Improving Standards for At-Risk Butterfly Translocations
Abstract
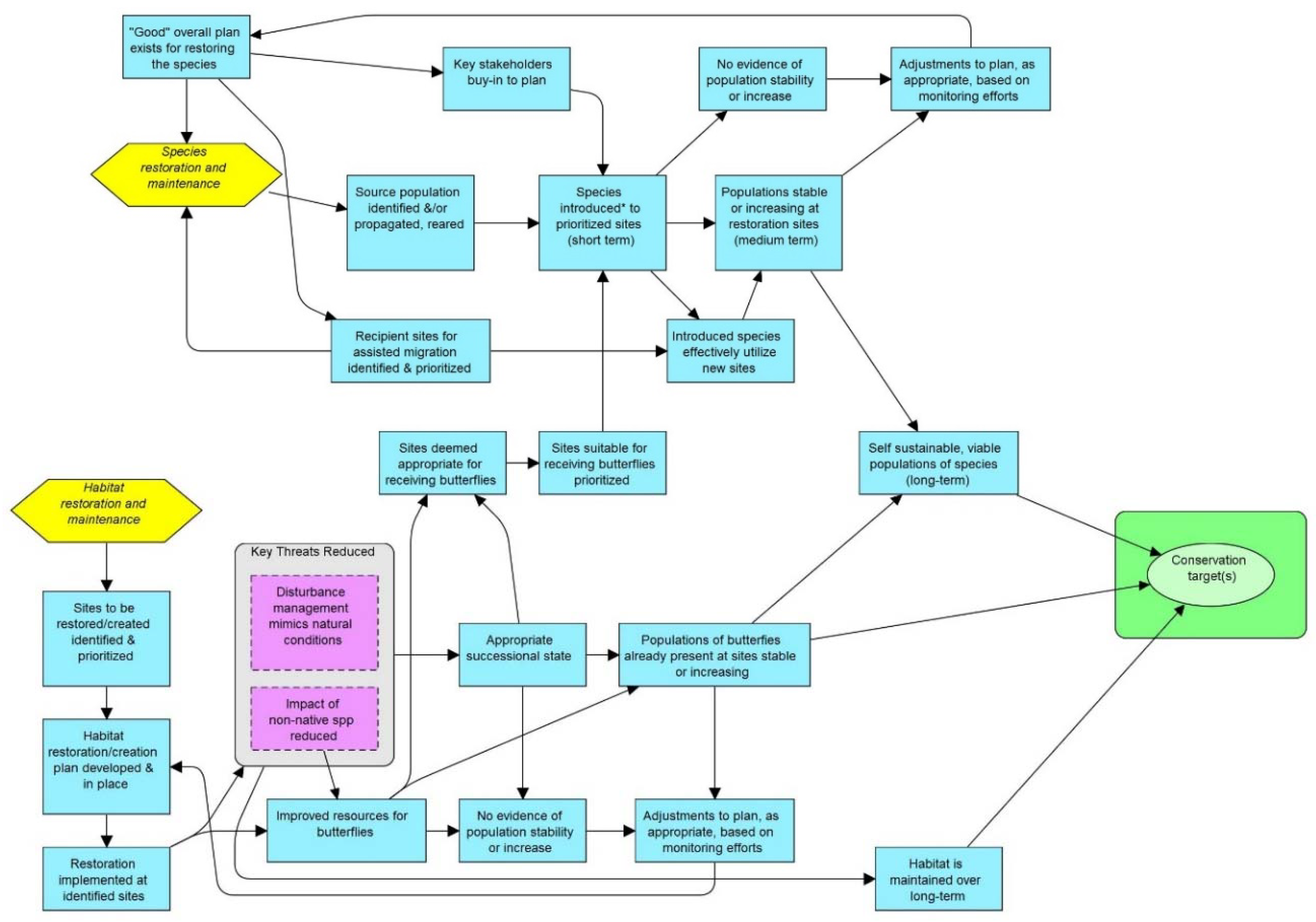
1. Introduction
2. Proposed Actions
2.1. Document All Release Events
2.2. Need for Better Communication and Coordination between Ex Situ and In Situ Program Components
2.3. Test and Develop More Robust Best Practices through Experimentation
2.4. Clearly Define Outcomes of Ex Situ Recovery Strategy A Priori
2.5. Promote Protocol Flexibility to Stimulate Innovation
2.6. Define Objectives of Post-Release Monitoring
2.7. Optimize the Contributions of Citizen Scientists
2.8. More Robust Habitat Monitoring at Recipient Sites
2.9. Increase Use of Genetic and Health Screening
2.10. Employ Ecological Modeling to Help Inform Practice Where Possible and Appropriate
2.11. Increase Information and Data Sharing
2.12. Make Use of Available Conservation Project Design, Management, and Monitoring Tools
3. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, J.A.; Telfer, M.G.; Roy, D.B.; Preston, C.D.; Greenwood, J.J.; Asher, J.; Fox, R.; Clarke, R.T.; Lawton, J.H. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 2004, 303, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Schmitt, T.; Weitzel, M.; Seitz, A. The severe decline of butterflies on western German calcareous grasslands during the last 30 years: A conservation problem. Biol. Conserv. 2005, 128, 542–552. [Google Scholar] [CrossRef]
- Thomas, C.D.; Franco, A.M.A.; Hill, J.K. Range retractions and extinction in the face of climate warming. Trends Ecol. Evol. 2006, 21, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Wallisdevries, M.F.; Van Swaay, C.A.M.; Plate, C.L. Changes in nectar supply: A possible cause of widespread butterfly decline. Curr. Zool. 2012, 58, 384–391. [Google Scholar] [CrossRef]
- Van Dyck, H.; Van Strein, A.J.; Maes, D.; Van Swaay, C.A. Declines in common, widespread butterflies in a landscape under intense human use. Conserv. Biol. 2009, 23, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Breed, G.A.; Stitcher, S.; Crone, E.E. Climate-driven changes in northeastern US butterfly communities. Nat. Clim. Chang. 2012, 3, 142–145. [Google Scholar] [CrossRef]
- Gilburn, A.S.; Bunnefeld, N.; Wilson, J.M.; Botham, M.S.; Brereton, T.M.; Fox, R.; Goulson, D. Are neonicotinoid insecticides driving declines of widespread butterflies? PeerJ 2015, 3, e1402. [Google Scholar] [CrossRef] [PubMed]
- Wilhere, G.F. Adaptive Management in Habitat Conservation Plans. Conserv. Biol. 2002, 16, 20–29. [Google Scholar] [CrossRef]
- Gregory, R.; Long, G. Using Structured Decision Making to Help Implement a Precautionary Approach to Endangered Species Management. Risk Anal. 2009, 29, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Runge, M.C. An Introduction to Adaptive Management for Threatened and Endangered Species. J. Fish Wildl. Manag. 2011, 2, 220–233. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013; ISBN 978-2-8317-1609-1. [Google Scholar]
- USFWS Environmental Conservation Online System. Listed Animals. U.S. Fish and Wildlife Service. Available online: https://ecos.fws.gov/ecp/ (accessed on 12 June 2018).
- Government of Canada. Species at Risk Registry. Available online: https://www.registrelep-sararegistry.gc.ca/species/schedules_e.cfm?id=1 (accessed on 15 June 2018).
- Butterfly Conservation. Available online: https://butterfly-conservation.org/3544/Species-ActionPlans.html (accessed on 1 June 2018).
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the Science of Reintroduction Biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Soorae, P.S. (Ed.) Global Re-Introduction Perspectives: Re-Introduction Case-Studies from Around the Globe; IUCN/SSC Reintroduction Specialist Group: Abu Dhabi, UAE, 2008; ISBN 978-2-8317-1113-3. [Google Scholar]
- Schultz, C.B.; Russel, C.; Wynn, L. Restoration, Reintroduction, and captive Propagation for at-risk Butterflies: A review of British and American Conservation Efforts. Isr. J. Ecol. Evol. 2008, 54, 41–61. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines on the Use of Ex Situ Management for Species Conservation; Version 2.0; IUCN Species Survival Commission: Gland, Switzerland, 2014; Available online: https://www.iucn.org/about/work/programmes/species/publications/iucn_guidelines_and__policy__statements (accessed on 15 June 2018).
- Daniels, J.C. (Ed.) Butterfly Conservation in North America: Efforts to Help Save our Charismatic Microfauna; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2015; ISBN 978-94-017-9851-8. [Google Scholar]
- Sutherland, W.J.; Armstrong, D.; Butchart, S.H.M.; Earnhardt, J.M.; Ewen, J.; Jamieson, I.; Jones, C.G.; Lee, R.; Newbery, P.; Nichols, J.D.; et al. Standards for documenting and monitoring bird reintroduction projects. Conserv. Lett. 2010, 3, 229–235. [Google Scholar] [CrossRef]
- Daniels, J.C. Cooperative conservation efforts to help recover an endangered south Florida butterfly. Insect Conserv. Divers. 2009, 2, 62–64. [Google Scholar] [CrossRef]
- Knight, A.T.; Cowling, R.M.; Campbell, B.M. An Operational Model for Implementing Conservation Action. Conserv. Biol. 2006, 20, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Crouse, D.T.; Mehrhoff, L.A.; Parkin, M.J.; Elam, D.R.; Chen, L.Y. Endangered species recovery and SCB Study: A U.S. Fish and Wildlife Service Perspective. Ecol. Appl. 2002, 12, 719–723. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service; National Marine Fisheries Service. Policy regarding controlled propagation of species listed under the Endangered Species Act. Fed. Regist. 2000, 65, 56916–56922. [Google Scholar]
- Moseby, K.E.; Hill, B.M.; Lavery, T.H. Tailoring Release Protocols to Individual Species and Sites: One Size Does Not Fit All. PLoS ONE 2014, 9, e99753. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bossart, J.L.; Carlton, C.E. Insect Conservation in America: Status and Perspectives. Am. Entomol. 2002, 48, 82–92. [Google Scholar] [CrossRef]
- Stephens, P.A.; Pettorelli, N.; Barlow, J.; Wittingham, M.J.; Cadotte, M.C. Management by proxy? The use of indices in applied ecology. J. Appl. Ecol. 2015, 52, 1–6. [Google Scholar] [CrossRef]
- Andersen, A.; Simcox, D.J.; Thomas, J.A.; Nash, D.R. Assessing reintroduction schemes by comparing genetic diversity of reintroduced and source populations: A case study of the globally threatened large blue butterfly (Maculinea arion). Biol. Conserv. 2014, 175, 34–41. [Google Scholar] [CrossRef]
- Bonney, R.; Cooper, C.B.; Dickinson, J.; Kelling, S.; Phillips, T.; Rosenberg, K.V.; Shirk, J. Citizen Science: A Developing Tool for Expanding Science Knowledge and Scientific Literacy. BioScience 2009, 59, 977–984. [Google Scholar] [CrossRef]
- Devictor, V.; Whittaker, R.J.; Beltrame, C. Beyond scarcity: Citizen science programmes as useful tools for conservation biogeography. Divers. Distrib. 2010, 16, 354–362. [Google Scholar] [CrossRef]
- Jue, D.K.; Daniels, J.C. A successful model for citizen scientist involvement in building a statewide at-risk butterfly database. J. Insect Conserv. 2014, 19, 421–431. [Google Scholar] [CrossRef]
- Chandler, M.; See, L.; Copas, K.; Bonde, A.M.Z.; López, B.C.; Danielsen, F.; Legind, J.K.; Masinde, S.; Miller-Rushing, A.J.; Newman, G.; et al. Contribution of citizen science towards international biodiversity monitoring. Biol. Conserv. 2016, 213, 280–294. [Google Scholar] [CrossRef]
- Hess, R.J.; Hess, A.N. Conserving Karner blue butterflies in Wisconsin: A development of management techniques. Am. Entomol. 2005, 61, 96–113. [Google Scholar] [CrossRef]
- Hudgins, J.A.; Hudgins, E.J.; Ali, K.; Mancini, A. Citizen science surveys elucidate key foraging and nesting habitat for two endangered marine turtle species within the Republic of Maldives. Herpetol. Notes 2017, 10, 463–471. [Google Scholar]
- Kobori, H.; Dickinson, J.L.; Washitani, I.; Sakurai, R.; Amano, T.; Komatsu, N.; Kitamura, W.; Takagawa, S.; Koyama, K.; Ogawara, T.; et al. Citizen science: A new approach to advance ecology, education, and conservation. Ecol. Res. 2016, 31, 1–19. [Google Scholar] [CrossRef]
- Tye, C.A.; McCleery, R.A.; Fletcher, R.J., Jr.; Greene, D.U.; Butryn, R.S. Evaluating citizen vs. professional data for modelling distributions of a rare squirrel. J. Appl. Ecol. 2017, 54, 628–637. [Google Scholar] [CrossRef]
- Cheyne, S.M. Wildlife reintroduction: Considerations of habitat quality at the release site. BMC Ecol. 2006, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ewen, J.G.; Armstrong, D.P. Strategic monitoring of reintroductions in ecological restoration programmes. Ecoscience 2007, 14, 401–409. [Google Scholar] [CrossRef]
- Schultz, C.B.; Dlugosh, K.M. Nectar and hostplant scarcity limit populations of an endangered Oregon butterfly. Oecologia 1999, 119, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Wesselingh, R.A.; Van Dyck, H. Floral resource limitation severely reduces butterfly survival, condition and flight activity in simplified agricultural landscapes. Oecologia 2016, 180, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kuussaari, M.; Heikkinen, R.K.; Heliölä, J.; Luoto, M.; Mayer, M.; Rytteri, S.; von Bagh, P. Successful translocation of the threatened Clouded Apollo butterfly (Parnassius mnemosyne) and metapopulation establishment in southern Finland. Biol. Conserv. 2015, 190, 51–59. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T.G.; Van Dyck, H. Towards a functional resource-based concept for habitat: A butterfly biology viewpoint. Oikos 2003, 102, 417–426. [Google Scholar]
- Dennis, R.L.H.; Shreeve, T.G.; Van Dyck, H. Habitats and Resources: The need for a resource-based definition to conserve butterflies. Biodivers. Conserv. 2006, 15, 1943–1966. [Google Scholar] [CrossRef]
- Lovari, S.; Ferretti, F.; Corazza, M.; Milder, I.; Troiani, N.; Ferrari, C.; Saddi, A. Unexpected consequences of reintroductions: Competition between increasing red deer and threatened Apennine chamois. Anim. Conserv. 2014, 17, 359–370. [Google Scholar] [CrossRef]
- Hayward, M.W.; Hayward, G.J. Activity patterns of reintroduced lion Panthera leo and spotted hyaena Crocuta crocuta in the Addo Elephant National Park, South Africa. Afr. J. Ecol. 2007, 45, 135–141. [Google Scholar] [CrossRef]
- Frankham, R. Where are we in conservation genetics and where do we need to go? Conserv. Genet. 2010, 11, 661–663. [Google Scholar] [CrossRef]
- Saarinen, E. Butterfly Conservation Genetics. In Butterfly Conservation in North America; Daniels, J., Ed.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2015; pp. 75–101. ISBN 978-94-017-9851-8. [Google Scholar]
- Dyson, E.A.; Kamath, M.K.; Hurst, G.D.D. Wolbachia infection associated with all-female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): Evidence for horizontal transmission of a butterfly male killer. Heredity 2002, 88, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.A.; Handley, C.A.; Pike, A.; Forister, M.L.; Fordyce, A.J.; Nice, C.C. Wolbachia infection and Lepidoptera of conservation concern. J. Insect Sci. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Araujo-Jnr, E.V.; Welch, J.J.; Kawahara, A.Y. Wolbachia in butterflies and moths: Geographic structure in infection frequency. Front. Zool. 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Bouchon, D.; Bouton, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Duplouy, A.; Hornett, E. Uncovering the hidden players in Lepidoptera biology: The heritable microbial endosymbionts. PeerJ 2018, 6, e4629. [Google Scholar] [CrossRef] [PubMed]
- Clem, R.J.; Passarelli, A.L. Baculoviruses: Sophisticated Pathogens of Insects. PLoS Pathog. 2013, 9, e1003729. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.H.; Murphy, W.J.; Ostrander, E.A.; Wayne, R.K. Genomics and conservation genetics. Trends Ecol. Evol. 2006, 21, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Joost, S.; Bonin, A.; Bruford, M.W.; Despres, L.; Conord, C.; Erhardt, G. A spatial analysis method (SAM) to detect candidate loci for selection: Towards a landscape genomics approach to adaptation. Mol. Ecol. 2007, 16, 3955–3969. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, E.V.; Hellmann, J.J. Genetic differentiation across a latitudinal gradient in two co-occurring butterfly species: Revealing population differences in a context of climate change. Mol. Ecol. 2007, 17, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Moa, R.; Yang, J.; Miao, C.; Li, Z.; Qiu, Y. Ten Years of Landscape Genomics: Challenges and Opportunities. Front. Plant Sci. 2010, 8, 2136. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; Hohenlohe, P.A.; Luikart, G. Genomics and the future of conservation genetics. Nat. Rev. Genet. 2010, 11, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.B.; Hammond, P.C. Using population viability analysis to develop recovery criteria for endangered insects: Case study of the Fender’s blue butterfly. Conserv. Biol. 2003, 17, 1372–1385. [Google Scholar] [CrossRef]
- McIntire, E.J.B.; Schultz, C.B.; Crone, E.E. Designing a network for butterfly habitat restoration: Where individuals, populations, and landscapes interact. J. Appl. Ecol. 2007, 44, 725–736. [Google Scholar] [CrossRef]
- Radchuk, C.; Johst, K.; Groeneveld, J.; Turlure, C.; Grimm, V.; Schtickzelle, N. Appropriate resolution in time and model structure for population viability analysis: Insights from a butterfly metapopulation. Biol. Conserv. 2014, 169, 345–354. [Google Scholar] [CrossRef]
- Coulson, T.; Mace, G.M.; Hudson, E.; Possingham, H. The use and abuse of population viability analysis. Trends Ecol. Evol. 2001, 16, 219–221. [Google Scholar] [CrossRef]
- Brook, B.W.; Burgman, M.A.; Akçakaya, H.R.; O’Grady, J.J.; Frankham, R. Critiques of PVA ask the wrong questions: Throwing the heuristic baby out with the numerical bath water. Conserv. Biol. 2002, 16, 262–263. [Google Scholar] [CrossRef]
- Reed, M.J.; Mills, L.S.; Dunning, J.B., Jr.; Menges, E.S.; McKelvey, K.S.; Frye, R.; Beissinger, S.R.; Anstett, M.; Miller, P. Emerging Issues in Population Viability Analysis. Conserv. Biol. 2002, 16, 7–19. [Google Scholar] [CrossRef]
- Wolf, S.; Hartl, B.; Carroll, C.; Neel, M.C.; Greenwald, D.N. Beyond PVA: Why Recovery under the Endangered Species Act Is More than Population Viability. BioScience 2015, 65, 200–207. [Google Scholar] [CrossRef]
- Lincoln Park Zoo. Avian Reintroduction and Translocation Database. Available online: http://www.lpzoo.org/ARTD (accessed on 20 June 2018).
- Schwartz, K.R.; Parsons, E.C.M.; Rockwood, L.; Wood, T.C. Integrating In-Situ and Ex-Situ Data Management Processes for Biodiversity Conservation. Front. Ecol. Evol. 2017, 5, 120. [Google Scholar] [CrossRef]
- Sarkar, S.; Pressey, R.L.; Faith, D.P.; Margules, C.R.; Fuller, T.; Stoms, D.M.; Moffett, A.; Wilson, K.A.; Williams, K.J.; Williams, P.H.; et al. Biodiversity Conservation Planning Tools: Present Status and Challenges for the Future. Annu. Rev. Environ. Resour. 2006, 31, 123–159. [Google Scholar] [CrossRef]
- Moilanen, E. Generalized Complementarity and Mapping of the Concepts of Systematic Conservation Planning. Conserv. Biol. 2008, 22, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Dietz, L.A.; Brown, M.; Swaminathan, V. Increasing the impact of conservation projects. Am. J. Primatol. 2010, 72, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Deiner, K.; Forrester, T.; Grof-Tisza, P.; Muir, M.J.; Santos, M.J.; Souza, L.E.; Wilkerson, M.L.; Zylberberg, M. Perspectives on the Open Standards for the Practice of Conservation. Biol. Conserv. 2012, 155, 169–177. [Google Scholar] [CrossRef]
- Conservation Measures Partnership. Available online: http://www.conservationmeasures.org/ (accessed on 30 May 2018).
- Miradi Share. Available online: https://www.miradishare.org/ (accessed on 2 June 2018).
- Sutherland, W.J.; Pullin, A.S.; Dolman, P.M.; Knight, T.M. The need for evidence-based conservation. Trends Ecol. Evol. 2004, 19, 305–308. [Google Scholar] [CrossRef] [PubMed]
|
|
|
|
|
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, J.C.; Nordmeyer, C.; Runquist, E. Improving Standards for At-Risk Butterfly Translocations. Diversity 2018, 10, 67. https://doi.org/10.3390/d10030067
Daniels JC, Nordmeyer C, Runquist E. Improving Standards for At-Risk Butterfly Translocations. Diversity. 2018; 10(3):67. https://doi.org/10.3390/d10030067
Chicago/Turabian StyleDaniels, Jaret C., Cale Nordmeyer, and Erik Runquist. 2018. "Improving Standards for At-Risk Butterfly Translocations" Diversity 10, no. 3: 67. https://doi.org/10.3390/d10030067
APA StyleDaniels, J. C., Nordmeyer, C., & Runquist, E. (2018). Improving Standards for At-Risk Butterfly Translocations. Diversity, 10(3), 67. https://doi.org/10.3390/d10030067




