Bee Diversity and Solanum didymum (Solanaceae) Flower–Visitor Network in an Atlantic Forest Fragment in Southern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Design
2.3. Pollen Collection
2.4. Assemblage of Floral Bee Visitors of S. didymum
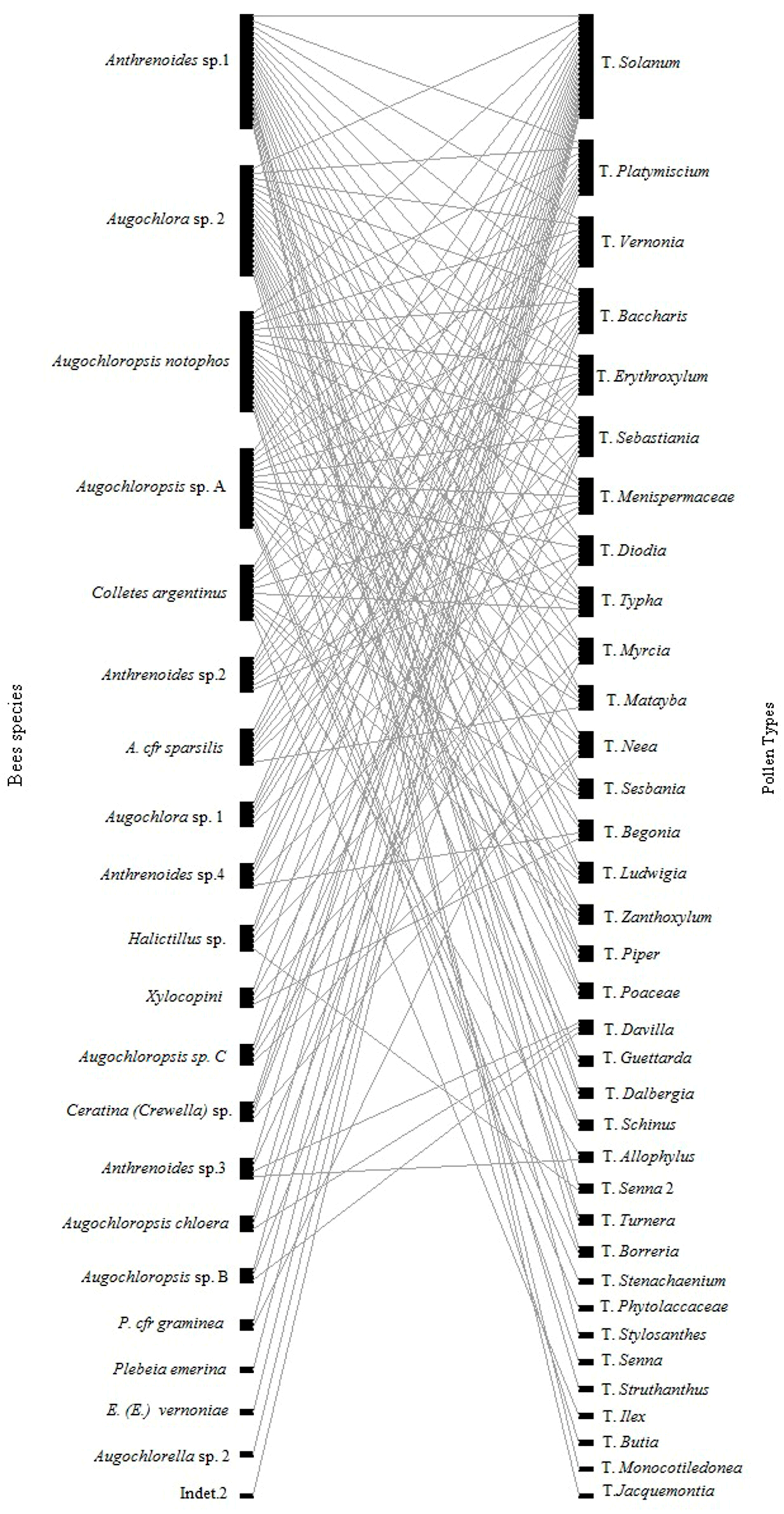
2.5. Interaction Network
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Araújo, J.L.O.; Quirino, Z.G.M.; Gandelha Neto, P.C.; Araújo, A.C. Síndromes de polinização ocorrentes em uma área de Mata Atlântica, Paraíba, Brasil. Biotemas 2009, 22, 83–94. [Google Scholar] [CrossRef]
- Torezan-Silingard, H.M. Flores e animais: Uma introdução à história natural dapolinização. In Eologia das Interações Plantas-Animais: Uma Abordagem Ecológico-Evolutiva; Technical Books Editora: Rio de Janeiro, Brazil, 2012; pp. 111–139. [Google Scholar]
- Buchmann, S.L.; Hurley, J.P. A biophysical model for buzz pollination in angiosperms. J. Theor. Biol. 1978, 72, 639–657. [Google Scholar] [CrossRef]
- Olmstead, R.; Palmer, J.D. Implications for the phylogeny, classification, and biogeography of Solanum from cpDNA restriction site variation. Syst. Bot. 1997, 22, 19–29. [Google Scholar] [CrossRef]
- Slaa, E.J.; Sánchez Chaves, L.A.; Malagodi-Braga, K.S.; Hofstede, F.E. Stingless bees in applied pollination: Practice and perspectives. Apidologie 2006, 37, 293–315. [Google Scholar] [CrossRef]
- Buchmann, S.L. Buzz pollination in angiosperms. In Handbook of Experimental Pollination Biology; Van Nostrand Reinhold Company: New York, NY, USA, 1983; pp. 63–113. [Google Scholar]
- Smith, S.D.; Knapp, S. The natural history of reproduction in Solanum and Lycianthes (Solanaceae) in a subtropical moist forest. Bull. Nat. Hist. Mus. Lond. (Bot.) 2002, 32, 125–136. [Google Scholar] [CrossRef]
- Tabarelli, M.; Mantovani, W.; Peres, C.A. Effects of habitat fragmentation on Plant guild structure in the montane Atlantic forest of southeastern Brazil. Biol. Conserv. 1999, 91, 119–127. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Fontes, M.A.L. Patterns of Floristic Differentiation among Atlantic Forests in Southeastern Brazil and the Influence of Climate. Biotropica 2000, 32, 793–810. [Google Scholar] [CrossRef]
- Duarte, L.S.; Dillenburg, L.R. Ecophysiological responses of Araucaria angustifolia (Araucariaceae) seedlings to different irradiance levels. Aust. J. Bot. 2000, 48, 531–537. [Google Scholar] [CrossRef]
- Rambo, B.O. elemento andino pinhal rio-grandense. Anais Bot. Herb. “Barbosa Rodrigues” 1951, 3, 3–39. [Google Scholar]
- Waechter, J.L. Padrões fitogeográficos na flora atual do Rio Grande do Sul. Ciênc. Amb. 2002, 24, 93–108. [Google Scholar]
- Smith, L.B.; Downs, R. Solanaceas. Flora Ilustrada Catarinense; Sola: Itajaí, Brazil, 1966. [Google Scholar]
- Jordano, P.; Bascompte, J.; Olesen, J.M. Invariant properties in coevolutionary networks of plant-animal interactions. Ecol. Lett. 2003, 6, 69–81. [Google Scholar] [CrossRef]
- Petanidou, T.; Kallimanis, A.S.; Tzanopoulos, J.; Sgardelis, S.P.; Pantis, J.D. Long-term observation of a pollination network: Fluctuation in species and interactions, relative invariance of network structure and implications for estimates of specialization. Ecol. Lett. 2008, 11, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Jordano, P.; Bascompte, J.; Olesen, J.M. The ecological consequences of complex topology and nested structure in pollination webs. In Plant–Pollinator Interactions, from Specialization to Generalization; University of Chicago Press: Chicago, IL, USA, 2006; pp. 173–199. [Google Scholar]
- Secretaria Municiapal do Meio Ambiente—SEMAFLOR. Prefeitura Municipal de Guarapuava. Available online: htpp://www.prefeituramunicipaldeguarapuava (accessed on 7 March 2013).
- Maack, R. Geografia física do Estado do Paraná, 2nd ed.; José Olympio/Sec. da cultura e do esporte do Governo do Estado do Paraná: Rio de Janeiro, Brazil, 1981.
- Erdtman, G. The acetolizes method: A revised description. Svensk Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Vergeron, P. Interprétation statistique des resultants en matièred’analyse pollinique des miels. Ann. Abeille 1964, 7, 349–364. [Google Scholar] [CrossRef]
- Silveira Neto, S.; Nakano, O.; Barbin, D.; Villa Nova, N.A. Manual de Ecologia dos Insetos; Ceres: Piracicaba, Brazil, 1976. [Google Scholar]
- Palma, S. Contribuciónal studio de lossifonoforos encontrados frente a la costa de Valparaiso. In Memorias del II Simpósio Latino Americano Sobre Oceanografia Biológica; Universidad de Oriente: Cumaná, Venezuela, 1975; pp. 119–133. [Google Scholar]
- Jordano, P. Patterns of mutualistic interactions in pollination and seed dispersal: Connectance, dependence asymmetries and coevolution. Am. Nat. 1987, 129, 657–677. [Google Scholar] [CrossRef]
- Blüthgen, N. Why network analysis is often disconnected from community ecology: A critique and an ecologist’s guide. Basic Appl. Ecol. 2010, 11, 185–195. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 1–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blüthgen, N.; Fründ, J.; Vázquez, D.; Menzel, F. What do interaction Network metrics tell us about specialization and biological traits? Ecology 2008, 89, 3387–3399. [Google Scholar]
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Guimarães, P.R.; Guimarães, P. Improving the analyses of nestedness for large sets of matrices. Environ. Model. Softw. 2006, 21, 1512–1513. [Google Scholar] [CrossRef]
- Bortoli, C.; Laroca, S. Melissocenologia no Terceiro Planalto Paranaense. I: Abundância relativa das abelhas silvestres (Apoidea) de um biótopo urbano de Guarapuava (PR, Brasil). Acta Biol. Parana. 1997, 26, 51–86. [Google Scholar] [CrossRef]
- Gonçalves, R.B.; Melo, G.A.R. A comunidade de abelhas (Hymenoptera, Apidae) em uma área restrita de campo natural no Parque Estadual de Vila Velha, Paraná: Diversidade, fenologia e fontes florais de alimento. Rev. Bras. Entomol. 2005, 49, 557–571. [Google Scholar] [CrossRef]
- Gonçalves, R.B.; Melo, G.A.R. A assembléia de abelhas (Hymenoptera, Apidae) de uma área restrita de campos naturais do Parque Estadual de Vila Velha, Paraná e comparações com áreas de campos e cerrado. Pap. Avulsos Zool. 2009, 49, 163–181. [Google Scholar]
- Freitas, L.; Sazima, M. Floral biology and pollination mechanisms in two Viola species-from nectar to pollen flowers? Ann. Bot. 2003, 91, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L. Biologia da polinização em campos de altitude no Parque Nacional da Serra da Bocaina, SP. Ph.D. Thesis, Universidade Estadual de Campinas, São Paulo, Brazil, 2002. [Google Scholar]
- Aguiar, C.M.L. Utilização de recursos florais por abelhas (Hymenoptera, Apoidea) em uma área de Caatinga (Itatim, Bahia, Brasil). Rev. Bras. Zool. 2003, 20, 457–467. [Google Scholar] [CrossRef]
- Zillikens, A.; Steiner, S.; Mihalkó, Z. Nests of Augochlora (A.) esox in Bromeliads, a Previously Unknown site for Sweat Bees (Hymenoptera: Halictidae). Stud. Neotrop. Fauna Environ. 2001, 36, 137–142. [Google Scholar] [CrossRef]
- Moure, J.S.; Hurd, P.D. An Annotated Catalog of the Halictid Bees of the Western Hemisphere (Hymenoptera: Halictidae); Smithsonian Institution: Washington, DC, USA, 1987. [Google Scholar]
- Dalmazzo, M.; Vossler, F.G. Assessment of the pollen diet in a wood-dwelling augochlorine bee (Halictidae) using different approaches. Apidologie 2015, 46, 478–488. [Google Scholar] [CrossRef]
- Hesse, M. An exine architeture model for viscin threads. Grana 1984, 23, 69–75. [Google Scholar] [CrossRef]
- Cruden, R.W. Pollen-ovule ratio, pollen size and the ratio of stigmatic area to the pollen-bearing area of the pollinator: An hypothesis. Evolution 1981, 35, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, M. Pollinating bees and other visitors of Ludwigia elegans (Onagraceae) flowers at a tropical site in Brazil. Stud. Neotrop. Fauna Environ. 1997, 32, 81–88. [Google Scholar] [CrossRef]
- Schlindwein, C. Are oligolectic bees always the most effective pollinators? In Solitary Bees. Conservation, Rearing and Management for Pollination; Imprensa Universitária: Fortaleza, CE, Brazil, 2004; pp. 231–240. [Google Scholar]
- Michener, C.D. Biogeography of the bees. Ann. Mo. Bot. Gard. 1979, 66, 277–347. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World; The Johns Hopkins University Press: Baltimore, MD, USA, 2000. [Google Scholar]
- Gimenes, M.; Benedito-Silva, A.A.; Marques, M.D. Chronobiologic aspects of a coadaptive process: The interaction of Ludwigia elegans flowers and its more frequent bee visitors. Chronobiol. Int. 1993, 10, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, M.; Benedito-Silva, A.A.; Marques, M.D. Circadian rhythms of pollen and nectar collection by bees on the flowers of Ludwigia elegans. Biol. Rhythm Res. 1996, 27, 281–290. [Google Scholar] [CrossRef]
- Gimenes, M. Interaction between visiting bees (Hymenoptera, Apoidea) and flowers of Ludwigia elegans (Camb.) Hara (Onagraceae) during the year in two different areas in São Paulo, Brazil. Braz. J. Biol. 2003, 63, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Buschini, M.L.T.; Rigon, J.; Cordeiro, J. Plants used by Megachile (Moureapis) sp. (Hymenoptera: Megachilidae) in the provisioning of their nests. Braz. J. Biol. 2009, 69, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.; Buschini, M.L.T. Bees diversity and their interaction networks with Ludwigia sericea (Cambessides) H. Hara and Ludwigia peruviana (L.) H. Hara (Onagraceae) flowers in Atlantic Forest Area in Southern Brazil. Sociobiology 2017, 64, 57–68. [Google Scholar] [CrossRef]
- Aguiar, C.M.L.; Garófalo, A.G. Nesting biology of Centris (Hemisiella) tarsata Smith (Hymenoptera, Apidae, Centridini). Rev. Bras. Zool. 2004, 21, 477–486. [Google Scholar] [CrossRef]
- Buschini, M.L.T. Species diversity and community structure in trap-nesting bees in Southern Brazil. Apidologie 2006, 37, 58–66. [Google Scholar] [CrossRef]
- Buschini, M.L.T.; Wolff, L.L. Nesting biology of Centris (Hemisiella) tarsata Smith in SouthernBrazil (Hymenoptera, Apidae, Centridini). Braz. J. Biol. 2006, 66, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.; Silva, C.I.; Buschini, M.L.T. Collection of Pollen Grains by Centris (Hemisiella) tarsata Smith (Apidae: Centridini): Is C. tarsata an Oligolectic or Polylectic Species? Zool. Stud. 2012, 51, 195–203. [Google Scholar]
- Diniz, M.E.R.; Buschini, M.L.T. Pollen analysis and interaction networks of floral visitor bees of Eugenia uniflora L. (Myrtaceae), in Atlantic Forest areas in southern Brazil. Arthropod Plant Interact. 2015, 9, 623–632. [Google Scholar] [CrossRef]
- Diniz, M.E.R.; Buschini, M.L.T. Diversity of flower visiting bees of Eugenia uniflora L. (Myrtaceae) in fragments of Atlantic Forest in South Brazil. Sociobiology 2016, 63, 982–990. [Google Scholar] [CrossRef]
- Antonini, Y.; Martins, R.P. The Flowering-Visiting Bees at the Ecological Station of the Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil. Neotrop. Entomol. 2003, 32, 565–575. [Google Scholar] [CrossRef]
- Imperatriz-Fonseca, V.L.; Santos, I.A.; Santos-Filho, P.S.; Engels, W.; Ramalho, M.; Wilms, W.; Aguilar, J.B.; Pinheiro-Machado, C.A.; Alves, D.A.; Kleinert, A.M.P. Checklist das abelhas e plantas melitófilas no Estado de São Paulo, Brasil. Biota Neotrop. 2011, 11, 632–655. [Google Scholar] [CrossRef]
- Santos, G.M.; Aguiar, C.M.L.; Mello, M.A.R. Flower-visiting guild associated with the Caatinga flora: Trophic interaction networks formed by social bees and social wasps with plants. Apidologie 2010, 41, 466–475. [Google Scholar] [CrossRef]
- Santos, K.C.B.S.; Gonçalvez, A.B.; Cereda, M.P. Polens importantes na flora apícola em uma região de Cerrado em Campo Grande-MS. Rev. Biol. Neotrop. 2015, 12, 81–85. [Google Scholar] [CrossRef]
- Faria-Mucci, G.M.; Melo, M.A.; Campos, L.A.O. A fauna de abelhas (Hymenoptera, Apoidea) e plantas utilizadas como fonte de recursos florais, em um ecossistema de campos rupestris em Lavras Novas, Minas Gerais, Brasil. In Apoidea Neotropica: Homenagem aos 90 Anos de Jesus Santiago Moure; Editora UNESC: Criciúma, SC, Brazil, 2003; pp. 241–256. [Google Scholar]
- Sakagami, S.F.; Laroca, S.; Moure, J.S. Wild bees biocenotics in São José dos Pinhais (PR), South Brazil. Preliminary report. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1967, 16, 253–291. [Google Scholar]
- Barros, M.A.G. Sistemas reprodutivos e polinizac: Ao em especies simpatricas de Erythroxylum P. Br. (Erythroxylaceae) do Brasil. Rev. Bras. Bot. 1998, 21, 159–166. [Google Scholar] [CrossRef]
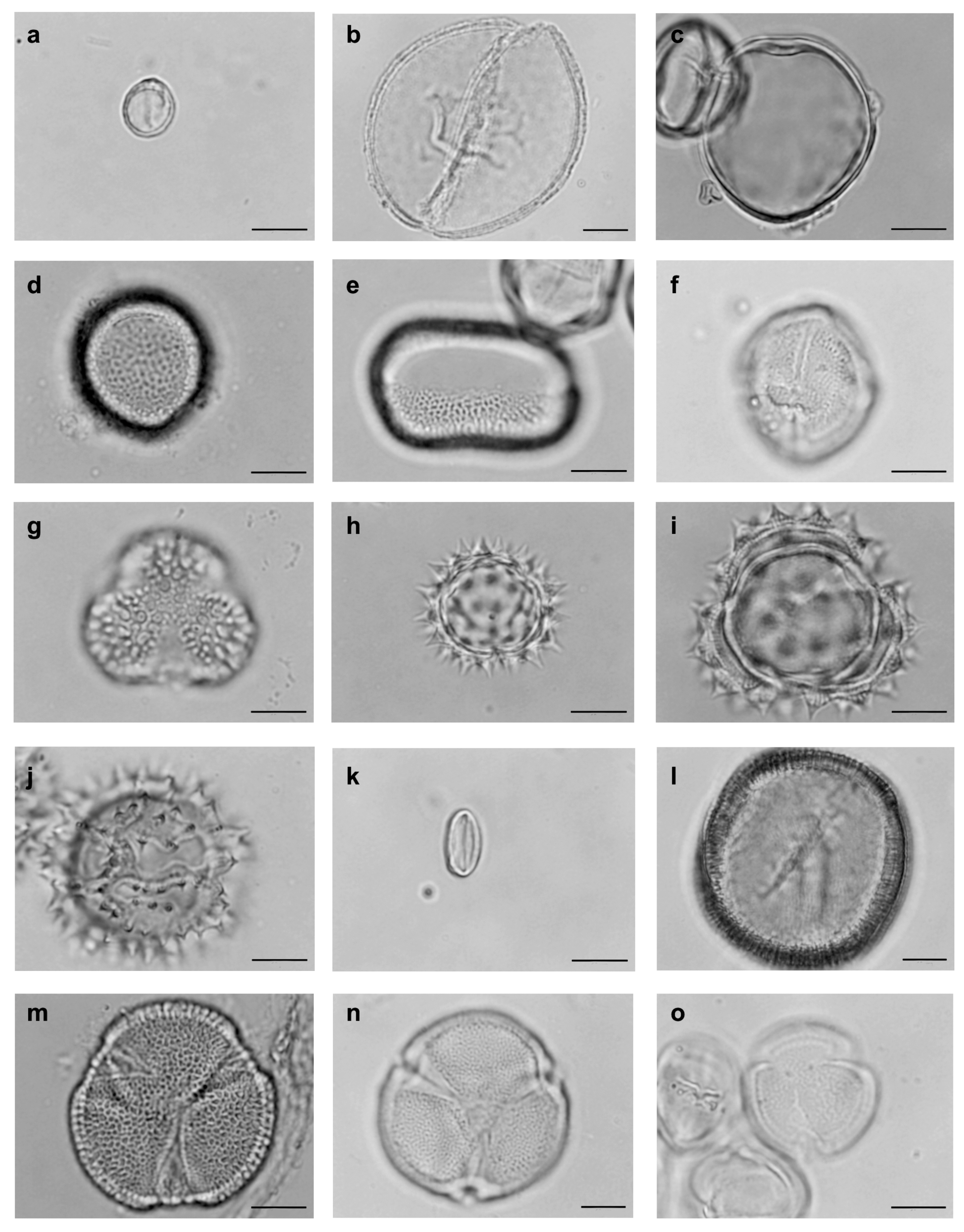
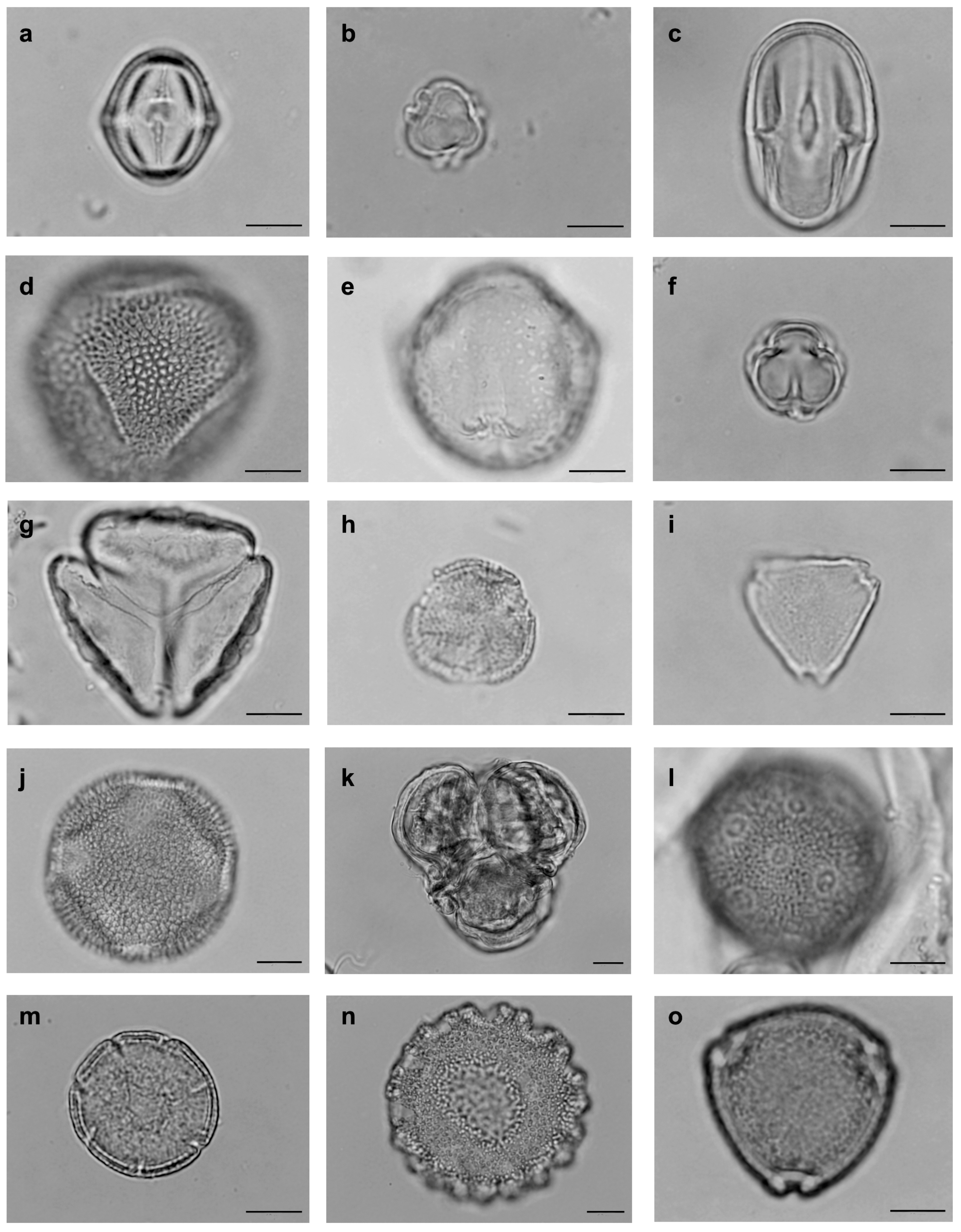
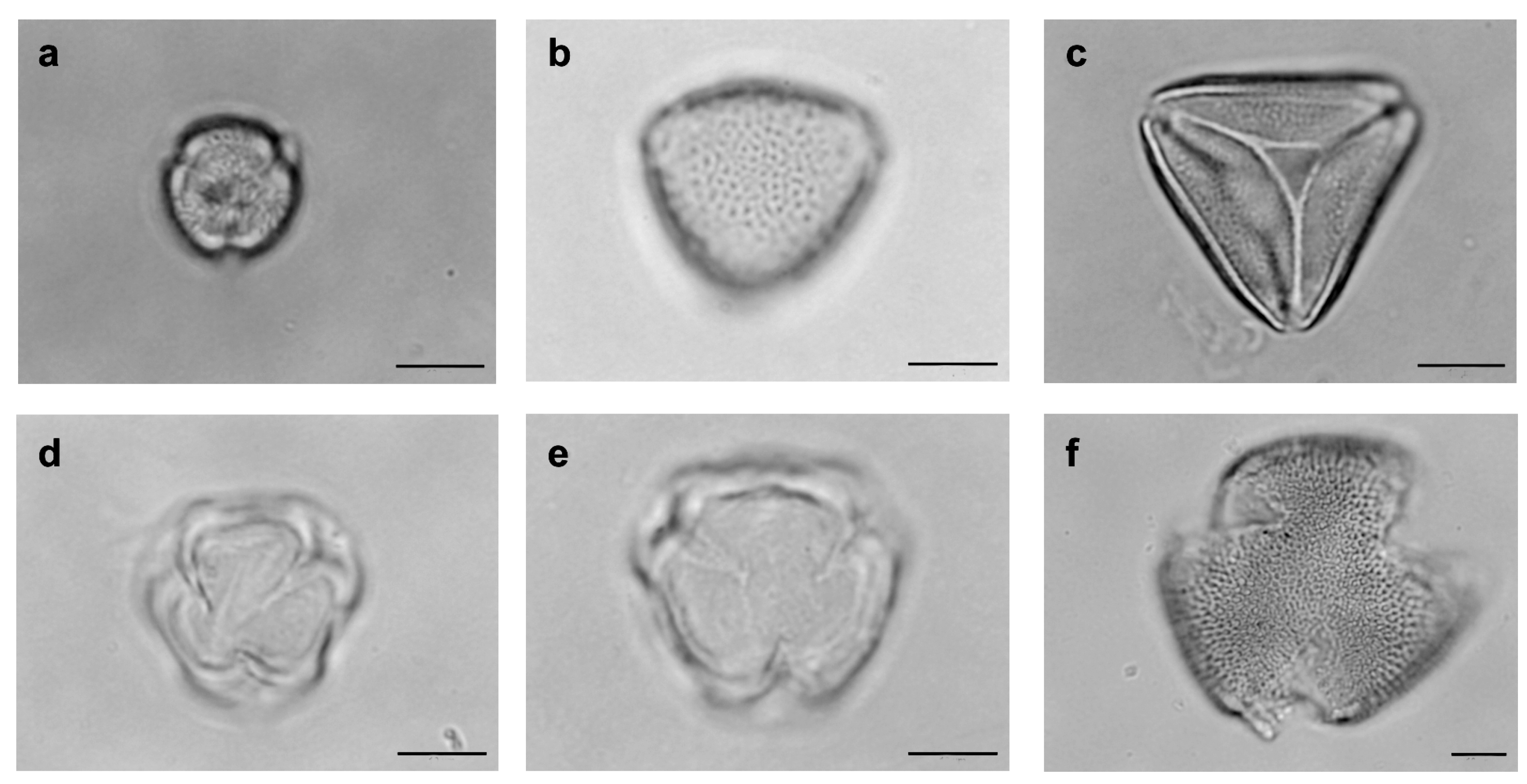
| Family | Tribe | Species | N | D% | FO% | Classification |
|---|---|---|---|---|---|---|
| Andrenidae | Protandrenini | Anthrenoides sp. 1 | 67 | 0.134 | 0.469 | Intermediate |
| Protandrenini | Anthrenoides sp. 2 | 16 | 0.032 | 0.063 | Intermediate | |
| Protandrenini | Anthrenoides sp. 3 | 2 | 0.004 | 0.063 | Rare | |
| Protandrenini | Anthrenoides sp. 4 | 16 | 0.032 | 0.25 | Rare | |
| Protandrenini | Chaeturginus sp. | 1 | 0.002 | 0.031 | Rare | |
| Apidae | Apini | Apis mellifera Linnaeus, 1758 | 1 | 0.002 | 0.031 | Rare |
| Xylocopini | Ceratina (Crewella) sp. | 1 | 0.002 | 0.031 | Rare | |
| Exomalopsini | Exomalopsis (Phanomalopsis) aureosericea Friese, 1899 | 1 | 0.002 | 0.031 | Rare | |
| Exomalopsini | Exomalopsis (Diomalopsis) bicellularis Michener and Moure, 1957 | 2 | 0.004 | 0.063 | Rare | |
| Exomalopsini | Exomalopsis perikalles Silveira e Almeida. 2009 | 1 | 0.002 | 0.031 | Rare | |
| Exomalopsini | Exomalopsis (Exomalopsis) vernoniae Schrottky. 1909 | 2 | 0.004 | 0.031 | Rare | |
| Meliponini | Plebeia emerina (Friese, 1900) | 1 | 0.002 | 0.031 | Rare | |
| Eucerini | Thygater sp. | 1 | 0.002 | 0.031 | Rare | |
| Xylocopini | - | 2 | 0.004 | 0.063 | Rare | |
| Colletidae | Colletes argentinus Friese. 190 | 1 | 0.002 | 0.031 | Rare | |
| Halictidae | Augochlorini | Augochlorella sp. 1 | 1 | 0.002 | 0.031 | Rare |
| Augochlorini | Augochlorella sp. 2 | 2 | 0.004 | 0.063 | Rare | |
| Augochlorini | Augochloropsis cfr sparsilis Vachal. 1903 | 13 | 0.026 | 0.219 | Rare | |
| Augochlorini | Augochloropsis chloera (Moure. 1940) | 3 | 0.006 | 0.063 | Rare | |
| Augochlorini | Augochloropsis notophos Vachal. 1903 | 171 | 0.342 | 0.656 | Common | |
| Augochlorini | Augochloropsis sp. A | 29 | 0.058 | 0.469 | Intermediate | |
| Augochlorini | Augochloropsis sp. B | 3 | 0.006 | 0.094 | Rare | |
| Augochlorini | Augochloropsis sp. C | 4 | 0.008 | 0.125 | Rare | |
| Augochlorini | Augochlora sp. 1 | 25 | 0.05 | 0.469 | Intermediate | |
| Augochlorini | Augochlora sp. 2 | 101 | 0.202 | 0.594 | Common | |
| Augochlorini | Ceratalictus sp. | 25 | 0..050 | 0.281 | Intermediate | |
| Augochlorini | Halictillus sp. | 1 | 0.002 | 0.031 | Rare | |
| - | Indet.1 | 1 | 0.002 | 0.031 | Rare | |
| Augochlorini | Pseudaugochlora cfr graminea Fabricius. 1804 | 3 | 0.006 | 0.094 | Rare | |
| Augochlorini | Pseudaugochlora simulata Almeida. 2008 | 1 | 0.002 | 0.031 | Rare | |
| Megachilidae | - | Indet.2 | 1 | 0.002 | 0.031 | Rare |
| Megachilini | Megachile sp. | 1 | 0.002 | 0.031 | Rare |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lando, F.; Lustosa, P.R.; P. da Luz, C.F.; Buschini, M.L.T. Bee Diversity and Solanum didymum (Solanaceae) Flower–Visitor Network in an Atlantic Forest Fragment in Southern Brazil. Diversity 2018, 10, 3. https://doi.org/10.3390/d10010003
Lando F, Lustosa PR, P. da Luz CF, Buschini MLT. Bee Diversity and Solanum didymum (Solanaceae) Flower–Visitor Network in an Atlantic Forest Fragment in Southern Brazil. Diversity. 2018; 10(1):3. https://doi.org/10.3390/d10010003
Chicago/Turabian StyleLando, Francieli, Priscila R. Lustosa, Cyntia F. P. da Luz, and Maria Luisa T. Buschini. 2018. "Bee Diversity and Solanum didymum (Solanaceae) Flower–Visitor Network in an Atlantic Forest Fragment in Southern Brazil" Diversity 10, no. 1: 3. https://doi.org/10.3390/d10010003
APA StyleLando, F., Lustosa, P. R., P. da Luz, C. F., & Buschini, M. L. T. (2018). Bee Diversity and Solanum didymum (Solanaceae) Flower–Visitor Network in an Atlantic Forest Fragment in Southern Brazil. Diversity, 10(1), 3. https://doi.org/10.3390/d10010003





