(7aR*,7bR*)-7a,7b-Dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene
Abstract
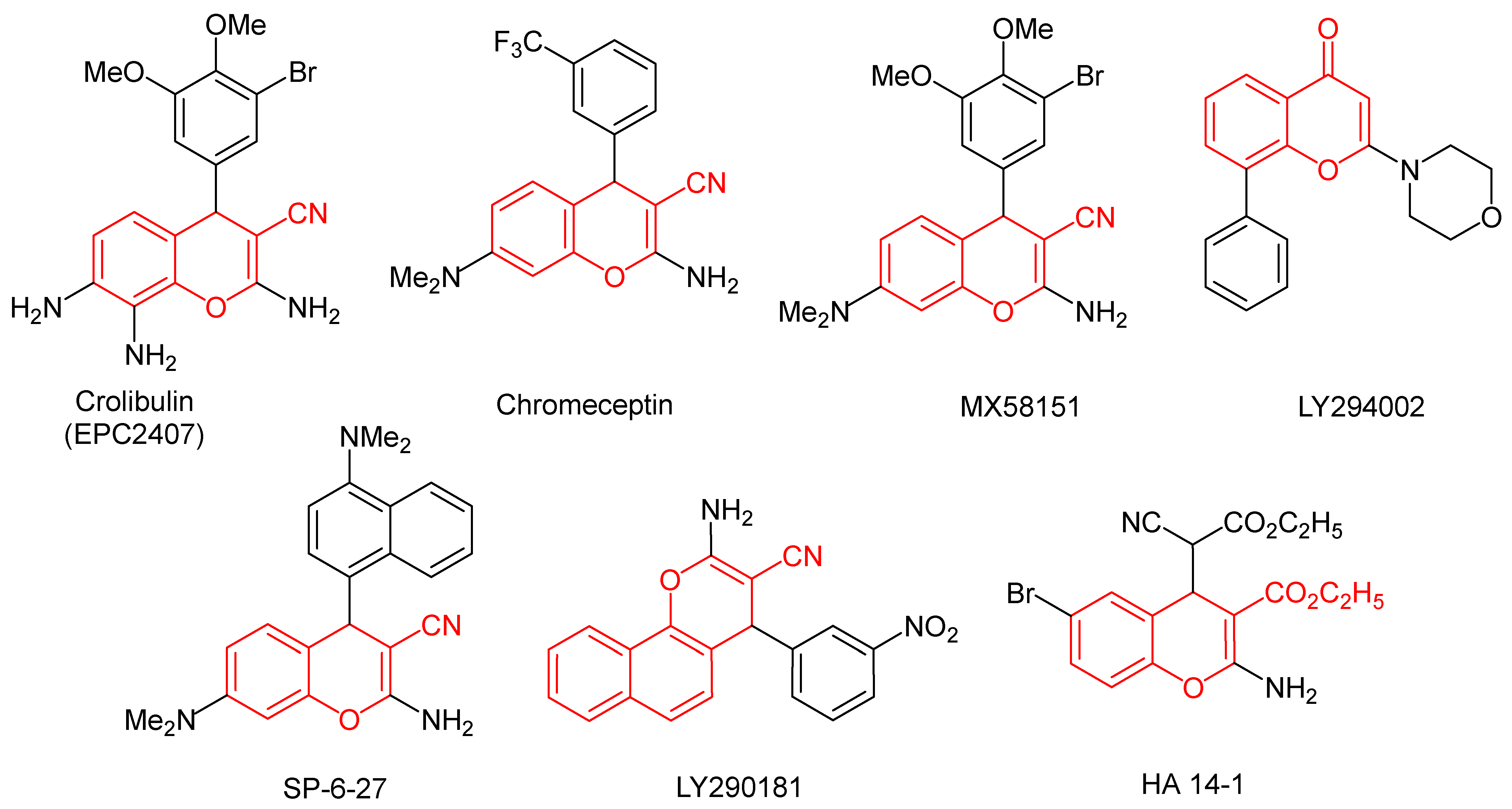
1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectroscopy
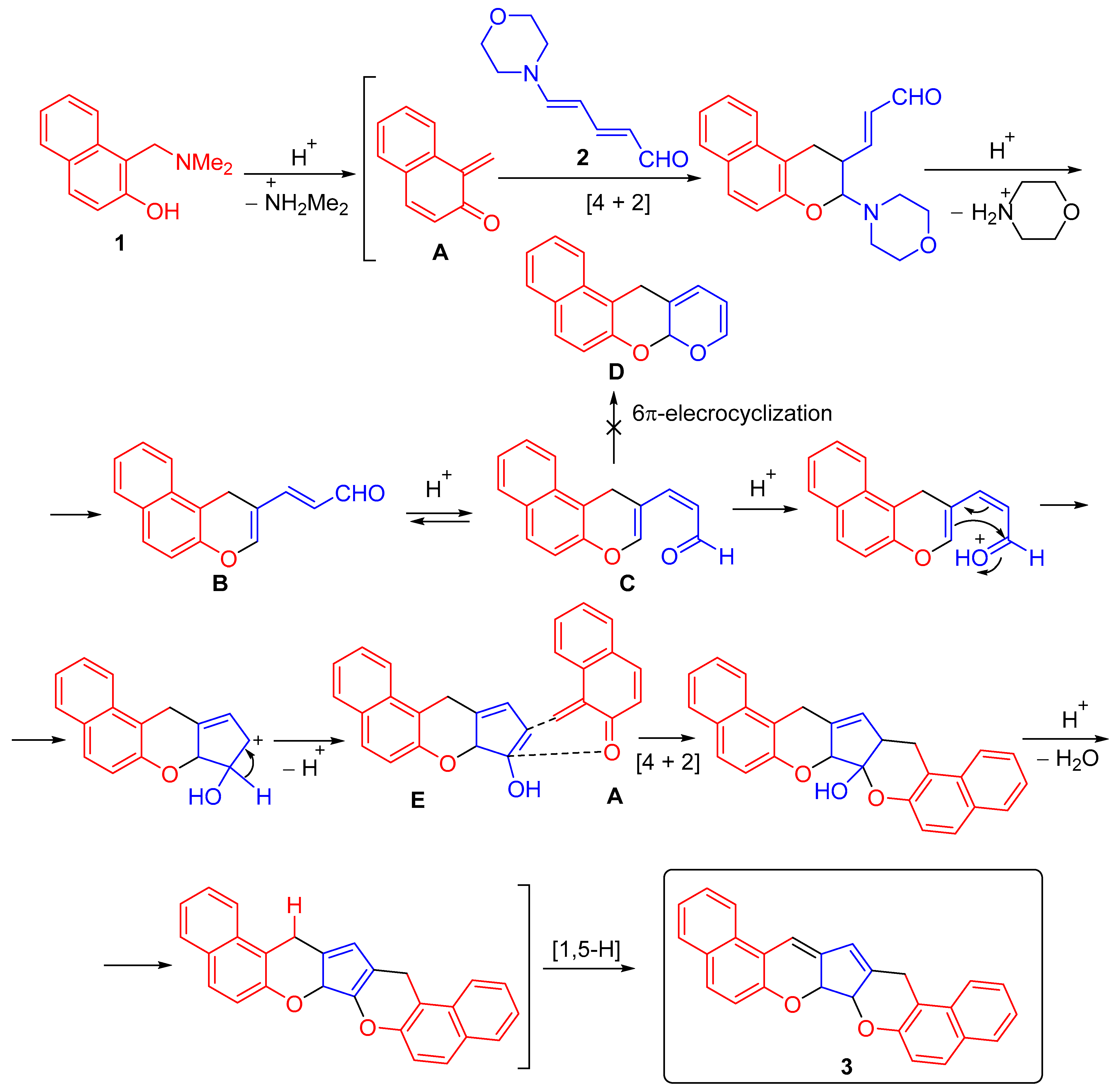
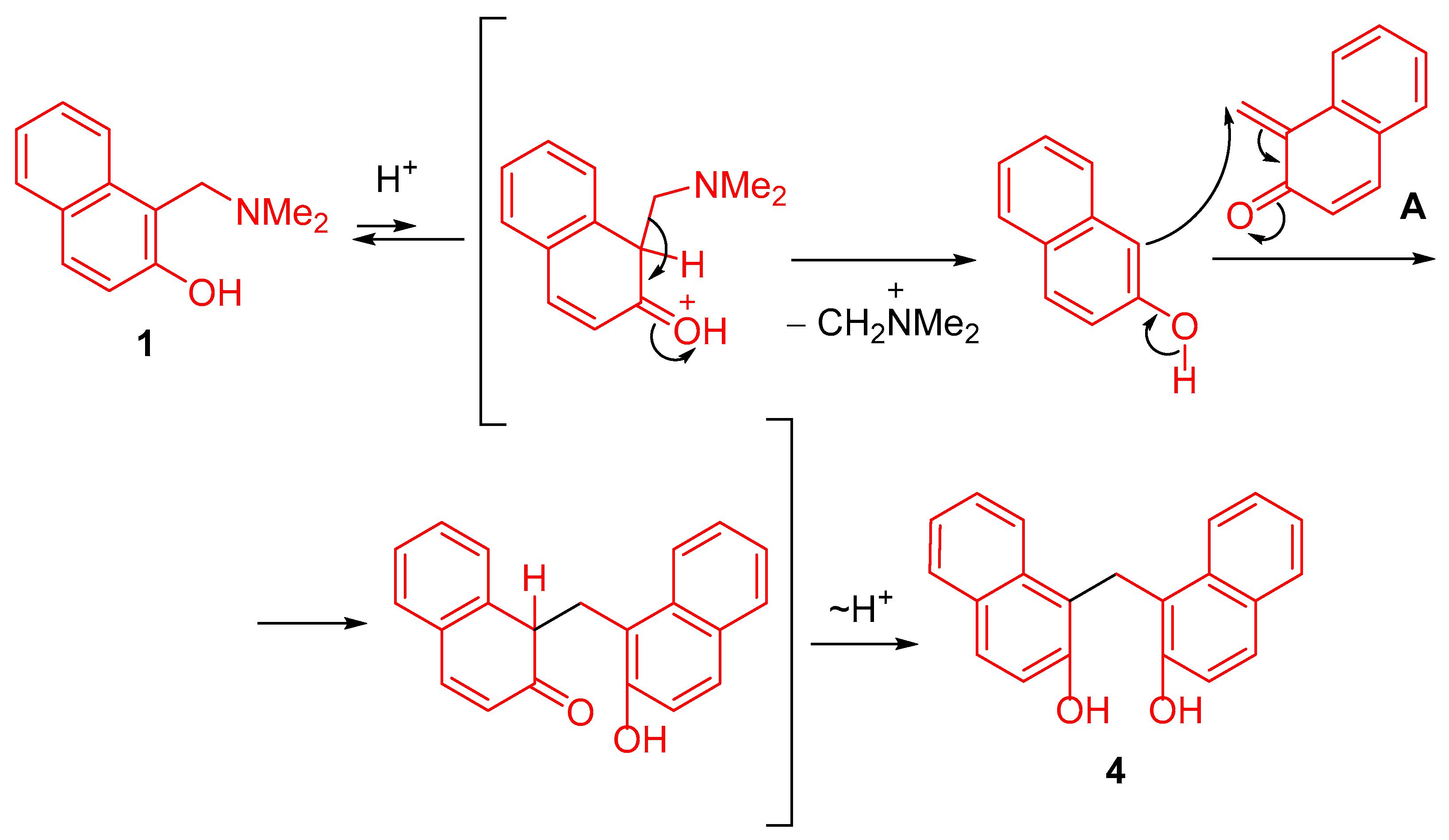
2.2. Proposed Mechanism
3. Materials and Methods
3.1. Synthesis and Characterization of (7aR*,7bR*)-7a,7b-Dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene (3)
3.2. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMR | Nuclear Magnetic Resonance |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| o-QM | ortho-Quinone Methide |
References
- Wen, Z.; Yang, K.-C.; Deng, J.-F.; Chen, L. Advancements in the Preparation of 4H-Chromenes: An Overview. Adv. Synth. Catal. 2023, 365, 1290–1331. [Google Scholar] [CrossRef]
- Chaudhary, A.; Singh, K.; Verma, N.; Kumar, S.; Kumar, D.; Sharma, P.P. Chromenes—A Novel Class of Heterocyclic Compounds: Recent Advancements and Future Directions. Mini Rev. Med. Chem. 2022, 22, 2736–2751. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Lee, J. 2H/4H-Chromenes—A Versatile Biologically Attractive Scaffold. Front. Chem. 2020, 8, 623. [Google Scholar] [CrossRef]
- Katiyar, M.K.; Dhakad, G.K.; Shivani; Arora, S.; Bhagat, S.; Arora, T.; Kumar, R. Synthetic strategies and pharmacological activities of chromene and its derivatives: An overview. J. Mol. Struct. 2022, 1263, 133012. [Google Scholar] [CrossRef]
- Sharon, K.N.; Padmaja, P.; Reddy, P.N. A Brief Review on the Synthesis of 4H-Chromene-Embedded Heterocycles. ChemistrySelect 2024, 9, e202400565. [Google Scholar] [CrossRef]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Osipov, D.V.; Osyanin, V.A.; Klimochkin, Y.N. Reactions of 5-formyl- and 5-acyl-3,4-dihydro-2H-pyrans and their annelated analogs with nucleophiles. Targets Heterocycl. Syst. 2019, 22, 436–467. [Google Scholar] [CrossRef]
- Lorza, A.M.A.; Ravi, H.; Philip, R.C.; Galons, J.-P.; Trouard, T.P.; Parra, N.A.; Von Hoff, D.D.; Read, W.L.; Tibes, R.; Korn, R.L.; et al. Dose–response assessment by quantitative MRI in a phase 1 clinical study of the anti-cancer vascular disrupting agent crolibulin. Sci. Rep. 2020, 10, 14449. [Google Scholar] [CrossRef]
- Choi, Y.; Shimogawa, H.; Murakami, K.; Ramdas, L.; Zhang, W.; Qin, J.; Uesugi, M. Chemical genetic identification of the IGF-linked pathway that is mediated by STAT6 and MFP2. Chem. Biol. 2006, 13, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Patil, R.; Pfeffer, L.M.; Miller, D.D. Chromenes: Potential new chemotherapeutic agents for cancer. Future Med. Chem. 2013, 5, 1647–1660. [Google Scholar] [CrossRef]
- Semba, S.; Itoh, N.; Ito, M.; Harada, M.; Yamakawa, M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer cells. Clin. Cancer Res. 2002, 8, 1957–1963. [Google Scholar]
- Kulshrestha, A.; Katara, G.K.; Ibrahim, S.A.; Patil, R.; Patil, S.A.; Beaman, K.D. Microtubule inhibitor, SP-6-27 inhibits angiogenesis and induces apoptosis in ovarian cancer cells. Oncotarget 2017, 8, 67017–67028. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Singh, J.P.; Wilson, L. Suppression of Microtubule Dynamics by LY290181: A potential mechanism for its antiproliferative action. J. Biol. Chem. 1997, 272, 7681–7687. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.M.; Tian, D.; Xing, C. Structure−Activity Relationship Studies of Ethyl 2-Amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (HA 14-1), an Antagonist for Antiapoptotic Bcl-2 Proteins To Overcome Drug Resistance in Cancer. J. Med. Chem. 2006, 49, 7731–7739. [Google Scholar] [CrossRef]
- Osyanin, V.A.; Lukashenko, A.V.; Osipov, D.V. Cycloaddition reactions of o-quinone methides with polarized olefins. Russ. Chem. Rev. 2021, 90, 324–373. [Google Scholar] [CrossRef]
- Lukashenko, A.V.; Osyanin, V.A.; Osipov, D.V.; Klimochkin, Y.N. Reaction of Push-Pull Enaminoketones and in Situ Generated ortho-Quinone Methides: Synthesis of 3-Acyl-4H-chromenes and 2-Acyl-1H-benzo[f]chromenes as Precursors for Hydroxybenzylated Heterocycles. J. Org. Chem. 2017, 82, 1517–1528. [Google Scholar] [CrossRef]
- Osyanin, V.A.; Osipov, D.V.; Semenova, I.A.; Korzhenko, K.S.; Lukashenko, A.V.; Demidov, O.P.; Klimochkin, Y.N. Eco-friendly synthesis of fused pyrano [2,3-b]pyrans via ammonium acetate-mediated formal oxa-[3 + 3] cycloaddition of 4H-chromene-3-carbaldehydes and cyclic 1,3-dicarbonyl compounds. RSC Adv. 2020, 10, 34344–34354. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.A.; Pergomet, J.L.; Gandon, V.; Riveira, M.J. Iodine-Catalyzed Iso-Nazarov Cyclization of Conjugated Dienals for the Synthesis of 2-Cyclopentenones. Org. Lett. 2018, 20, 7298–7303. [Google Scholar] [CrossRef]
- Riveira, M.J.; Marsili, L.A.; Mischne, M.P. The iso-Nazarov reaction. Org. Biomol. Chem. 2017, 15, 9255–9274. [Google Scholar] [CrossRef]
- Attanonchai, S.; Poonswat, K.; Ruchirawat, S.; Ploypradith, P. Indenoindenes, Indenoindoles, and Indenobenzofurans via the Interrupted iso-Nazarov Reaction. Asian J. Org. Chem. 2025, 14, e202400655. [Google Scholar] [CrossRef]
- La-Venia, A.; Passaglia, L.; Gurgone, L.; Gandon, V.; Riveira, M.J. Acid-Promoted Iso-Nazarov Cyclization of Conjugated trans-Dienones and Dienals for the Synthesis of 2-Cyclopentenones. J. Org. Chem. 2022, 87, 13469–13479. [Google Scholar] [CrossRef]
- Marques, A.-S.; Marrot, J.; Chataigner, I.; Coeffard, V.; Vincent, G.; Moreau, X. In Situ Generation of Cyclopentadienol Intermediates from 2,4-Dienals. Application to the Synthesis of Spirooxindoles via a Domino Polycyclization. Org. Lett. 2018, 20, 792–795. [Google Scholar] [CrossRef]
- Finsen, L.; Becher, J.; Buchardt, O.; Koganty, R.R. Derivatives and Reactions of Glutaconaldehyde. XI. N-Substituted 5-Amino-2,4-Pentadienenals, their Oximes, and 5-Amino-2,4-Pentadienenitriles. Structural Analysis by 1H and 13C NMR Spectroscopy. Acta Chem. Scand. B 1980, 34, 513–518. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.38.41; Rigaku Oxford Diffraction: Abingdon, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipov, D.V.; Demidov, O.P.; Osyanin, V.A. (7aR*,7bR*)-7a,7b-Dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene. Molbank 2025, 2025, M2096. https://doi.org/10.3390/M2096
Osipov DV, Demidov OP, Osyanin VA. (7aR*,7bR*)-7a,7b-Dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene. Molbank. 2025; 2025(4):M2096. https://doi.org/10.3390/M2096
Chicago/Turabian StyleOsipov, Dmitry V., Oleg P. Demidov, and Vitaly A. Osyanin. 2025. "(7aR*,7bR*)-7a,7b-Dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene" Molbank 2025, no. 4: M2096. https://doi.org/10.3390/M2096
APA StyleOsipov, D. V., Demidov, O. P., & Osyanin, V. A. (2025). (7aR*,7bR*)-7a,7b-Dihydro-15H-dibenzo[f,f′]cyclopenta[1,2-b:5,4-b′]dichromene. Molbank, 2025(4), M2096. https://doi.org/10.3390/M2096





