various degrees, on the membranous, unpigmented tegminal ring, as seen in T. perrinae (Figures 12A,B,D and 13), T. saotomense (Figure 15A–C), and T. turneri (Figure 17A–C). The plates may also extend apical and basally (e.g., T. geiseri) or apically only (e.g., T. perrinae) (penis position as reference). The plates bear tendons that are similar to those found throughout the apically-expanded, asymmetrically-lobed tegminal ring, albeit smaller. The tegminal plates are not considered homologous to the parameroid lobes, but a novel apomorphic structure, due to the differences of the structures’ position and morphology.
The tegmen is more sclerotised and less membranous in Titilayo than in most other Anchonini.
Titilayo gen. nov. can be easily distinguished from Aethiopacorep, which lacks tegminal plates, displays no tendons on the expanded, membranous tegmen and it possesses an underdeveloped laminar extension at the apex of the spiculum gastrale, which is not thickened and concave as in Titilayo species. The membrane of the bursa copulatrix in Titilayo is thinner and bears faint longitudinal ridges (cf. many Central and South American Anchonini species where the females present the same type of elongate bursa with well-marked ridges). In addition, most species of Titilayo have a dorsal notch at the base of the rostrum where it meets the head capsule (not T. perrinae); Aethiapocorep does not have such a notch.
Diagnostic characters.T. geiseri is most similar to T. garnerae. It differs from this species in the following characters: pronotum with widest part approximately 0.4 of length from anterior margin (cf. 0.3 of length); pronotal punctures smaller (ca. one-fifteenth length of pronotum on midline cf. one tenth of length); endophallus with long dorsal pigmented lobe (cf. lacking pigmented lobe); entrance of ductus seminalis pigmented near endophallus; ratio of penis depth at anterior of ostium to penis body length more than 0.31 (cf. less than 0.26). It can be distinguished from T. takanoi by the following character: basal tubercle of 5th interstria less than one elytral puncture diameter from the basal margin (cf. more than one puncture diameter).
Description. Length 4.4 mm (n = 1). Apterous. Black.
Head. Eye with height approximately 0.75 of depth of rostrum at base; dorsal margin below dorsal margin of rostrum at base; oval, 0.4 times as wide as high; ommatidia well-defined. Rostrum curved ventrad, more strongly basally than distally; transversely notched dorsally where it meets head capsule, and laterally before eyes; scrobe visible laterally for less than half its length, opening ventrally in basal half, in male visible dorsally at antennal insertion. Rostral punctation deep, large, irregularly distributed; longitudinal carinae not developed; golden scale in each puncture at most same length as puncture diameter, shorter in most areas. Head capsule shallowly concave and rugose dorsally to eyes, rugose areas meeting dorsally behind short medial glossy triangular area. Antennae rufous. 1st and 2nd funicle antennomeres subequal in length.
Thorax. Prothorax approximately 0.42 length of elytra dorsally, 1.8 times wider than long (n = 1); abruptly widening from collar to widest point at approximately 0.4 of length; punctures dorsally large (ca. one-fifteenth length of pronotum on midline), deep, circular, with rugose and sometimes glossy ridges between them; anterior margin of pronotum weakly emarginate; collar punctate dorsally and laterally, with submarginal prominence dorsally either side of midline; dorsal punctures each with erect scale as long as diameter of puncture, scales shorter laterally; weak tuft of erect scales on submarginal prominences.
Elytra. Weakly convex dorsally, apical declivity steep. Approximately 1.3 times longer than wide (n = 1). Tubercles at base of interstria 3 submarginal, at base of interstria 5 very nearly reaching basal margin of elytra and no more than diameter of one interstrial puncture from it; interstrial tubercles elliptical, all of similar height, each with row of yellow erect scales slightly shorter than those found anteriorly on pronotum, but longer than strial scales. Strial punctures small, distinct, separate, each with scale shorter than puncture diameter.
Legs. Tibial punctures confluent longitudinally forming neat rows, each with a row of erect scales, these more hair-like ventrally. Tibiae lacking ventral longitudinal row of teeth. Tarsomere 3 symmetrical.
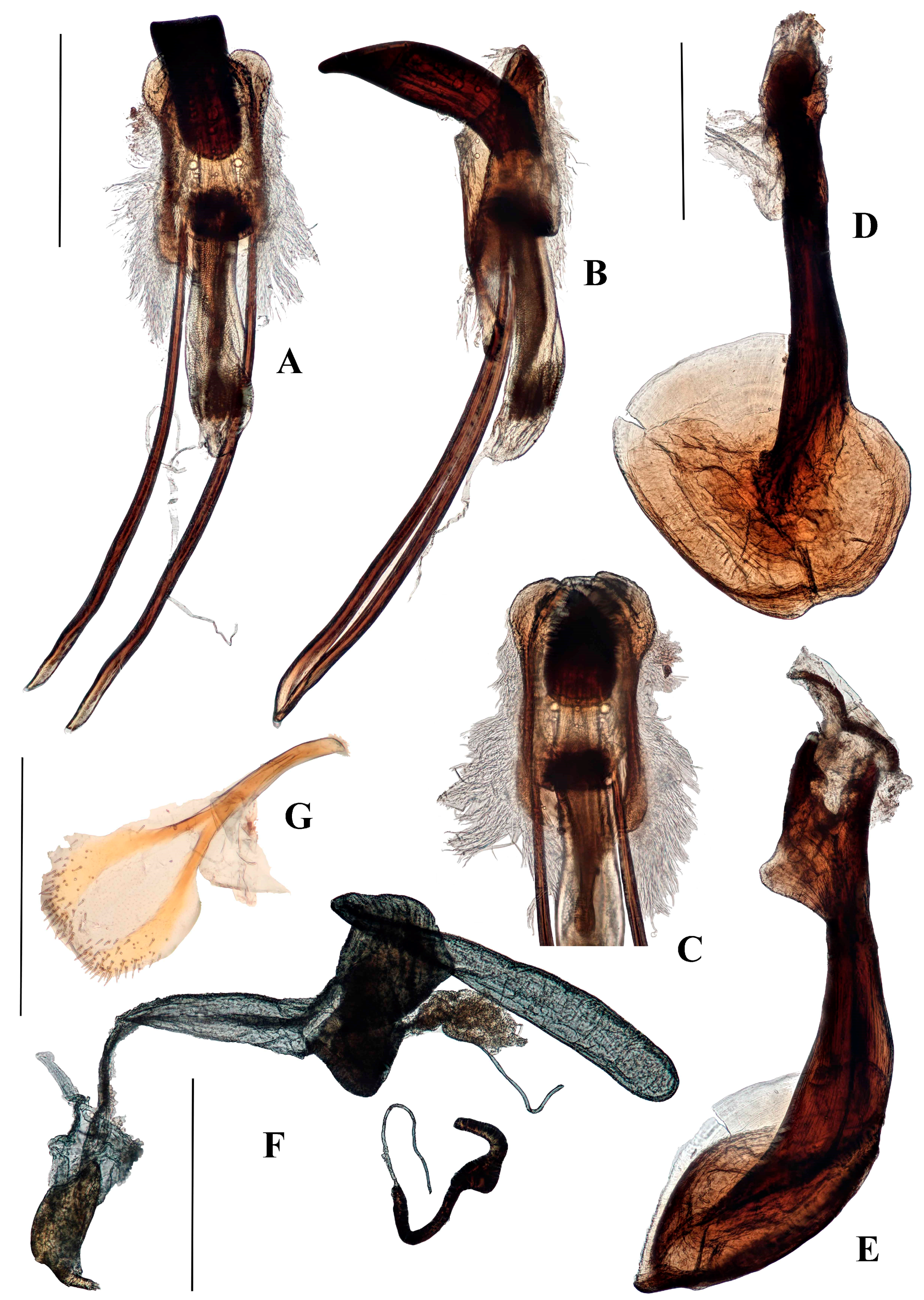
Genitalia. Male (
Figure 6A–F). Penis cylindrical, sclerotized and pigmented reddish-brown, curved ventrad basally and at apex. Ostium extending to first quarter of penis length; depth of penis at ostium 0.31–0.37 of ventral length of penis body. Endophallus with contiguous small, granular teeth particularly distally (when everted); dorsal elongate pigmented lobe present; ductus seminalis inserted ventro-apically, pigmented near endophallus. Tegmen with ring weakly pigmented reddish-brown; dorsally with two well-developed tegminal plates that are connected dorso-medially (
Figure 6E), ventrally with short pigmented lobe asymmetrically on either side of apodeme; tegminal apodeme present, approximately one-quarter of length of tegmen, including apodeme; tendons well-developed. Female not known.
Type locality: Ivory Coast, Mount Nimba.
Type material: HOLOTYPE ♂, with labels: “Holo- / type” (red-bordered disk) and “IVORY COAST 1100–1430m / Mt Nimba. Track and crest 5Km / SW of Richard Molard peak. / From: 7°35’24’’N, 08°25’’43W / To: 7°35’28’’N, 08°26’09’’W” and “6.V.2016. General / collecting. Aristophanous, M., Geiser, M., Moretto, P., leg. / BMNH(E)2016-109 / Trip Ref: CI-003(ANHRT 17)” and “[QR code] / “NHMUK010871077” and “HOLOTYPE / Titilayo / geiseri / Cristóvão & Lyal 2018”. PARATYPE ♂, same data as holotype and with labels: “Para- / type” (yellow-bordered disk) and “[QR code] / “NHMUK010871078” and “PARATYPE / Titilayo / geiseri / Cristóvão & Lyal 2018”.
Depository: BMNH.
Etymology. This species is named after our friend and colleague Dr Michael Geiser, who caught the first series of Anchonini from mainland Africa.
Diagnostic characters.T. garnerae is most similar to
T. geiseri. It differs from this species in the following characters: pronotum with widest part approximately 0.3 of length from anterior margin (cf. 0.4 of length); pronotal punctures larger (ca. one-tenth length of pronotum on midline cf. one-fifteenth of length); endophallus lacking dorsal longitudinal pigmented lobe (
Figure 8A) (cf. with a weakly pigmented longitudinal dorsal lobe (
Figure 6A,F); entrance of ductus seminalis not pigmented; ratio of penis depth at anterior of ostium to penis body length less than 0.26 (cf. more than 0.31). It can be distinguished from
T. takanoi by the following characters: basal tubercle of 5th interstria less than one elytral puncture diameter from basal margin (cf. more than one puncture diameter); female genitalia (
Figure 8F) lacking large expansion at apex of bursal atrium, at base of bursal duct (cf. large expansion present, Figure 10B); collum of spermatheca only slightly elongate (cf. longer than cornu).
Description. Length 3.8–5.3 mm (mean 4.91 mm, n = 5). Apterous. Black.
Head. Eye with height approximately 0.75 of depth of rostrum at base; dorsal margin lower than top of rostrum basally; oval, 0.4 times as wide as high; ommatidia well-defined. Rostrum fairly evenly curved ventrad; transversely notched dorsally where it meets head capsule, and laterally before eyes; scrobe visible laterally for less than half its length, opening ventrally in basal half, in male visible dorsally at antennal insertion. Rostral punctation deep, large, irregularly distributed, punctures smaller and shallower in female; longitudinal carinae not developed; golden scale in each puncture approximately same length as puncture diameter, shorter in some areas. In females, rostrum does not broaden apically to antennal insertions. Head capsule shallowly concave and rugose dorsally to eyes, rugose areas meeting dorsally or separated by two weak ridges. Antennae rufous; 2nd funicle antennomere slightly longer than 1st.
Thorax. Prothorax 0.43–0.47 length of elytra dorsally (mean 0.45, n = 5), 1.12–1.19 times wider than long (mean 1.15, n = 5); widening abruptly from collar to widest point at approximately 0.3 of length; punctures dorsally large (ca. one-tenth length of pronotum on midline), deep, circular, sometimes confluent, with glossy ridges between them; anterior margin of pronotum weakly emarginate; collar punctate dorsally and laterally, with sometimes weak submarginal prominence dorsally on either side of midline; dorsal punctures each with erect scale as long as diameter of puncture, scales shorter laterally; weak tuft of erect scales on submarginal prominence.
Elytra. Weakly convex dorsally, apical declivity steep. Approximately 1.3–1.4 times longer than wide (mean 1.38, n = 5). Tubercles at base of interstriae 3 and 5 submarginal, that at base of interstria 5 separated from margin by no more than diameter of one interstrial puncture; interstrial tubercles elliptical, all of similar height, each with row of yellow erect scales of similar length to those of pronotal tufts. Strial punctures small, distinct, separate, and each with scale approximately half as long as puncture diameter.
Legs. Tibial punctures confluent longitudinally forming neat rows, each with row of erect scales, these slightly narrower ventrally. Tibiae with ventral longitudinal row of teeth present, very inconspicuous. Tarsomere 3 symmetrical.
Genitalia. Male (
Figure 8A–D). Penis cylindrical, sclerotized and pigmented reddish-brown, curved ventrad basally and at apex. Ostium extending to first quarter of penis length; depth of penis at ostium 0.25 of ventral length of penis body. Endophallus with contiguous small, granular teeth particularly distally (when everted), but lacking dorsal pigmented lobe; ductus seminalis inserted ventro-apically, not sclerotised near endophallus. Tegmen with ring weakly pigmented reddish-brown; dorsally with tegminal plates lacking pigmented connection, extending laterally anteriad (
Figure 8A); ventrally with short unpigmented lobe asymmetrically on either side of apodeme; apodeme present, approximately one quarter total length of tegmen including apodeme; tendons well-developed.
Female (
Figure 8E,F). Posterior margin of tergite VII emarginate. Spiculum ventrale with posterior margin convex. Vaginal membrane thin, weakly pigmented. Bursal atrium expanded laterally with few internal folds; bursal membrane thick, pigmented; numerous sharp teeth present within bursa; bursa with indistinct longitudinal folds. Spermathecal duct arising at base of bursa away from common oviduct. Spermatheca with collum bulbous (
Figure 8F).
Type locality: Ivory Coast, Mount Nimba.
Type material: HOLOTYPE ♂, with labels: “Holo- / type” (red-bordered disk) and “IVORY COAST 1100–1430m / Mt Nimba. Track and crest 5Km / SW of Richard Molard peak. / From: 7°35’24’’N, 08°25’’43W / To: 7°35’28’’N, 08°26’09’’W” and “6.V.2016. General / collecting. Aristophanous, M., Geiser, M., Moretto, P., leg. / BMNH(E)2016-109 / Trip Ref: CI-003(ANHRT 17)” and “HOLOTYPE / Titilayo / garnerae / Cristóvão & Lyal 2018”. PARATYPES: 4♀♀ 1♂ with same data as Holotype and with labels: “Para- / type” (yellow-bordered disk) and “PARATYPE / Titilayo / garnerae / Cristóvão & Lyal 2018”.
Depository: BMNH.
Etymology. This species is named after our friend and colleague Beulah Garner.
Diagnostic characters. T. takanoi is morphologically similar to T. geiseri and T. garnerae. For diagnostic characters see under those species.
Description. Length 5.35 mm (n = 1). Black.
Head. Eye with height approximately 0.8 × depth of rostrum at base; dorsal margin lower than top of rostrum basally; oval, 0.8 times as wide as high; ommatidia well-defined. Rostrum curved ventrad, more strongly basally than distally; transversely notched dorsally where it meets head capsule, and laterally before eyes, slightly expanded laterally before eyes; scrobe visible laterally for more than half its length, opening ventrally for basal half; punctation deep basally dorsally, punctures more shallow and smaller distal to antennal insertions, arranged in rows basally and laterally; golden erect scale in each puncture approximately as long as the diameter of puncture. Head capsule shallowly concave and rugose dorsally to eyes, rugose areas meeting dorsally. Antennae rufous; first funicle antennomere shorter than second.
Thorax. Prothorax slightly less than 1/2 the length of the elytra dorsally, abruptly widening from collar to widest point at basal 3/5th of length. Punctation dorsally deep, varying in size, sometimes confluent on collar, with matte ridges between them; weak tuft of elongate erect scales submarginally on either side of midline dorsally; scattering of similar scales dorsally anteriorly, otherwise scales shorter, one-third the length of the long scales, and subequal to the diameter of punctures.
Elytra. Weakly convex dorsally, apical declivity steep; 1.35 times longer than wide (n = 1). Tubercles at the base of interstriae 3 and 5 submarginal; interstrial tubercles elongate, rarely rounded, some twice as long as others; dorsal tubercles slightly higher than lateral tubercles; each with row or weak tuft of yellow erect scales slightly shorter than those found anteriorly on pronotum, but longer than strial scales. Strial punctures small, distinct, separate, each with scale approximately as long as puncture diameter.
Legs. Tibial punctures confluent longitudinally, forming neat rows, each with a row of erect scales. Tibiae with ventral longitudinal row of teeth present, inconspicuous (
Figure 9C). Tarsomere 3 symmetrical.
Genitalia. Male unknown.
Female (
Figure 10A,B). Tergite VII with posterior margin emarginate. Spiculum ventrale with posterior margin weakly convex. Vaginal membrane thin and weakly pigmented. Bursal atrium greatly expanded ventrally, folded, more pigmented than vaginal membrane, and bearing numerous sharp small teeth internally. Bursa with numerous teeth, longer and larger than those of bursal atrium; membrane thick and well pigmented. Spermatheca weakly broadened in middle, collum elongate, cylindrical, curved (
Figure 10B).
Type locality: Sierra Leone, Loma Mountains.
Type material: HOLOTYPE ♀, with labels: “Holo- / type” (red-bordered disk) and “SIERRA LEONE 1050m / Loma Mountains / Closed-canopy forest / N09°10’35’’, W11°05’25’’ / 7-10.vi.16 General Coll. / leg. Takano, Miles & Goff” and “BMNH(E) / 2016-196” and “[QR code] / “NHMUK010871079” and “HOLOTYPE / Titilayo / takanoi / Cristóvão & Lyal 2018”.
Depository: BMNH.
Etymology. This species is named after our friend and colleague Hitoshi Takano, who caught this first recorded Anchonini from Sierra Leone.
Remarks. Despite the male being unknown, this species is being described in Titilayo gen. nov. due to its geographical proximity and external similarity to T. geiseri sp. nov. as well as to the close similarity of the female genitalia to the other species being described in this genus.
Diagnostic characters. This species can be separated from other Titilayo species by its short, weakly defined paired tufts of scales on the collar constriction and by the broad, irregular, very glossy longitudinal raised areas between the deep punctures dorsally on the pronotum.
Description. Length 3.3–4.8 mm (mean 4.29mm, n = 8). Black.
Head. Eye with height slightly less than depth of rostrum at base; dorsal margin lower than top of rostrum basally; oval, 0.66 times as wide as high; ommatidia well defined. Rostrum strongly curved ventrad in basal quarter, more weakly curved distally; meeting head capsule at obtuse angle, lacking dorsal transverse notch at the base or before eyes; scrobe visible laterally only in its distal quarter, opening ventrally for most of length. Rostral punctation moderately deep dorsally and laterally, some punctures merging creating short, neat longitudinal rows separated by very weak longitudinal carinae, especially in male; golden scale in each puncture approximately same length as puncture diameter. Antennae rufous; first funicle antennomere shorter than second.
Thorax. Prothorax 0.42–0.5 of length of elytra dorsally (mean 0.45, n = 8); 1.16–1.36 times wider than long (mean 1.29, n = 8), broadest at anterior quarter of length, abruptly widening from collar dorsolaterally only, more ventrally lacking abrupt widening; collar strong dorsolaterally and dorsally; dorsally convex in profile (
Figure 11B); collar impunctate, dorsal punctures behind collar large, deep, confluent longitudinally on disc and anteriorly, with broad glossy raised areas between them, these sometimes appearing to be irregular longitudinal flattened carinae; paired weak tufts of long, golden scales either side of pronotum where it is broadest; sparse tuft of long, golden erect scales submarginally on either side of midline anteriorly, scales in tufts up to three times longer than the other pronotal scales.
Elytra. Weakly convex dorsally, apical declivity steep. Approximately 1.2–1.36 times longer than wide (mean 1.29, n = 8). Tubercles at base of interstriae 3 and 5 more elevated and elongate than other elytral tubercles, tubercle on 3 reaching basal margin, that on 5 extending anteriad to base of elytra; other elytral tubercles numerous, elliptical, larger at the apical declivity than elsewhere, only weakly symmetrical between elytra; each tubercle with tuft of golden erect scales of similar length to pronotal tuft scales. Strial punctures weak, separate, each with scale approximately as long as puncture diameter; striae indistinct.
Legs. Femora black with sparse erect golden scales, more numerous distally. Tibiae dark rufous; with punctures confluent longitudinally forming rows, each with row of narrow erect golden scales or setae; ventral longitudinal row of teeth present, weak at apical quarter of all tibiae. Tarsomere 3 asymmetrical, outer lobe slightly broader than inner lobe.
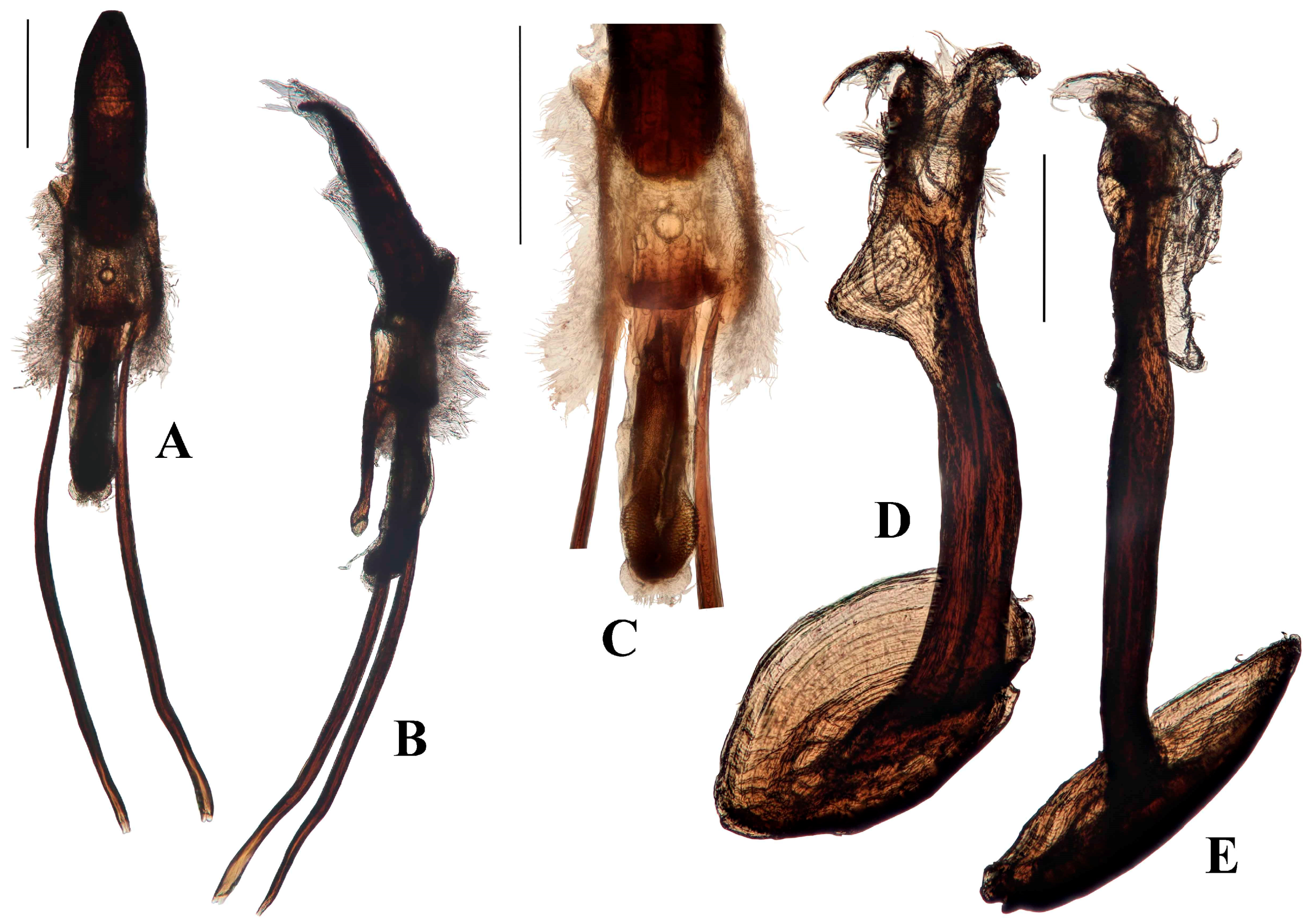
Genitalia. Male (
Figure 12A–F and
Figure 13). Penis cylindrical, sclerotized, and pigmented reddish-brown; curved ventrad basally and apically; penis apodemes pigmented reddish-brown. Posterior of ostium at approximately 0.4 length of penis from apex of penis body. Endophallus with matrix of small, granular teeth. Ductus seminalis arising ventrally, sub-apically. Tegminal ring lightly pigmented, expanded dorsally and ventrally, with visible tendons and two asymmetrical, short lobes; tegminal plates weakly expanded dorso-posteriorly and sclerotisations not connected dorso-medially (
Figure 12D and
Figure 13); tegminal apodeme present, pigmented, less than half total length of tegmen, including apodeme.
Female (
Figure 12G–H). Tergite VII with posterior margin emarginate. Spiculum ventrale with posterior margin convex. Vaginal membrane thin, unpigmented. Bursal atrium expanded laterally, lobate, internally folded, and pigmented. Common oviduct arising from bursal atrium. Spermathecal duct arising on bursal atrium away from its junction with the common oviduct. Bursa elongate, constricted at base; membrane thick and pigmented; numerous sharp teeth present internally. Spermatheca slightly broadened at base of ramus, collum elongate, curved, more or less of constant diameter (
Figure 12G).
Type locality: São Tomé e Príncipe, São Tomé Island.
Type material: HOLOTYPE ♂, with labels: “Holo- / type” (red-bordered disk) and “San Thomè” and “Muséum Paris / ex Coll. / R. Oberthür / 1952” and “HOLOTYPE / Titilayo / perrinae / Cristóvão & Lyal 2018”. PARATYPES 1♂ 1♀ with labels: “Para- / type” (yellow-bordered disk) “San Thomè” and “Muséum Paris / Coll. M. Pic” and “PARATYPE / Titilayo / perrinae / Cristóvão & Lyal 2018”; 1♂ 3♀♀ with labels: “Para- / type” (yellow-bordered disk) and “San Thomé” and “Museum Paris / Collection Léon Fairmaire / 1906” and “PARATYPE / Titilayo / perrinae / Cristóvão & Lyal 2018”; 1♀ with labels: “Para- / type” (yellow-bordered disk) and “San Thomè” and “Muséum Paris / ex Coll. / R. Oberthür / 1952” and “PARATYPE / Titilayo / perrinae / Cristóvão & Lyal 2018”.
Depository: MNHN.
Etymology. This is one of the first anchonine species described from the island of São Tomé. It is named after Madame Hélène Perrin who brought these specimens to our attention.
Diagnostic characters. This species can be distinguished from all other Titilayo by its smaller size, pronotum less than 1.1 times as wide as long (some specimens of T. barclayi have a smaller ratio) with convex shoulders immediately behind the collar, pronotum with inconspicuous setae, as well as erect scales, and by its very small, inconspicuous interstrial tubercles.
Description. Length 3.03–3.38 mm (mean 3.16mm, n = 3). Black and rufous.
Head. Eye with height approximately 0.85 depth of rostrum at base; dorsal margin lower than top of rostrum basally; oval, 0.64 times as wide as high; ommatidia well defined. Rostrum curved ventrad, more strongly basally than distally; transversely very weakly notched dorsally where it meets head capsule, more strongly so in male than female and notch may not be apparent; baso-lateral part of rostrum weakly expanded, with weak lateral notch before eyes. Scrobe visible laterally for half its length, opening ventrally in its basal half. Rostral punctation shallow, obscure, especially in female, punctures often longitudinally confluent, delimiting longitudinal carinae in male; punctation present distal to antennal insertions, shallower in females. Scales in punctures short, in neat rows, especially laterally. Antennae rufous; first and second funicle antennomeres subequal in length.
Thorax. Prothorax 0.49–0.50 times length of elytra dorsally, 1.03–1.09 (mean 1.07, n = 3) times as wide as long; widest antero-medially (at 0.4 of length), widened from collar, shoulders evenly convex. Anterior margin dorsally weakly emarginate. Punctation deep, circular, punctures not confluent dorsally, reticulation between them not glossy, punctation very weak and shallow on collar; very weak tuft of erect and semi-erect golden scales submaginally on either side of midline anteriorly, sparser tuft on each shoulder, these scales approximately same length as those elsewhere on pronotum.
Elytra. Black, with rufous patches. Weakly convex dorsally, apical declivity not steep. 1.22–1.35 longer than wide (mean 1.28, n = 3). Tubercles at base of interstriae 3 and 5 weak, submarginal although close to margin; other interstrial tubercles very weak, sparse, very weakly symmetrical between elytra; tubercles marked mainly by erect golden setae of a similar size to those in pronotal tufts; strial punctures very shallow, indistinct, mostly lacking short scales or setae.
Legs. Rufous. Femora with longitudinal rows of erect golden setiform scales ventrally, shorter scales present distally dorsally and laterally. Tibial punctures confluent longitudinally, forming neat rows, each with row of erect scales laterally and dorsally, these more setiform ventrally. Ventral longitudinal row of teeth absent. Tarsomere 3 symmetrical.
Genitalia. Male (
Figure 15A–E). Penis cylindrical, sclerotised and pigmented brown, including apodemes; curved ventrad strongly in basal third and again apically. Endophallus with band of very small quadrate sclerotised reticulations along ventral side and as band around the endophallus near entrance of ductus seminalis. Ductus seminalis arising apically, slightly ventrally. Tegmen pigmented dark-brown from lateral parts of the tegminal ring through to tegminal lobes, otherwise pigmented light-brown; ring membranous, expanded; tegminal plates (
Figure 15C) with weak sclerotised connection dorsally; apodeme present, pigmented dark-brown, approximately one-third total length of tegmen, including apodeme; tendons large and present from tegminal plates to lobes of tegminal ring. Darkly pigmented ring in membrane between tegmen and penis (shown in
Figure 15B at same level as junction of penis apodemes and membrane).
Female (
Figure 15F–G). Tergite VII with posterior margin deeply concave. Spiculum ventrale with posterior margin weakly smoothly emarginate. Vaginal membrane thin, unpigmented. Bursal atrium expanded laterally, lobate, internally folded, and pigmented. Bursa elongate, constricted at the base; membrane unfolded and unpigmented; internal teeth absent. Spermathecal duct arising next to common oviduct. Spermatheca expanded, bulbous at ramus, collum elongate, cylindrical, curved (
Figure 15F).
Type locality: São Tomé, São Tomé e Príncipe.
Type material: HOLOTYPE ♂, with labels: “Holo- / type” (red-bordered disk) and “SÃO TOMÉ, 1324m, / Antenna, Bom Successo, / 00°16’31.6‘‘N 6°36’13.7‘‘E, / (20-29).x.2016” and “Banana trap, / Turner, C.R., Tasane, T., / BMNH(E) 2017-11, / TripRef: ST-001 (ANHRT 21)” and “[QR code] / “NHMUK010599878” and “HOLOTYPE / Titilayo / saotomense / Cristóvão & Lyal 2018”. PARATYPES: 2♀♀, same data as Holotype except “Para- / type” (yellow-bordered disk) and with QR code labels numbering “NHMUK010599879” and “NHMUK010599880” respectively and “PARATYPE / Titilayo / saotomense / Cristóvão & Lyal 2018”.
Depository: BMNH.
Etymology. This species is named after the island on which it was caught.
Remarks. The locality should properly be spelled “Bom Sucesso”.
Diagnostic characters. In some characters, T. turneri ♂ is similar to T. perrinae: pronotal collar very short, so that pronotum appears convex in outline from close to anterior margin to its widest point in dorsal view; anterior submarginal scale tufts of pronotum weak; basal tubercle on elytral interstria 5 large and projecting anterior to base of elytra. It differs in lacking the broad glossy raised areas on the pronotum, the punctures being more oval and well-defined, and having a weak setal tuft on either side of the midline on the pronotal disc. Titilayo barclayi is also similar, but it is a less robust insect (Pronotal width:length 1.23 in T. turneri, 1.04–1.16 in T. barclayi; elytral length:width 1.24 in T. turneri, 1.27–1.33 in T. barclayi; in T. turneri the basal tubercles of elytral interstria 5 extend strongly over the basal margin of the elytra, which they do not in T. barclayi.
Description. Length 4.19 mm (n = 1). Black.
Head. Height of eye 0.8 times depth of rostrum where it meets head capsule; dorsal margin lower than top of rostrum basally; oval, width 0.6 times height; ommatidia well-defined, separately convex. Rostrum strongly curved ventrad in basal half, nearly straight ventrally distal to antennal insertion; meeting head capsule at obtuse angle, lacking dorsal transverse notch and with very weak vertical notch before eyes; scrobe visible laterally in distal half, opening ventrally for most of length. Rostral punctures sometimes confluent dorsally and laterally basal to antennal insertions, with five matte longitudinal carinae dorsal to scrobe; more scattered distal to antennal insertions; each puncture with small golden scale subequal in length to puncture diameter. Antennae rufous; first funicle antennomere longer and broader than second.
Thorax. Prothorax 0.47 length of elytra, 1.23 × wider than long, widest at anterior 0.4 of length, abruptly widening from collar only dorsolaterally, more ventrally lacking abrupt widening; collar strong dorsolaterally; dorsally convex; strong convex shoulders present dorsolaterally; pronotum with anterior margin entire; punctures deep except on collar, where shallow, all similar in size, rarely confluent, with weakly raised matte reticulation between them; paired weak tufts of semi-erect golden scales either side of midline where pronotum is broadest, on shoulders and submarginally on either side of midline anteriorly, scales in tufts only slightly longer than other pronotal scales.
Elytra. Very weakly convex dorsally, apical declivity very steep. Length 1.23 times maximum width. Tubercles at base of interstriae 3 and 5 more elevated and elongate than other elytral tubercles, tubercle on 3 reaching basal margin, that on 5 extending strongly anteriad to base of elytra; other elytral tubercles numerous, elliptical, only weakly symmetrical between elytra; each tubercle with tuft of golden semi-erect scales of a similar length to pronotal scales away from tufts. Strial punctures weak, separate, each with scale approximately as long as puncture diameter; striae indistinct.
Legs. Black. Femora with semi-erect scales dorsally and laterally, long slender erect scales ventrally. Tibial punctures confluent longitudinally forming rows, each with row of narrow erect golden scales, these more elongate and hair-like ventrally; ventral teeth absent. Tarsomere 3 symmetrical.
Genitalia. Male (
Figure 17A–E). Penis dorso-ventrally compressed, sclerotised, and pigmented brown; curved abruptly ventrad basally and apically; penis apodemes pigmented dark brown; ostium vertical with respect to long axis of penis, opening into distal U-shaped furrow in penis, which extends nearly half length of penis. Endophallus with longitudinal bands of densely-packed small quadrate teeth distally, these forming broadened pads of teeth dorsally near gonopore; gonopore ventral, subapical. Microtrichiae externally at apex of endophallus.
Tegmen pigmented light brown on the tegminal plates, laterally and ventrally; tegminal plates asymmetrical, not meeting medially; apodeme free for about 0.4 total length of tegmen; membranous collar folds over approximately half way down tegmen, marked by darker band in membrane to which penis apodemes attach; membranous lobes on tegmen not present, although dense patches of tendons give a similar impression; tendons large and present from tegminal plates to level of base of apodeme (
Figure 17C).
Spiculum gastrale with large, concave apical laminate extension; basal arms of similar size with one possessing a large triangular sclerotized lobe.
Female not known.
Type locality: São Tomé
Type material: HOLOTYPE ♂, with labels: “Holo- / type” (red-bordered disk) and “SÃO TOMÉ, 1324m / Antenna, Bom Successo / 00°16’31.6‘‘N 6°36’13.7‘‘E / (20-29). x.2016” and “Carrion pitfall / Turner, C.R., Tasane, T. / BMNH(E) 2017-11 / TripRef: ST-001 (ANHRT 21)” and “[QR code] / “NHMUK010599883” and “HOLOTYPE / Titilayo / turneri / Cristóvão & Lyal 2018”.
Depository: BMNH.
Etymology. This species is named after Dr. Clive Turner who collected this species and several other specimens and species described here from the island of São Tomé.
Remarks. The locality should properly be spelled “Bom Sucesso”.
Diagnostic characters.
T. barclayi is most similar to
T. saotomense and to
T. turneri. It can be distinguished from the latter by: basal tubercles on elytral interstria 5 submarginal to very weakly projecting anteriad to the elytral margin (cf. very strongly projecting, overlapping base of pronotum); no scale tufts on disc of pronotum (cf. weak scale tufts either side of pronotal midline); ratio of pronotal length to maximum width less than 1.17 (cf. more than 1.2); height of eye slightly more than three-quarters of depth of rostrum where it meets head capsule (cf. subequal). It can be distinguished from
T. saotomense by: elytral tubercles raised, prominent, basal tubercle on interstria 5 submarginal to weakly projecting anteriad to basal margin of elytron (cf. elytral tubercles only very weakly raised, not prominent, basal tubercle on interstria 5 not projecting anteriad); pronotum raised either side of midline (cf. pronotum not raised either side of midline). The form of the tibiae with a ventral projection at the base (
Figure 18C) is not known from other species.
Description. Length 3.8–4.1 mm (mean 3.92 mm, n = 3). Black.
Head. Height of eye slightly more than three-quarters depth of rostrum where it meets head capsule, just less than twice width of eye; dorsal margin lower than top of rostrum basally; ommatidia well-defined, separately convex. Rostrum curved ventrad strongly in basal third, more weakly distally; meeting head capsule at obtuse angle, lacking dorsal transverse notch, and with very weak vertical notch before eyes; scrobe visible laterally in distal half, opening ventrally for most of length. Rostrum densely punctate dorsally, more sparsely so distal to antennal insertions, punctures weakly arranged in irregular longitudinal rows, these more apparent laterally where punctures often confluent; each puncture with small golden setiform scale. Antennae rufous; first funicle antennomere longer and broader than second.
Thorax. Prothorax; 0.45–0.52 times length of elytra (mean 0.48, n = 3), 1.04–1.16 wider than long (mean 1.11, n = 3), widest at anterior third of length, abruptly widening from collar; collar visible laterally and dorsally; dorsally with median longitudinal depression behind collar, raised and convex on either side; collar with weak rounded tubercle on either side of midline submarginally; pronotum with anterior margin weakly emarginate; collar with punctures laterally, not dorsally, otherwise pronotum deeply densely punctate, glossy highlights on some reticulation between punctures, otherwise matte; tuft of semi-erect golden scales on prominences on collar, otherwise each puncture with single golden decumbent scale.
Elytra. Very weakly convex dorsally, apical declivity very steep. Length 1.27–1.33 maximum width (mean 1.30, n = 3). Tubercles at base of interstria 3 submarginal, that at base of interstria 5 submarginal or weakly projecting anteriad to base of elytra; other interstrial tubercles numerous, each being crowned with small glossy points, only weakly symmetrical between elytra; each tubercle with sparse tuft of erect or semi-erect golden scales. Strial punctures weak, sparse; striae poorly defined.
Legs. Mainly rufous. Femora with small semi-erect narrow scales, longer and more setiform ventrally. Tibiae with strong ventral projection basally and sparse ventral row of teeth (
Figure 18C); tibial punctures confluent longitudinally forming rows, each with row of narrow erect pale scales, more setiform and longer ventrally than dorsally. Tarsomere 3 symmetrical.
Genitalia. Male not known.
Female (
Figure 19A–C). Tergite VII posterior margin with deep concave emargination (
Figure 19A). Spiculum ventrale with posterior margin weakly convex. Vaginal membrane thin, lightly pigmented. Bursal atrium expanded, with few internal folds; unpigmented externally, pigmented brown in folded area. Common oviduct, bursa, and spermathecal duct arise from bursal atrium, with bursa arising apically, spermathecal duct arising anterior to common oviduct on ventral side of bursal atrium. Bursa arising on dorsal side of bursal atrium, bursal duct narrow, as long as bursa, bursa elongate, with longitudinal folds, thickened and lightly pigmented; fine teeth visible internally. Spermatheca expanded at base of ramus, collum short (
Figure 19B).
Type material: HOLOTYPE ♀, with labels: “Holo- / type” (red-bordered disk) and “SÃO TOMÉ, 1324m / Antenna, Bom Successo, / 00°16’31.6‘‘N 6°36’13.7‘‘E / (20-29). x. 2016” and “Carrion pitfall / Turner, C.R., Tasane, T. / BMNH(E) 2017-11 / TripRef: ST-001 (ANHRT 21)” and “[QR code] / “NHMUK010599884” and “HOLOTYPE / Titilayo / barclayi / Cristóvão & Lyal 2018”.
PARATYPES: 2♀♀ with same data as Holotype except with QR code labels numbering “NHMUK010599886” and “NHMUK010599885” respectively, and with labels: “Para- / type” (yellow-bordered disk) and “PARATYPE / Titilayo / barclayi / Cristóvão & Lyal 2018”.
Depository: BMNH.
Etymology. This species is named after our friend and colleague Max Barclay in recognition of his assistance to J.P.C.
Remarks. The locality should properly be spelled “Bom Sucesso”.
Key to species of Aethiopacorep and Titilayo
- 1.
Completely rufous. Tegmen completely membranous, unpigmented, lacking tendons, asymmetrical ventral lobes, and tegminal plates. Apex of spiculum gastrale spatulate. Bursa lacking basal-apical folds. Spermatheca of similar diameter throughout (
Figure 4D). Annobón Island……………………………………..…..…………….…….
Aethiopacorep africanus (Hustache)- -
Black to black and rufous. Tegmen pigmented, with strongly pigmented dorsal tegminal lobes; tendons present, sometimes densely gathered in asymmetrical ventral membranous lobes. Apex of spiculum gastrale concave, circular, laminar. Bursa bearing basal-apical folds. Spermatheca broadened at least near base of ramus. Ivory Coast, Sierra Leone and São Tomé island…………...………………………………………………………………………………….……... 2
- 2.
Pronotum with collar constriction not well-marked, shoulders with convex outline nearly reaching anterior margin of pronotum (
Figure 11A and
Figure 16A); basal tubercle on elytral interstria 5 strongly projecting over basal elytral margin …………………………………….………………….. 3
- -
Pronotum with collar constriction well-marked, with clear angle between collar and shoulders of pronotum (
Figure 5A,
Figure 7A,
Figure 9A,
Figure 14A and
Figure 18A); basal tubercle on elytral interstria 5 submarginal or weakly projecting over basal elytral margin .……………………………………………..…….….… 4
- 3.
Pronotum with broad glossy raised areas between punctures, sometimes giving the impression of longitudinal convex ridges; pronotal disc lacking setal tufts. São Tomé, São Tomé e Príncipe ………………………………………………………………………………… Titilayo perrinae sp.nov.
- -
Pronotum lacking broad glossy raised areas between punctures; pronotal disc with weak sparse setal tuft on either side of midline. São Tomé ………….………………….. Titilayo turneri sp.nov.
- 4.
Elytral tubercles conspicuous, occupying at least width of interstriae and sometimes interrupting striae; more than 3.8 mm long ……………………………………………………………………..……5
- -
Elytral tubercles inconspicuous, occupying less than width of interstria and very weakly or not raised, giving elytra smooth (although matte) appearance; striae faint but not interrupted; less than 3.4 mm long. São Tomé …………………………………….……. Titilayo saotomense sp.nov.
- 5.
Pronotum with collar constriction approx. 1/3rd length of pronotum, widest part of pronotum approximately 0.3 of length; endophallus (where known) lacking dorsal pigmented lobe.…….. 6
- -
Pronotum with collar constriction approx. 1/5th length of pronotum, widest part of pronotum approximately 0.4 of length; endophallus with dorsal pigmented lobe dorsally (
Figure 6F). Ivory Coast………………………………………………………………………………...….
T. geiseri sp.nov.- 6.
Tibiae with ventral margin more or less straight or weakly sinuate, lacking ventral projection basally (
Figure 9C); pronotal shoulders more angular (
Figure 7A and
Figure 9A)……………………...........7
- -
Tibiae deeper basally than apically, with strong ventral projection (
Figure 18C); pronotal shoulders more rounded. São Tomé ……………………..………….……
Titilayo barclayi sp.nov.- 7.
Basal tubercle of 5th interstria less than one elytral puncture diameter from basal; female genitalia (
Figure 8F) lacking large expansion at apex of bursal atrium, at the base of bursal duct; collum of spermatheca only slightly elongate (
Figure 8F). Ivory Coast ..……...…
Titilayo garnerae sp.nov.- -
basal tubercle of 5th interstria more than one elytral puncture diameter from basal margin; female genitalia (
Figure 10B) with large expansion at apex of bursal atrium, at base of bursal duct; collum of spermatheca. longer than cornu (
Figure 10B). Sierra Leone ………….
Titilayo takanoi sp.nov.