2,5-Dimethylbenzyl 2-{(4,6-diaminopyrimidin-2-yl)thio}acetate
Abstract
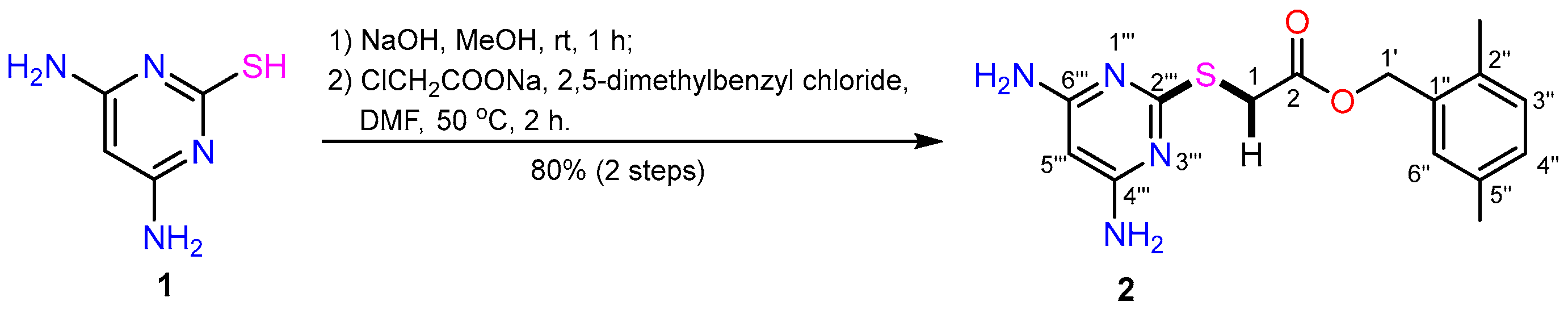
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Instrumentation
3.2. 2-{(4,6-Diaminopyrimidin-2-yl)thio}acetate (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natarajan, R.; Anthoni Samy, H.N.; Sivaperuman, A.; Subramani, A. Structure–Activity Relationships of Pyrimidine Derivatives and Their Biological Activity—A Review. Med. Chem. 2023, 19, 10–30. [Google Scholar]
- Sochacka, E.; Frątczak, I. Efficient Desulfurization of 2-Thiopyrimidine Nucleosides to the Corresponding 4-Pyrimidinone Analogues Using trans-2-(Phenylsulfonyl)-3-Phenyloxaziridine. Tetrahedron Lett. 2004, 45, 6729–6731. [Google Scholar] [CrossRef]
- Radwan, M.A.A.; Abbas, E.M.H. Synthesis of Some Pyridine, Thiopyrimidine, and Isoxazoline Derivatives Based on the Pyrrole Moiety. Monatsh. Chem. 2009, 140, 229–233. [Google Scholar] [CrossRef]
- Nammalwar, B.; Bunce, R.A. Recent Advances in Pyrimidine-Based Drugs. Pharmaceuticals 2024, 17, 104. [Google Scholar] [CrossRef]
- Haffez, H.; Taha, H.; Rabie, M.A.; Awad, S.M.; Zohny, Y.M. Synthesis, Biological Evaluation and Molecular Docking Studies of Novel Thiopyrimidine Analogue as Apoptotic Agent with Potential Anticancer Activity. Bioorg. Chem. 2020, 104, 104249. [Google Scholar] [CrossRef] [PubMed]
- Helwa, A.A.; Ryad, N.M.; Youssef, A.; Omar, Y.M.; Attia, K.M.; El Etrawy, A.A.S. Pyrimidine Derivatives as Anticancer Agents. J. Pharm. Sci. Dev. Med. 2024, 1, 54–68. [Google Scholar] [CrossRef]
- Bhasin, D.; Chettiar, S.N.; Etter, J.P.; Mokale, S.N.; Cheng, X.; Hartman, T.L.; Mitsuya, H.; Rice, W.G. Synthesis and anti-HIV activity of 2-thiopyrimidine derivatives as novel non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 747–751. [Google Scholar]
- Abdelkhalek, A.S.; Attia, M.S.; Kamal, M.A. Triazolopyrimidine Derivatives: An Updated Review on Recent Advances in Synthesis, Biological Activities and Drug Delivery Aspects. Curr. Med. Chem. 2024, 31, 1896–1919. [Google Scholar] [CrossRef]
- Gais, H.J.; Jagusch, T.; Spalthoff, N.; Gerhards, F.; Frank, M.; Raabe, G. Highly Selective Palladium-Catalyzed Kinetic Resolution and Enantioselective Substitution of Racemic Allylic Carbonates with Sulfur Nucleophiles: Asymmetric Synthesis of Allylic Sulfides, Allylic Sulfones, and Allylic Alcohols. Chem. Eur. J. 2003, 9, 4202–4221. [Google Scholar] [CrossRef] [PubMed]
- El-Kalyoubi, S.; El-Sebaey, S.A.; Elfeky, S.M.; Al-Ghulikah, H.A.; El-Zoghbi, M.S. Novel aminopyrimidine-2,4-diones, 2-thiopyrimidine-4-ones, and 6-arylpteridines as dual-target inhibitors of BRD4/PLK1: Design, synthesis, cytotoxicity, and computational studies. Pharmaceuticals 2023, 16, 1303. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Ren, X.; Tan, Y.; Jin, L.; Zhou, X. Design, synthesis and bioactivity of novel pyrimidine sulfonate esters containing thioether moiety. Int. J. Mol. Sci. 2023, 24, 4691. [Google Scholar] [CrossRef] [PubMed]
- Hekel, A.J.; Awad, S.M.; Nosseir, O.; El-Shehry, M.F.; El-Rashedy, A.A.; Hammad, S.F. Synthesis and Antimicrobial Screening of Novel 2-Thiopyrimidine Derivatives Bearing Pyrazole Moiety. Egypt. J. Chem. 2025, 68, 645–652. [Google Scholar]
- Desai, S.; Sastry, G.; Chatrapati, K.S. Synthesis, Characterization, Antimicrobial and Antitubercular Activity of Some New Pyrimidine Derivatives. Indian J. Chem. 2023, 62, 11–15. [Google Scholar] [CrossRef]
- Bhalgat, C.M.; Ramesh, B. Synthesis, Antimicrobial Screening and Structure–Activity Relationship of Novel Pyrimidines and Their Thioethers. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 259–267. [Google Scholar] [CrossRef]
- Baluja, S.; Kacchadia, N.; Chanda, S. Thiopyrimidine Derivatives: Synthesis and Antibacterial Activity. Pharm. Chem. J. 2012, 46, 117–121. [Google Scholar] [CrossRef]
- Patrick-Armand, A.; Siomenan, C.; Doumade, Z.; Adéyolé, T.; Eric, B.; Daouda, T.; Ané, A. Synthesis and Antibacterial Activities of New 2-(Benzylthio)pyrimidines and 2-(Benzimidazolylmethylthio)pyrimidines Derivatives. Open J. Med. Chem. 2021, 11, 27–39. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, G.; Jia, S.; Wu, J.; Zhang, Y.; Tao, Y.; Huang, W.; Song, M.; Ding, K.; Ma, D.; et al. Design, synthesis and evaluation of thieno[3,2-d]pyrimidine derivatives as potent CDK7 inhibitors. Bioorg. Chem. 2024, 148, 107456. [Google Scholar] [CrossRef]
- Xie, W.; Yang, S.; Liang, L.; Wang, M.; Zuo, W.; Lei, Y.; Zhang, Y.; Tang, W.; Lu, T.; Chen, Y.; et al. Discovery of 2-Amino-7-sulfonyl-7H-pyrrolo[2,3-d]pyrimidine derivatives as potent reversible FGFR inhibitors with gatekeeper mutation tolerance. J. Med. Chem. 2022, 65, 16570–16588. [Google Scholar] [CrossRef]
- Salieva, G.; Uktamova, M.; Torikai, K.; Kholikov, T. 2-(Heptylthio)pyrimidine-4,6-diamine. Molbank 2025, 2025, M1965. [Google Scholar] [CrossRef]
- Barmaki, M.; Valiyeva, G.; Maharramov, A.A.; Allaverdiyev, M.M. Synthesis of 2,3-Dihydro-6-Methyl-2-Thiopyrimidin-4(1H)-One (6-Methylthiouracil) Derivatives and Their Reactions. J. Chem. 2013, 2013, 176213. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Goyal, R.N.; Lahoti, A.M.; Singh, N.; Shukla, R.; Raghubir, R. Synthesis and Biological Evaluation of 2-Thiopyrimidine Derivatives. Bioorg. Med. Chem. 2005, 13, 3185–3195. [Google Scholar] [CrossRef]
- Severina, H.I.; Skupa, O.O.; Khairulin, A.; Voloshchuk, N.I.; Georgiyants, V.A. Synthesis and Anticonvulsant Activity of 6-Methyl-2-Thioxo-2,3-Dihydropyrimidin-4(1H)-One Acetamides. J. Appl. Pharm. Sci. 2019, 9, 33–39. [Google Scholar]
- Salieva, G.; Kholikov, T.; Okmanov, R.Y.; Matchanov, A.; Khodjaniyazov, K.U.; Kadirova, S.; Torambetov, B. Synthesis, Crystal Structure and Hirshfeld Surface Analysis of 2-[(2,4-Dimethylbenzyl)sulfanyl]pyrimidine-4,6-diamine. Struct. Rep. 2025, 81, 4. [Google Scholar] [CrossRef]
- He, Z.X.; An, Q.; Wei, B.; Zhou, W.J.; Wei, B.F.; Gong, Y.P.; Zhao, W. Discovery of Potent and Selective 2-(Benzylthio)pyrimidine-Based DCN1-UBC12 Inhibitors for Anticardiac Fibrotic Effects. J. Med. Chem. 2021, 65, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Martis, G.J.; Mugali, P.S.; Gaonkar, S.L. Recent Progress in the Synthesis, Functionalization, and Biological Outlook of Pyrimidines. ACS Omega 2025, 10, 46248–46271. [Google Scholar] [CrossRef] [PubMed]
- Sroor, F.M.; Tohamy, W.M.; Zoheir, K.M.; Abdelazeem, N.M.; Mahrous, K.F.; Ibrahim, N.S. Design, Synthesis, In Vitro Anticancer, Molecular Docking and SAR Studies of New Series of Pyrrolo[2,3-d]pyrimidine Derivatives. BMC Chem. 2023, 17, 106. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salieva, G.; Uktamova, M.; Otajonov, S.; Torikai, K.; Kholikov, T. 2,5-Dimethylbenzyl 2-{(4,6-diaminopyrimidin-2-yl)thio}acetate. Molbank 2025, 2025, M2091. https://doi.org/10.3390/M2091
Salieva G, Uktamova M, Otajonov S, Torikai K, Kholikov T. 2,5-Dimethylbenzyl 2-{(4,6-diaminopyrimidin-2-yl)thio}acetate. Molbank. 2025; 2025(4):M2091. https://doi.org/10.3390/M2091
Chicago/Turabian StyleSalieva, Gulrukh, Malokhat Uktamova, Sardorbek Otajonov, Kohei Torikai, and Tursunali Kholikov. 2025. "2,5-Dimethylbenzyl 2-{(4,6-diaminopyrimidin-2-yl)thio}acetate" Molbank 2025, no. 4: M2091. https://doi.org/10.3390/M2091
APA StyleSalieva, G., Uktamova, M., Otajonov, S., Torikai, K., & Kholikov, T. (2025). 2,5-Dimethylbenzyl 2-{(4,6-diaminopyrimidin-2-yl)thio}acetate. Molbank, 2025(4), M2091. https://doi.org/10.3390/M2091





