Diversity and Conservation of Cave-Dwelling Bats in the Brunca Region of Costa Rica
Abstract
1. Introduction
2. Materials and Methods
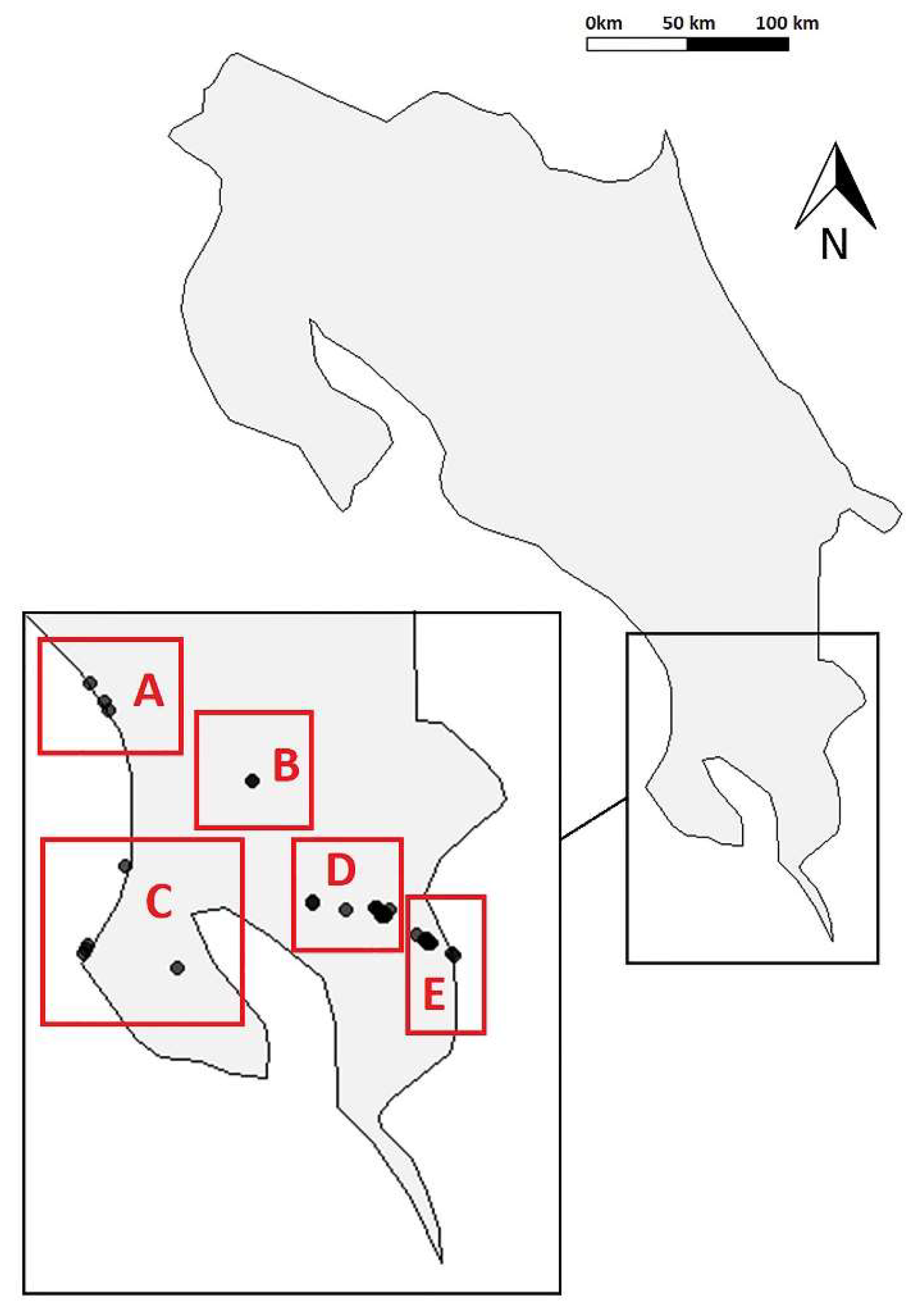
2.1. Study Region
2.2. Cave Surveys
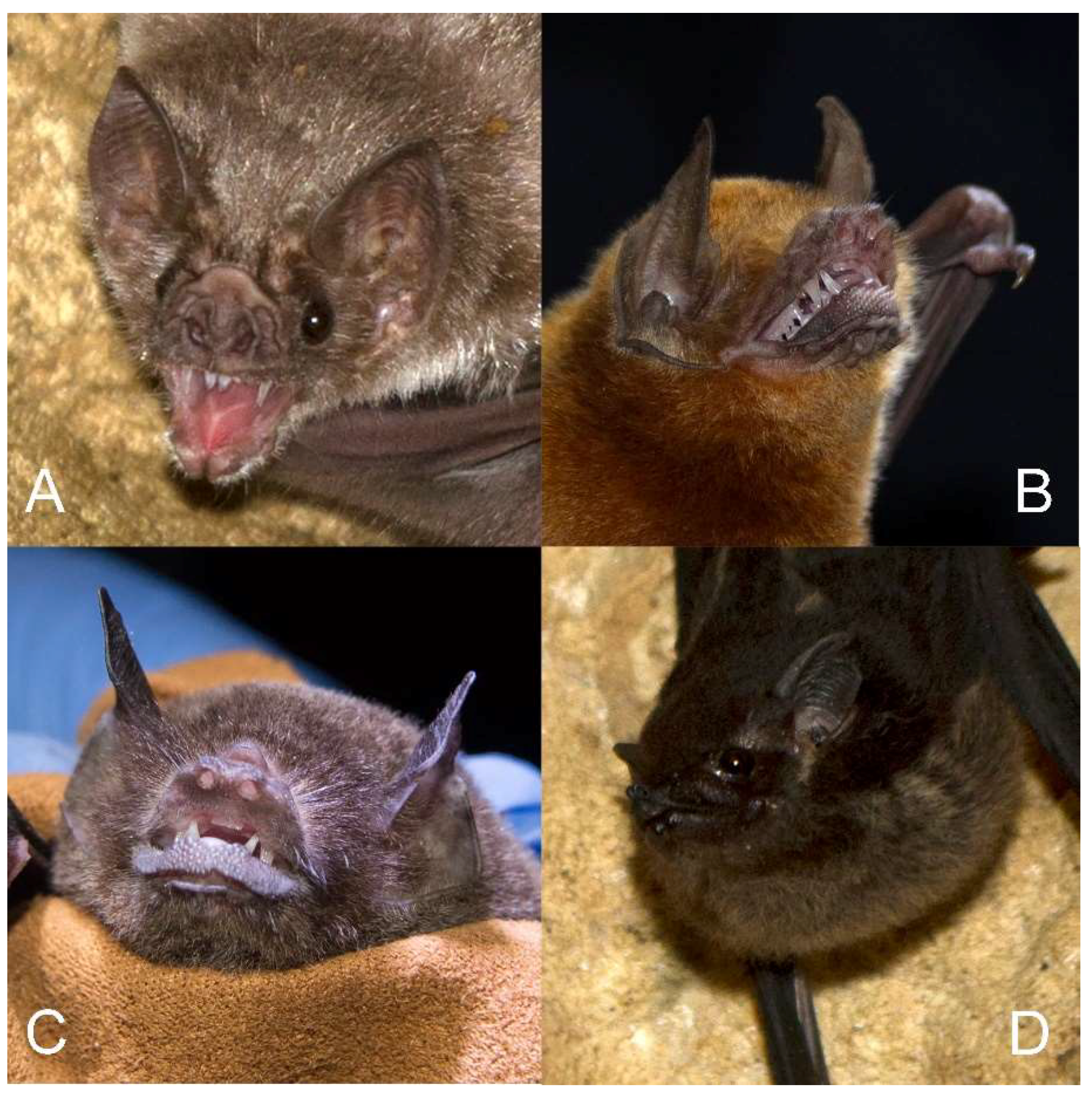
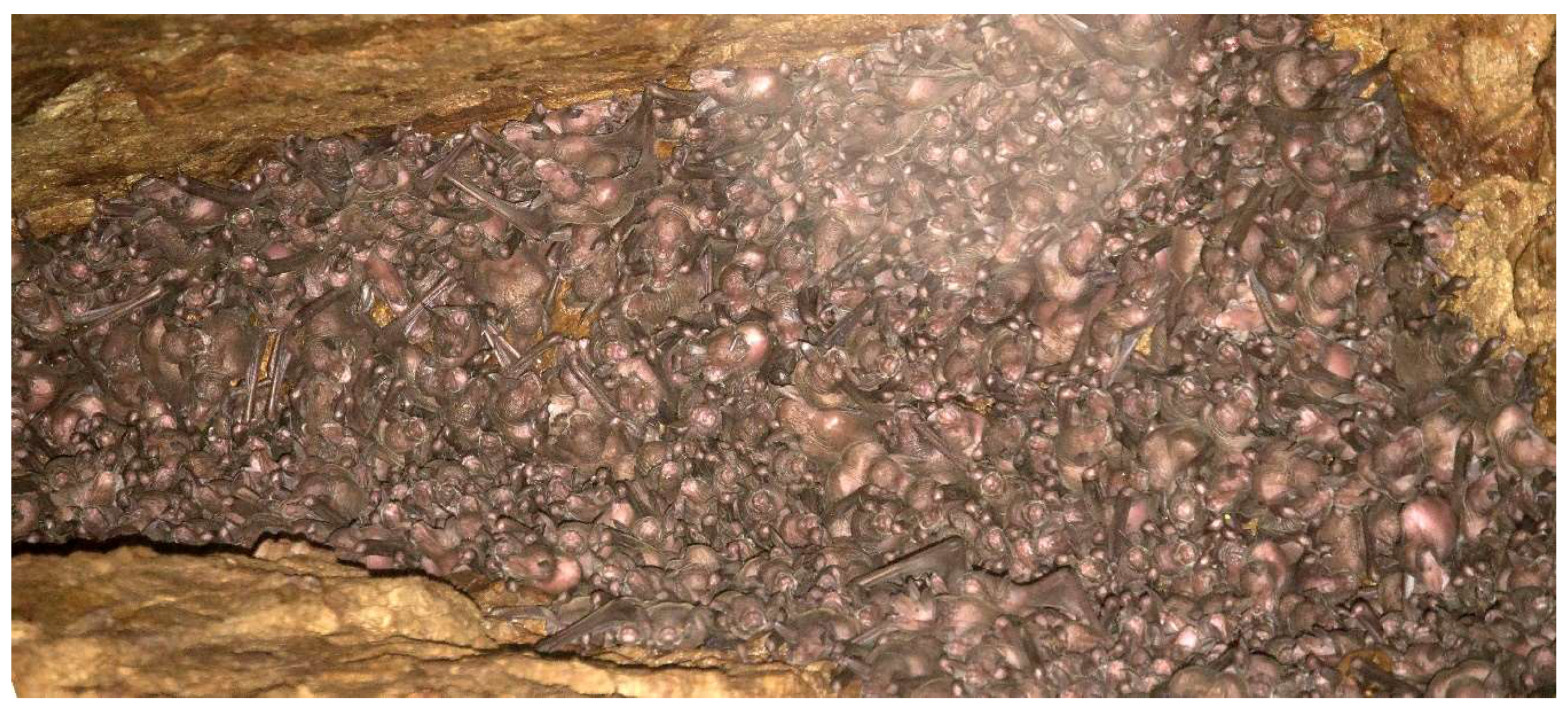
2.3. Bat Surveys
2.4. Assessing Conservation Priority
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Cave | Area | Visits/mm/yy | BCVI | No of Species | A sp. ** | A jam | C per | C sow | C aur | D rot | G sor | L con | L rob | L aur | N mex | P kap | P mac | P dis | P has | P gym | P par | P per | S bil | T cir |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFRODIZIACO POZO * | D | 03/16 | 4 C | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ALMA | E | 02/16, 05/16, 02/17. 12/17 | 3 B | 5 | 0 | 0 | 10 | 0 | 0 | 6 | 3 | 0 | 0 | 0 | 0 | 59 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 0 |
| APRENDIZAJE POZO * | D | 03/16 | 4 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ARBOL CAIDO * | D | 03/16 | 4 C | 2 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ARCO * | A | 02/16, 05/16 | 4 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ARELIS * | D | 01/16; 04/16 | 1 B | 4 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 45 | 0 | 36 | 0 | 0 | 0 | 0 | 55 | 0 |
| BAMBOO POZO * | D | 03/17 | 4 C | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 |
| BANANAL | E | 01/17 | 4 C | 1 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BANANO QUEMADO | E | 03/16 | 4 C | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BOMBASA * | C | 02/18 | 1 B | 6 | 0 | 0 | 350 | 0 | 0 | 1 | 40 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 1 |
| BUENA CUEVA * | D | 03/16 | 4 C | 2 | 0 | 0 | 74 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CABALLO MUERTO * | D | 01/16, 03/16 | 4 C | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CAMPANARIO * | C | 05/17, 02/18 | 1 C | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2000 | 5000 | 600 | 0 | 0 |
| CARMA | E | 02/16 | 2 C | 1 | 0 | 179 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CASTILLO REAL | E | 04/16 | 4 C | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CINCO MILLIONES * | D | 03/16 | 4 C | 4 | 0 | 0 | 15 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0 |
| CORREDORES | E | 01/16, 03.16, 02/16, 12/17 | 1 B | 8 | 0 | 14 | 49 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 235 | 0 | 0 | 0 | 0 | 500 | 700 | 0 | 8 | 0 |
| CUEVA 1 NO NAME * | D | 01/16 | 4 C | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CUEVA 3 NO NAME * | D | 01/16 | 4 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CUEVA 5 NO NAME * | D | 01/16 | 4 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CUEVA CERCA COR | E | 02/16 | 4 C | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DOS BRAZOS * | C | 03/17, 02/18 | 1 B | 2 | 0 | 0 | 663 | 0 | 0 | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EMUS | D | 01/16, 04/16, 12/17 | 1 B | 7 | 0 | 0 | 813 | 0 | 0 | 44 | 0 | 0 | 0 | 0 | 10 | 9 | 2 | 0 | 0 | 0 | 200 | 0 | 26 | 0 |
| FINAL 7 POZO * | D | 03/16 | 4 C | 1 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GRAN GALERIA | E | 03/16, 10/16, 12/17 | 3 B | 5 | 0 | 49 | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 1 | 0 | 0 | 0 | 0 | 0 | 24 | 0 |
| GRAN MADRE * | D | 03/16, 03/16 | 4 C | 5 | 0 | 0 | 16 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 62 | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 0 |
| ICE 1 TUNNEL | B | 02/17 | 4 C | 1 | 0 | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ICE 2 TUNNEL | B | 02/17, 12/17 | 1 B | 5 | 0 | 320 | 70 | 0 | 0 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 150 | 0 | 12 | 0 |
| LA TROJA | E | 04/16 | 4 C | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LAGRIMA POZO * | D | 03/16 | 4 C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LAGUNA PERDIDA * | D | 10/16, 10/16, 12/17 | 1 B | 7 | 100 | 0 | 1239 | 0 | 0 | 176 | 0 | 0 | 118 | 0 | 0 | 0 | 0 | 0 | 57 | 0 | 350 | 0 | 1 | 0 |
| LOS SUEÑOS * | D | 11/16, 02/18 | 4 B | 4 | 0 | 0 | 250 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 65 | 0 |
| METROS 12 NO NAME * | D | 01/16 | 4 C | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MIRAMAR POZO * | E | 12/15, 01/16 | 1 C | 5 | 0 | 0 | 134 | 1 | 0 | 1 | 0 | 11 | 0 | 108 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MONTEADORES * | E | 01/16 | 4 C | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| PIEDRAS BLANCAS 2 * | D | 10/17 | 3 D | 1 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| RECTANGULO | E | 04/16 | 4 C | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| SAN JOSECITO * | C | 05/17, 02/18 | 4 B | 3 | 0 | 0 | 35 | 0 | 0 | 19 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SAN PEDRILLO * | C | 05/17 | 1 B | 5 | 0 | 34 | 5 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| SAPO GORDO POZO * | D | 03/16 | 4 C | 1 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SERPIENTE DORMIDA | E | 03/17 | 4 C | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TITI MONO * | D | 03/16 | 4 C | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TORTUGA * | A | 05/16, 10/16, 02/17 | 1 A | 3 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 427 | 400 | 0 | 0 | 0 |
| VENTANA * | A | 05/16 | 4 A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Common Name | Latin Name | IUCN Status | Population Trend | Cave-Dwelling | Cave-Dependent |
|---|---|---|---|---|---|
| Handley’s tailless bat | Anoura cultrata | LC | decreasing | Yes | No |
| Geoffroy’s tailless bat | Anoura geoffroyi | LC | stable | Yes | No |
| Jamaican fruit bat | Artibeus jamaicensis | LC | stable | Yes | No |
| Great fruit-eating bat | Artibeus lituratus | LC | stable | Yes | No |
| Chestnut short-tailed bat | Carollia castanea | LC | stable | Yes | No |
| Seba’s short-tailed bat | Carollia perspicillata | LC | stable | Yes | No |
| Sowell’s short-tailed bat | Carollia sowelli | LC | stable | Yes | No |
| Shaggy bat | Centronycteris centralis | LC | unknown | No | No |
| Wrinkle-faced bat | Centurio senex | LC | stable | No | No |
| Salvin’s big-eyed bat | Chiroderma salvini | LC | stable | No data | No |
| Hairy big-eyed bat | Chiroderma villosum | LC | stable | No | No |
| Godman’s long-tailed bat | Choeroniscus godmani | LC | unknown | No data | No data |
| Big-eared wooly bat | Chrotopterus auritus | LC | stable | Yes | No |
| Wagner’s sac-winged bat | Cormura brevirostris | LC | unknown | No | No |
| Aztec fruit-eating bat | Dermanura azteca | LC | unknown | Yes | No |
| Toltec fruit-eating bat | Dermanura tolteca | LC | unknown | Yes | No |
| Thomas’ fruit eating bat | Dermanura watsoni | LC | stable | No | No |
| Common vampire bat | Desmodus rotundus | LC | stable | Yes | No |
| White-winged vampire bat | Diaemus youngi | LC | unknown | Yes | No |
| Northern ghost bat | Diclidurus albus | LC | unknown | No | No |
| Hairy-legged vampire bat | Diphylla ecaudata | LC | stable | Yes | No |
| Velvety fruit-eating bat | Enchisthenes hartii | LC | unknown | No data | No data |
| Brazilian brown bat | Eptesicus brasiliensis | LC | unknown | No | No |
| Chirqui brown bat | Eptesicus chiriquinus | LC | unknown | No | No |
| Argentine brown bat | Eptesicus furinalis | LC | unknown | Yes | No |
| Big brown bat | Eptesicus fuscus | LC | increasing | Yes | No |
| Black bonneted bat | Eumops auripendulus | LC | unknown | No | No |
| Sanborn’s bonneted bat | Eumops hansae | LC | unknown | No data | No data |
| Commissaris’s long-tongued bat | Glossophaga commissarisi | LC | stable | Yes | No |
| Pallas’s long-tongued bat | Glossophaga soricina | LC | stable | Yes | No |
| Underwood’s long-tongued bat | Hylonycteris underwoodi | LC | stable | Yes | Yes |
| Yellow-throated big-eared bat | Lampronycteris brachyotis | LC | stable | Yes | No |
| Desert red bat | Lasiurus blossevillii | LC | unknown | No | No |
| Southern yellow bat | Lasiurus ega | LC | unknown | No | No |
| Dark long-tongued bat | Lichonycteris obscura | LC | unknown | No data | No data |
| Goldman’s nectar bat | Lonchophylla concava | LC | unknown | Yes | No data |
| Orange nectar bat | Lonchophylla robusta | LC | unknown | Yes | Yes |
| Tomes’s sword-nosed bat | Lonchorhina aurita | LC | stable | Yes | Yes |
| Pygmy round-eared bat | Lophostoma brasiliense | LC | stable | No | No |
| White-throated round-eared bat | Lophostoma silvicolum | LC | unknown | No | No |
| Long-legged bat | Macrophyllum macrophyllum | LC | unknown | Yes | No data |
| Hairy big-eared bat | Micronycteris hirsuta | LC | unknown | No | No |
| Common big-eared bat | Micronycteris microtis | LC | stable | Yes | No |
| White-bellied big-eared bat | Micronycteris minuta | LC | unknown | Yes | No |
| Schmidts’s big-eared bat | Micronycteris schmidtorum | LC | stable | No | No |
| Striped hairy-nosed bat | Mimon crenulatum | LC | stable | No | No |
| Coiban Mastiff Bat | Molossus coibensis | LC | unknown | No | No |
| Velvety free-tailed bat | Molossus molossus | LC | unknown | No | No |
| Miller’s mastiff bat | Molossus pretiosus | LC | unknown | Yes | No data |
| Black mastiff bat | Molossus rufus | LC | stable | No | No |
| Sinaloan mastiff bat | Molossus sinaloae | LC | stable | Yes | No |
| Silver-tipped myotis | Myotis albescens | LC | stable | Yes | No |
| Hairy-legged myotis | Myotis keaysi | LC | unknown | Yes | No |
| Black myotis | Myotis nigricans | LC | stable | Yes | No |
| Montane myotis | Myotis oxyotus | LC | unknown | No data | No data |
| Riparian myotis | Myotis riparius | LC | stable | No data | No data |
| Mexican funnel-eared bat | Natalus mexicanus | LC | unknown | Yes | Yes |
| Lesser bulldog bat | Noctilio albiventris | LC | stable | No | No |
| Greater bulldog bat | Noctilio leporinus | LC | unknown | Yes | No |
| Greater dog-like bat | Peropteryx kappleri | LC | unknown | Yes | No |
| Lesser doglike bat | Peropteryx macrotis | LC | stable | Yes | No |
| Pale spear-nosed bat | Phyllostomus discolor | LC | stable | Yes | No |
| Greater spear-nosed bat | Phyllostomus hastatus | LC | stable | Yes | No |
| Heller’s broad-nosed bat | Platyrrhinus helleri | LC | stable | Yes | No |
| Greater broad-nosed bat | Platyrrhinus vittatus | LC | unknown | Yes | No data |
| Naked-backed bat | Pteronotus davyi | LC | stable | Yes | Yes |
| Big naked-backed bat | Pteronotus gymnonotus | LC | stable | Yes | Yes |
| Parnell’s mustached bat | Pteronotus mesoamericanus | LC | unknown | Yes | Yes |
| Wagner’s mustached bat | Pteronotus personatus | LC | stable | Yes | Yes |
| Thomas’ yellow bat | Rhogeessa io | LC | unknown | No | No |
| Proboscis bat | Rhynchonycteris naso | LC | unknown | No | No |
| Greater sac-winged bat | Saccopteryx bilineata | LC | unknown | Yes | No |
| Lesser sac-winged bat | Saccopteryx leptura | LC | unknown | No | No |
| Talamancan yellow-shouldered bat | Sturnira mordax | NT | stable | No data | No data |
| Mexican free-tailed bat | Tadarida brasiliensis | LC | stable | Yes | No |
| Spix’s disk-winged bat | Thyroptera tricolor | LC | unknown | No | No |
| Stripe-headed round-eared bat | Tonatia saurophila | LC | stable | No | No |
| Fringe-lipped bat | Trachops cirrhosus | LC | stable | Yes | No |
| Niceforo’s big-eared bat | Trinycteris nicefori | LC | unknown | No | No |
| Tent-making bat | Uroderma bilobatum | LC | stable | No | No |
| Striped yellow-eared bat | Vampyriscus nymphaea | LC | unknown | No | No |
| Northern little yellow-eared bat | Vampyressa thyone | LC | unknown | No | No |
| Great stripe-faced bat | Vampyrodes major | LC | unknown | No | No |
| Spectral bat | Vampyrum spectrum | NT | decreasing | Yes | No |

References
- Culver, D.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Romero, A. Cave Biology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Kambesis, P. The importance of cave exploration to scientific research. J. Cave Karst Stud. 2007, 69, 46–58. [Google Scholar]
- Schilthuizen, M.; Cabanban, A.; Haase, M. Possible speciation with gene flow in tropical cave snails. J. Zool. Syst. Evol. Res. 2005, 43, 133–138. [Google Scholar] [CrossRef]
- Vidthayanon, C. Cryptotora thamicola (Waterfall Climbing Cave Fish) the IUCN Red List of Threatened Species 2011. Available online: http://www.iucnredlist.org/details/41407/0 (accessed on 15 March 2018).
- Brinkløv, S.; Elemans, C.; Ratcliffe, J. Oilbirds produce echolocation signals beyond their best hearing range and adjust signal design to natural light conditions. R. Soc. Open Sci. 2017, 4, 170255. [Google Scholar] [CrossRef] [PubMed]
- Ladle, R.; Firmino, J.; Malhado, A.; Rodríguez-Durán, A. Unexplored diversity and conservation potential of neotropical hot caves. Conserv. Biol. 2012, 26, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Maldonado, J. Female interactions in harem groups of the Jamaican fruit-eating bat, Artibeus jamaicensis (Chiroptera: Phyllostomidae). Acta Chiropterologica 2006, 8, 485–495. [Google Scholar] [CrossRef]
- McCracken, G.; Gustin, M. Nursing behavior in Mexican free-tailed bat maternity colonies. Ethology 2010, 89, 305–321. [Google Scholar] [CrossRef]
- Racey, P.; Furey, N. Conservation Ecology of cave bats. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C., Kingston, T., Eds.; Springer International Publishing: Berlin, Germany, 2016; pp. 463–500. [Google Scholar]
- Sagot, M.; Chaverri, G. Effects of roost specialization on extinction risk in bats. Conserv. Biol. 2015, 29, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Sewall, B.; Granek, E.; Trewhella, W. The endemic Comoros islands fruit bat Rousettus obliviosus: Ecology, conservation, and red list status. Oryx 2003, 37. [Google Scholar] [CrossRef]
- Hristov, N.; Betke, M.; Theriault, D.; Bagchi, A.; Kunz, T. Seasonal variation in colony size of brazilian free-tailed bats at Carlsbad cavern based on thermal imaging. J. Mammal. 2010, 91, 183–192. [Google Scholar] [CrossRef]
- McFarlane, D.; Rentergem, G.; Ruina, A.; Lundberg, J.; Christenson, K. Estimating colony size of the wrinkle-lipped bat, Chaerephon plicatus (Chiroptera: Molossidae) at Gomantong, Sabah, by quantitative image analysis. Acta Chiropterol. 2015, 17, 171–177. [Google Scholar] [CrossRef]
- Reid, F. A Field Guide to the Mammals of Central America & Southeast Mexico, 2nd ed.; Oxford University Press: Oxford, UK, 2009; pp. 72–176. [Google Scholar]
- Trimm, R.; LaVal, R. A Field Key to the Bats of Costa Rica; Occasional Publication Series; Center for Latin American Studies; University of Kansas: Lawrence, KS, USA, 1999; Volume 22, pp. 1–20. [Google Scholar]
- Ulloa, A.; Goicoechea, C. Geotourism Potential of Underground Sites in Costa Rica. Tour. Karst Areas 2013, 6, 43–56. [Google Scholar]
- Arita, H. Conservation Biology of the cave bats of Mexico. J. Mammal. 1993, 74, 693–702. [Google Scholar] [CrossRef]
- Bats Living in Caves, Barra Honda National Park. Available online: http://docs.projects-abroad.org/us/conservation-management-plan/data-and-reports/costa-rica/bats-living-in-caves.pdf (accessed on 15 March 2018).
- Kunz, T.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.; Hodgkison, R.; Weise, S. Methods for capturing and handling bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T., Parsons, S., Eds.; The Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 5–35. [Google Scholar]
- Flaquer, C.; Torre, I.; Arrizabalaga, A. Comparison of sampling methods for inventory of bat communities. J. Mammal. 2007, 88, 526–533. [Google Scholar] [CrossRef]
- Atanasov, N. Novata peshtera cave near the town of Peshtera. Priroda Nauka 1936, 3, 75–77. [Google Scholar]
- Petrov, B.; Helversen, O. Bats (Mammalia: Chiroptera) of the western Rhodopes Mountain (Bulgaria and Greece). In Biodiversity of Western Rhodopes (Bulgaria and Greece); Biodiversity of Bulgaria 4; Beron, P., Ed.; Pensoft & National Museum of Natural History: Sofia, Bulgaria, 2011. [Google Scholar]
- Battersby, J. Guidelines for Surveillance and Monitoring of European Bats; EUROBATS Publication Series No 5; UNEP/EUROBATS Secretariat: Bonn, Germany, 2010; Volume 5, p. 95. [Google Scholar]
- Cardiff, S.; Ratrimomanarivo, F.; Rembert, G.; Goodman, S. Hunting, disturbance and roost persistence of bats in caves at Ankarana, northern Madagascar. Afr. J. Ecol. 2009, 47, 640–649. [Google Scholar] [CrossRef]
- Foley, J.; Clifford, D.; Castle, K.; Cryan, P.; Ostfeld, R. Investigating and managing the rapid emergence of white-nose syndrome, a novel, fatal, infectious disease of hibernating bats. Conserv. Biol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Christenson, K.; Mcfarlane, D. An ecologically-significant range extension for Hahn’s short-tailed fruit bat (Carollia Subrufa) in Southwestern Costa Rica. Chiropt. Neotrop. 2007, 13, 319–321. [Google Scholar]
- Pacheco, J.; Ceballos, G.; Daily, G.; Ehrlich, P.; Suzán, G.; Rodríguez-Herrera, B.; Marcé, E. Diversidad, historia natural y conservación de los mamíferos de San Vito De Coto Brus, Costa Rica. Rev. Biol. Trop. 2014, 54, 219. [Google Scholar] [CrossRef]
- Landmann, A.; Walder, C.; Vorauer, A.; Bohn, S.; Weinbeer, M. Bats of the La Gamba Region, Esquinas Rainforest, Costa Rica: Species diversity, guild structure and niche segregation. In Natural and Cultural History of the Golfo Dulce Region, Costa Rica; Einbeer, M., Ed.; Oberösterreichisches Landesmuseum, Biologiezentrum: Linz, Austria, 2008; pp. 423–440. [Google Scholar]
- Hutson, A.M.; Mickleburgh, S.P. Racey, P.A. Michrochiropteran Bats: Global Status Survey and Conservation Action Plan; IUCN/SSC Chiroptera Specialist Group; IUCN: Gland, Switzerland; Cambridge, UK, 2001. [Google Scholar]
- Baker, A.; Genty, D. Environmental pressures on conserving cave speleothems: Effects of changing surface land use and increased cave tourism. J. Environ. Manag. 1998, 53, 165–175. [Google Scholar] [CrossRef]
- Medellin, R.; Wiederholt, R.; Lopez-Hoffman, L. Conservation relevance of bat caves for biodiversity and ecosystem services. Biol. Conserv. 2017, 211, 45–50. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sato, G.; Mochizuki, N.; Hirano, S.; Itou, T.; Carvalho, A.; Albas, A.; Santos, H.; Ito, F.; Sakai, T. Molecular and geographic analyses of vampire bat-transmitted cattle rabies in central Brazil. BMC Vet. Res. 2008, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, M.; Moreno, A. Cave-Dwelling Bats of Northern Mexico: Their Value and Conservation Needs; Bat Conservation International: Austin, TX, USA, 2005. [Google Scholar]
- BATS Magazine Article: Conserving Costa Rica’s Bats. Available online: http://www.batcon.org/resources/media-education/bats-magazine/bat_article/57 (accessed on 15 March 2018).
- Climate Ciudad Neily: Temperature, Climograph, Climate Table for Ciudad Neily–Climate-Data.org. Available online: https://en.climate-data.org/location/1005481/ (accessed on 14 February 2018).
- Gilbert, L.; Christen, C.; Altrichter, M.; Longino, J.; Sherman, P.; Plowes, R.; Swartz, M.; Winemiller, K.; Weghorst, J.; Vega, A.; et al. The Southern Pacific lowland evergreen moist forest of the Osa Region. In Costa Rican Ecosystems; Kappelle, M., Ed.; University of Chicago Press: Chicago, IL, USA, 2016; pp. 360–411. [Google Scholar]
- Registro Kárstico Nacional (RKN)|Grupo Espeleológico Anthros. Available online: http://anthros.org/es/anthros/registro-karstico-nacional/ (accessed on 15 February 2018).
- Peacock, N.; Hempel, J. Studies of the Rio Corredor Basin Bulletin 2–NSS Costa Rica Project; National Speleological Society Bulletin: Washington, DC, USA, 1993; Volume 55. [Google Scholar]
- Marbach, G.; Tourte, B. Alpine Caving Techniques; Speleo Projects: Allschwil, Switzerland, 2002. [Google Scholar]
- Paperless Cave Surveying. Available online: https://paperless.bheeb.ch/ (accessed on 15 February 2018).
- Hayes, J.; Ober, H.; Sherwin, E. Surveying and monitoring bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Kunz, T., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 115–132. [Google Scholar]
- Ferreira, T.; Rasband, W. Imagej. 2012. Available online: http://imagej.nih.gov/ij/docs/guide (accessed on 7 April 2018).
- La Val, R.; Rodriguez-Herrera, B.; Tschapka, M.; Quesada, F.; Suárez, C.A.; Sistachs, O. Costa Rica Bats, 1st ed.; Editorial Inbio: San Jose, CA, USA, 2002. [Google Scholar]
- Tanalgo, K.; Tabora, J.; Hughes, A. Bat Cave Vulnerability Index (BCVI): A Holistic Rapid Assessment Tool to Identify Priorities for Effective Cave Conservation in the Tropics. Ecol. Indic. 2018, 89, 852–860. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Version 2017-3. Available online: http://www.iucnredlist.org/search?page=1 (accessed on 7 April 2018).
- Rodriguez-Herrera, B.; Chinchilla, F.; May-Collado, L. Lista de especies, endemismo y conservación de los mamíferos de Costa Rica. Rev. Mex. Mastozool. 2002, 16, 21–57. [Google Scholar]
- Rodriguez-Duran, A. Nonrandom aggregations and distribution of cave-dwelling bats in Puerto Rico. J. Mammal. 1998, 79, 141–146. [Google Scholar] [CrossRef]
- Torquetti, C.; Silva, M.; Talamoni, S. Differences between caves with and without bats in a Brazilian karst habitat. Zoologia 2017, 34, 1–7. [Google Scholar] [CrossRef]
- Medellin, R.; Wilson, D.; Daniel, L. Micronycteris brachyotis. Mamm. Species 1985, 1. [Google Scholar] [CrossRef]
- Muñoz-Romo, M.; Herrera, E.; Kunz, T. Roosting behavior and group stability of the big fruit-eating bat Artibeus lituratus (Chiroptera: Phyllostomidae). Mamm. Biol. Z. Säugetierkunde 2008, 73, 214–221. [Google Scholar] [CrossRef]
- Dinets, V. Long-term cave roosting in the spectral bat (Vampyrum Spectrum). Mammalia 2017, 81. [Google Scholar] [CrossRef]
- Aguirre, L.; Mantilla, H.; Miller, B.; Dávalos, L. “Vampyrum Spectrum”. The IUCN Red List of Threatened Species 2008. Available online: http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T22843A9395576.en (accessed on 7 April 2018).
- Pineda, W.; Rodriguez, B. Macrophyllum macrophyllum: Rodriguez, IUCN Red List of Threatened Species 2015. Available online: http://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T12615A22025883.en (accessed on 7 April 2018).
- Rodríguez-Herrera, B.; Sánchez, R.; Pineda, W. First Record of Natalus lanatus (Chiroptera: Natalidae) in Costa Rica, and Current Distribution of Natalus in the Country. Ecotropica 2011, 17, 113–117. [Google Scholar]
- Rodríguez-Herrera, B.; Ramírez-Fernández, J.; Villalobos-Chaves, D.; Sánchez, R. Actualización De La Lista De Especies De Mamíferos Vivientes De Costa Rica. Mastozool. Neotrop. 2014, 21, 275–289. [Google Scholar]
- Áreas Importantes Para LA Conservación De Los Murciélagos (AICOMs) AICOMs y SICOMs–RELCOM–Red Latinoamericana y del Caribe Para la Conservación de Los Murciélagos. Available online: http://www.relcomlatinoamerica.net/index.php/que-hacemos/conservacion/18-relcom/33-aicomsysicoms (accessed on 7 April 2018).
- Sheffield, S.; Shaw, J.; Heidt, G.; McQenaghan, L. Guidelines for the protection of bat roosts. J. Mammal. 1992, 73, 707–710. [Google Scholar] [CrossRef]
| Family | Species | Roosts |
|---|---|---|
| Phyllostomidae | Anoura sp. | Laguna Perdida, Piedras Blancas 2 |
| Artibeus jamaicensis | Arelis, Carma, Corredores, Gran Galería, Túnel ICE 2, San Pedrillo | |
| Carollia perspicillata | Afrodiziaco Pozo, Alma, Árbol Caido, Bananal, Bombasa, Buena Cueva, Caballo Muerto, Cinco Millones, Corredores, Dos Brazos, Emús, Final 7 Pozo, Gran Galería, Gran Madre, Túnel ICE 1, Túnel ICE 2, San Josecito, Laguna Perdida, Los Sueños, Miramar Pozo, San Pedrillo, Sapo Gordo Pozo, Titi Mono, Tortuga | |
| Carollia sowelli | Miramar | |
| Chrotopterus auritus | Corredores | |
| Desmodus rotundus | Alma, Bombasa, Buena Cueva, Cinco Millones, Emús, Gran Madre, Túnel ICE 2, San Josecito, Laguna Perdida, Los Sueños, Miramar | |
| Glossophaga soricina | Alma, Bombasa, Corredores, Dos Brazos | |
| Lonchophylla concava | San Josecito, Miramar Pozo, San Pedrillo | |
| Lonchophylla robusta | Bombasa, Laguna Perdida | |
| Lonchorhina aurita | Gran Madre, Miramar | |
| Phyllostomus discolor | Arelis | |
| Phyllostomus hastatus | Laguna Perdida | |
| Trachops cirrhosus | Bombasa, San Pedrillo | |
| Emballonuridae | Peropteryx kappleri | Alma, Arbol Caido, Arelis, Bamboo Pozo, Banano Quemado, Caballo Muerto, Castillo Real, Cinco Millones, Cueva 1 No Name, Cueva Cerca Cor, Emús, Gran Galería, Gran Madre, La Troja, Metros 12, Monteadores, Rectángulo, Serpiente Dormida |
| Peropteryx macrotis | Emús, Gran Galería | |
| Saccopteryx bilineata | Alma, Arelis, Bamboo Pozo, Cinco Millones, Corredores, Emús, Gran Galería, Gran Madre, Túnel ICE 2, Laguna Perdida, Los Sueños, Monteadores, Rectángulo, San Pedrillo | |
| Natalidae | Natalus mexicanus | Corredores, Emus |
| Mormoopidae | Pteronotus gymnonotus | Campanario, Corredores, Tortuga |
| Pteronotus parnellii | Bombasa, Campanario, Corredores, Emus, Túnel ICE 2, Laguna Perdida, Los Sueños, Tortuga | |
| Pteronotus personatus | Campanario |
| BCVI | Priority | Description * | Roosts | |
|---|---|---|---|---|
| BP | BV | |||
| 1 | А | High | Large population, highest site accessibility, highly prone to disturbance. | Tortuga |
| 1 | B | High | Large population, high species diversity, high site accessibility, highly prone to disturbance. | Arelis, Bombasa, Corredores, Dos Brazos, Emus, Túnel Ice 2, Laguna Perdida, San Pedrillo |
| 1 | C | Medium | Large population, high species diversity, low site accessibility and less prone to disturbance. | Campanario, Miramar |
| 2 | C | Medium | Relatively high population, low species diversity, low site accessibility and less prone to disturbance. | Carma |
| 3 | B | Medium | Small populations, relatively high species diversity, high site accessibility, highly prone to disturbance. | Alma, Gran Galeria |
| 3 | D | Medium | Relatively large population, low species diversity, rare species present, low site accessibility, not prone to disturbance. | Piedras Blancas 2 |
| 4 | A | Low | No bats present, highest site accessibility, highly prone to disturbance. | Arco, Ventana |
| 4 | B | Low | Small populations, relatively high species diversity, high site accessibility. | San Jocesito Cataratas, Los Sueños, Gran Madre |
| 4 | C | Low | Very small population, low species diversity, lower site accessibility and less prone to disturbance. | Afrodiziaco, Aprendizaje, Arbol Caido, Bamboo, Bananal, Banano Quemado, Buena Cueva, Caballo Muerto, Castillo Real, Cinco millones, Cueva 1, Cueva 3, Cueva 5, Cueva cerca Corredores., Final 7, Túnel ICE 2, La Troja, Lagrima, Metros 12, Monteadores, Rectangulo, Sapo Gordo, Serpiente Dormida, Titi Mono. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deleva, S.; Chaverri, G. Diversity and Conservation of Cave-Dwelling Bats in the Brunca Region of Costa Rica. Diversity 2018, 10, 43. https://doi.org/10.3390/d10020043
Deleva S, Chaverri G. Diversity and Conservation of Cave-Dwelling Bats in the Brunca Region of Costa Rica. Diversity. 2018; 10(2):43. https://doi.org/10.3390/d10020043
Chicago/Turabian StyleDeleva, Stanimira, and Gloriana Chaverri. 2018. "Diversity and Conservation of Cave-Dwelling Bats in the Brunca Region of Costa Rica" Diversity 10, no. 2: 43. https://doi.org/10.3390/d10020043
APA StyleDeleva, S., & Chaverri, G. (2018). Diversity and Conservation of Cave-Dwelling Bats in the Brunca Region of Costa Rica. Diversity, 10(2), 43. https://doi.org/10.3390/d10020043





