Gas Phase Fragmentation of N,N-Ditosyl-2-aminodiphenylamine to Phenazine
Abstract
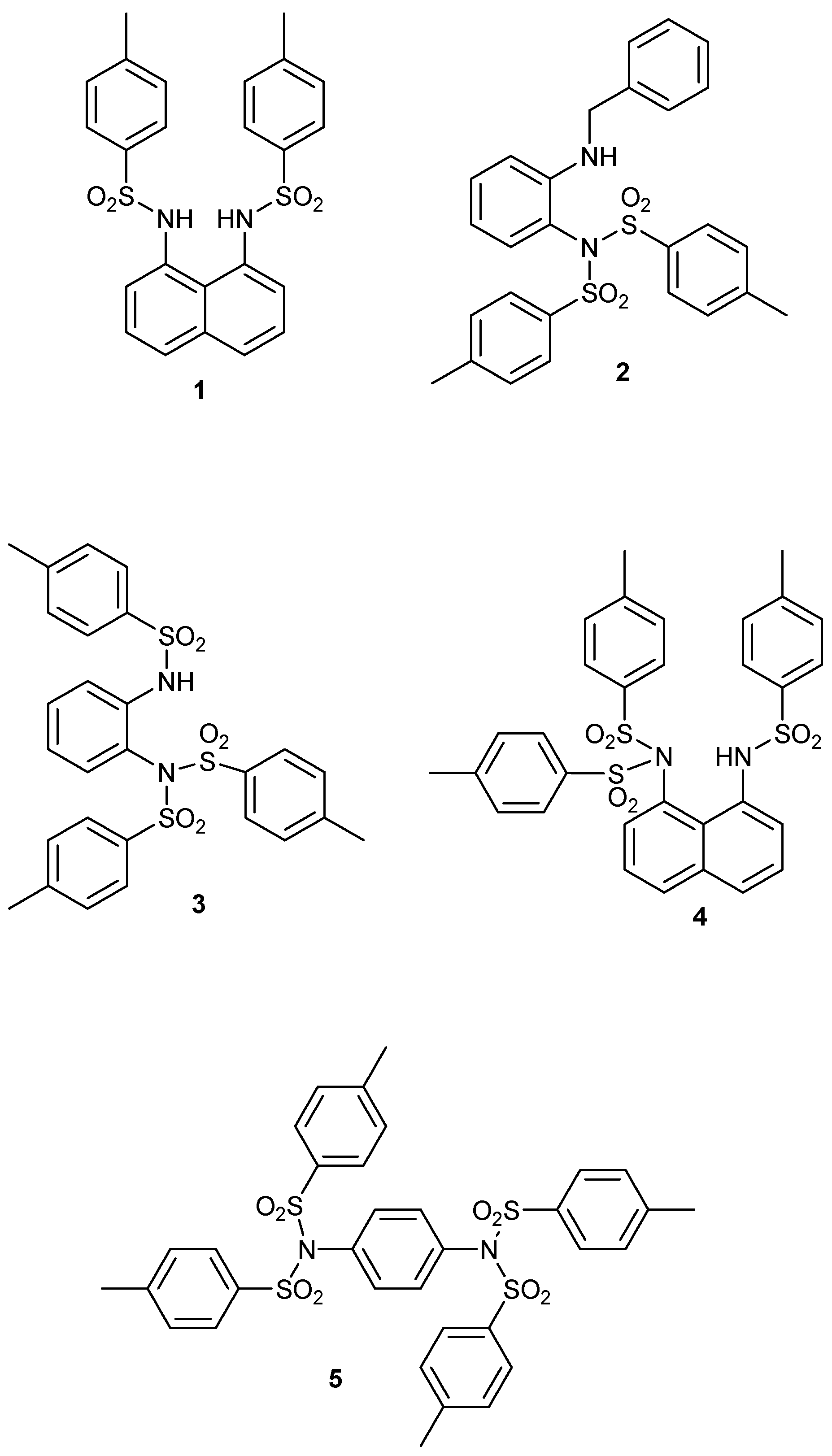
1. Introduction
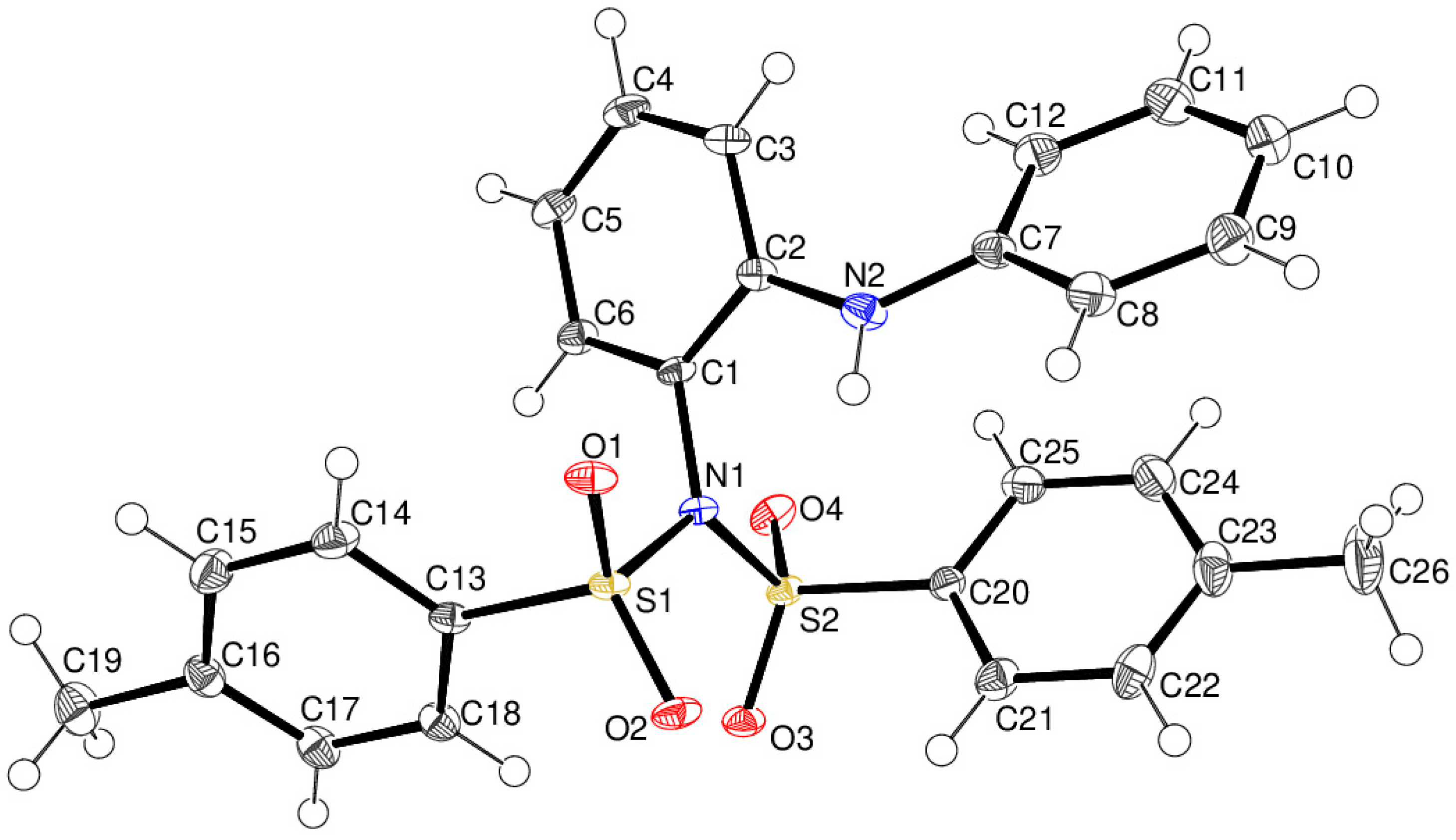
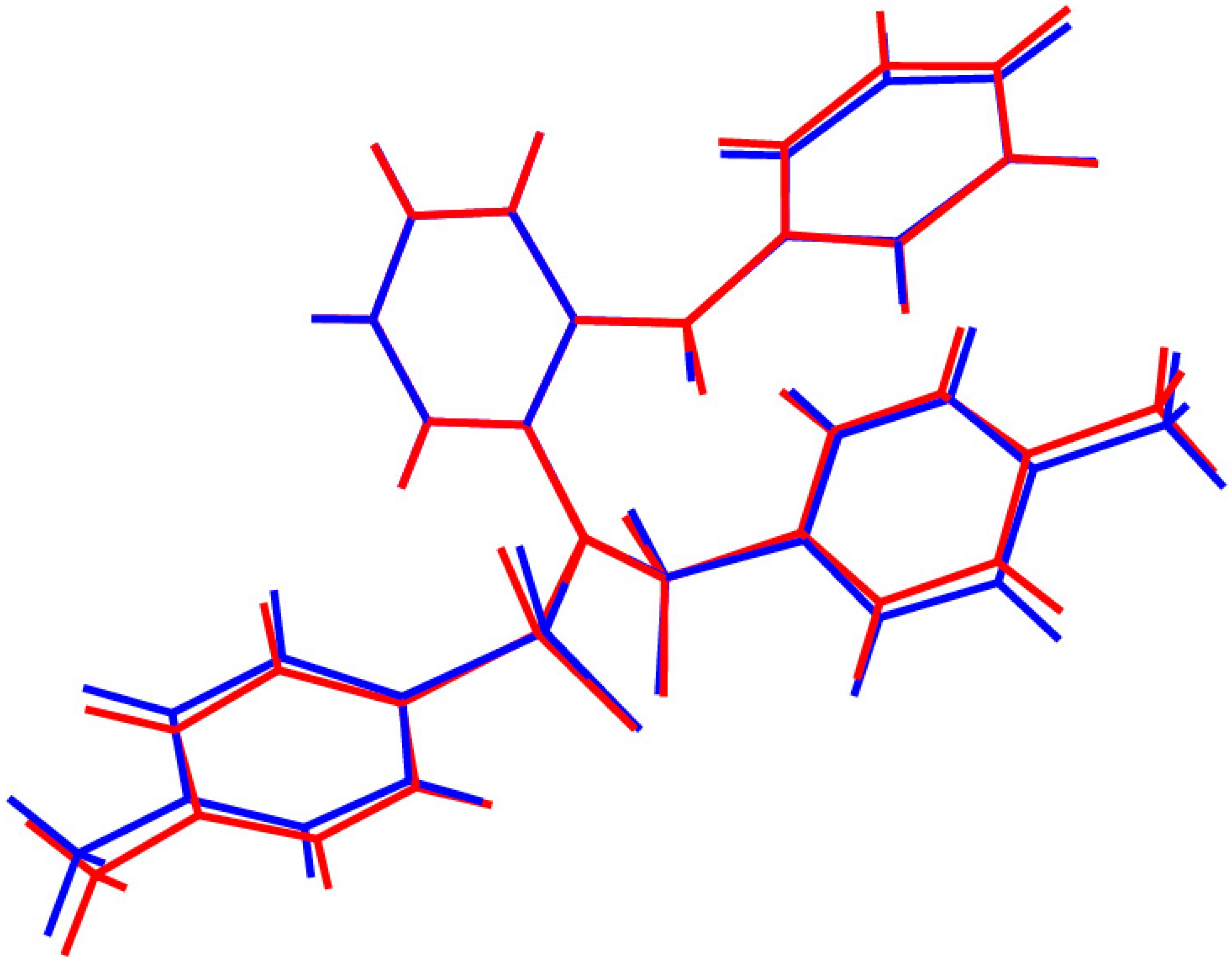
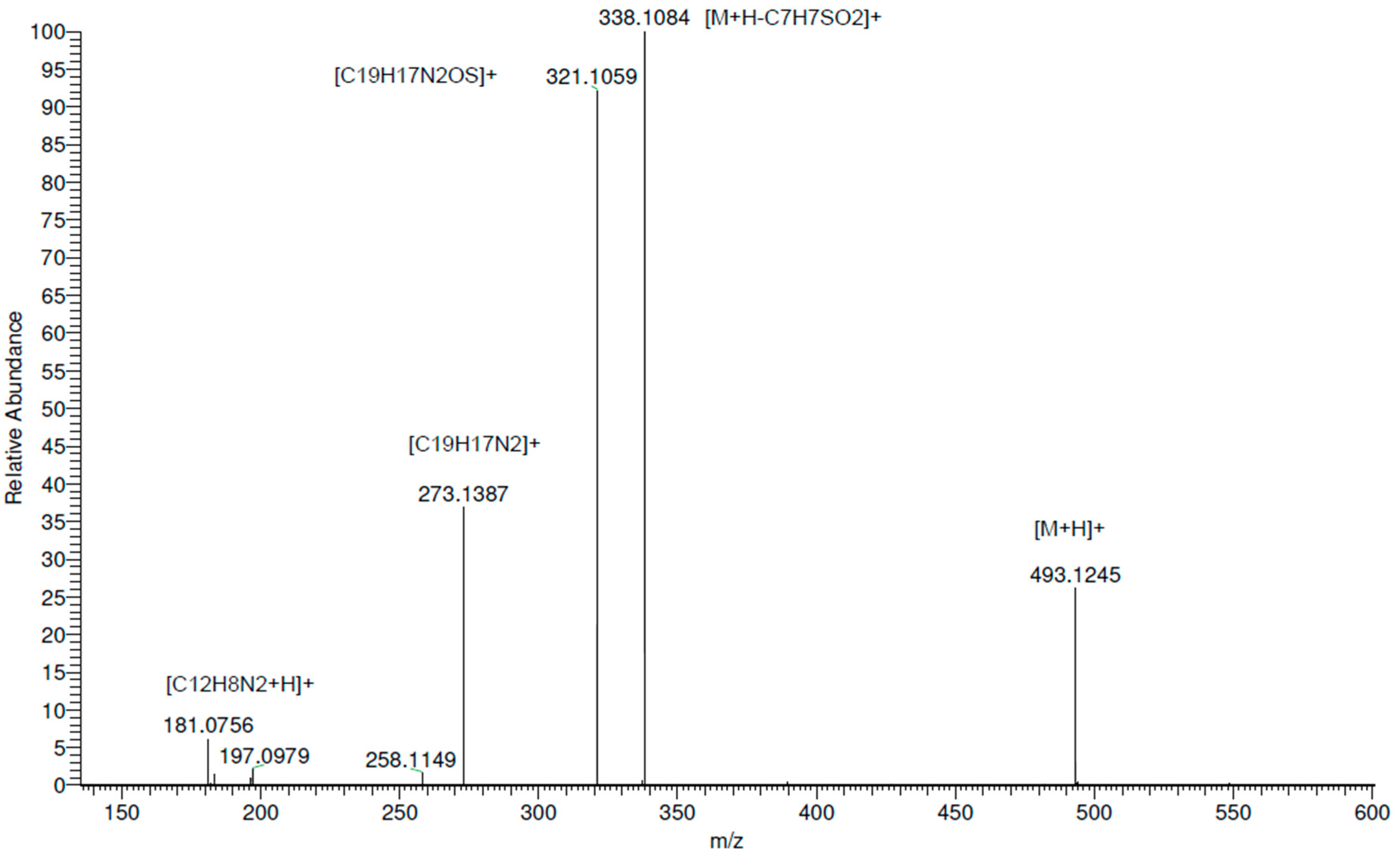
2. Discussion
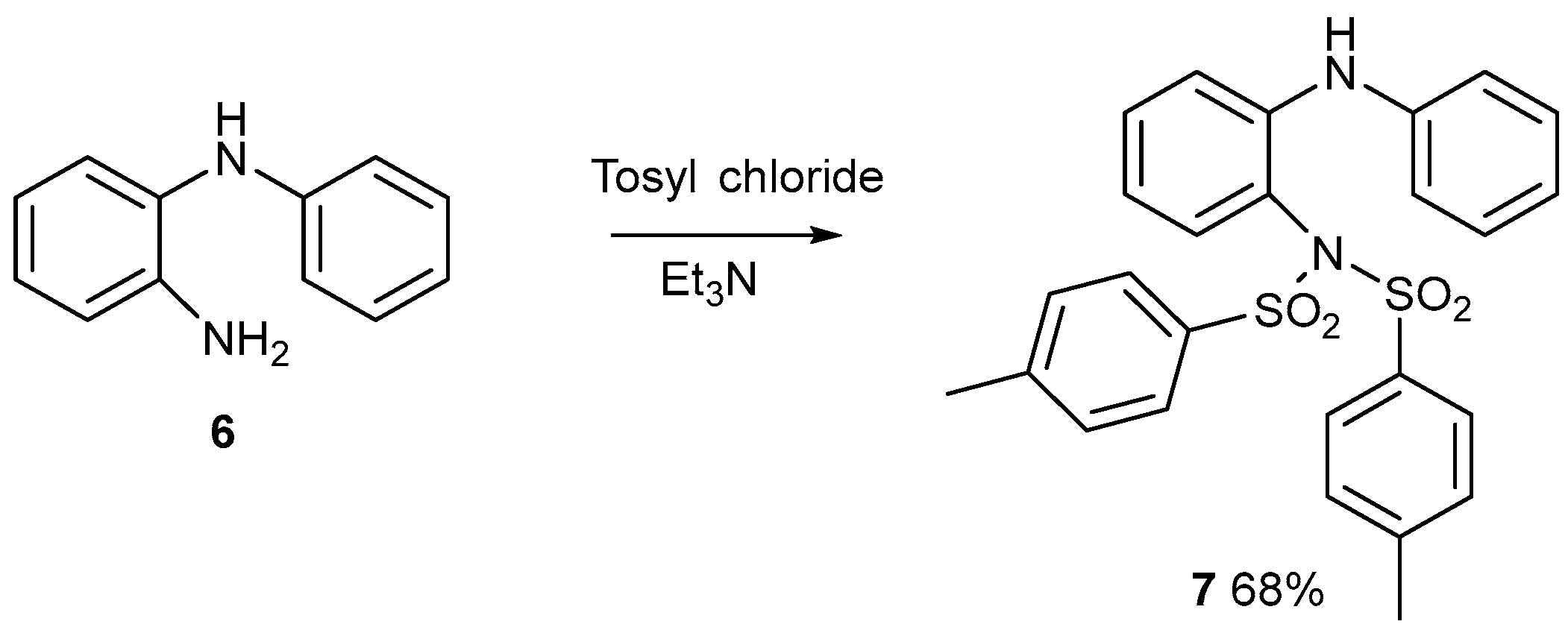
Gas-Phase Fragmentation of Compound 7
3. Conclusions
4. Experimental
4.1. General Procedure
N,N-Ditosyl-2-aminodiphenylamine
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plater, M.J.; Harrison, W.T.A. A study of mono-, di-, and tri-tosylated amines: An unexpected sulfonamide. J. Chem. Res. 2023, 47. [Google Scholar] [CrossRef]
- Oloyede, H.O.; Woods, J.A.O.; Gorls, H.; Plass, W.; Eseola, A.O. New cobalt(ii) coordination designs and the influence of varying chelate characters, ligand charges and incorporated group I metal ions on enzyme-like oxidative coupling activity. New J. Chem. 2020, 44, 14849–14858. [Google Scholar] [CrossRef]
- Bondarenko, N.A. Transition Metal Complexes Containing a Tosyl Group. Russ. J. Gen. Chem. 1996, 66, 584–589. [Google Scholar]
- Rivillo, D.; Gulyas, H.; Benet-Buchholz, J.; Escudero-Adan, E.C.; Zoraida, F.; van Leeuwen, P.W.N.M. Catalysis by design: Wide-bite-angle diphosphines by assembly of ditopic ligands for selective rhodium-catalyzed hydroformylation. Angew. Chem. Int. Ed. 2007, 46, 7247–7250. [Google Scholar] [CrossRef]
- Fu, S.; Jiang, H.; Deng, Y.; Zeng, W. Palladium-catalyzed intramolecular sulfonamidation/oxidation of imines: Access to multifunctional benzimidazoles. Adv. Synth. Catal. 2011, 353, 2795–2804. [Google Scholar] [CrossRef]
- Mertes, M.P.; Lin, A.J. Cofactor inhibition of thymidylate synthetase. Tetrahydrofolic acid analogs. J. Med. Chem. 1970, 13, 77–82. [Google Scholar] [CrossRef]
- Avdeenko, A.P. Synthesis and reactions of 3-arylsulfonylamido-N-arylsulfonyl-1,4-benzoquinonimines. Russ. J. Org. Chem. 1998, 34, 515–524. [Google Scholar]
- Saunders, A.; Sprake, J.M. Ring-substituted derivatives of 5,6,11,12-tetrahydrodibenzo[b,f][1,4]-diazocine. J. Chem. Soc. C. 1970, 1161–1165. [Google Scholar] [CrossRef]
- Squires, C.; Baxter, C.W.; Campbell, J.; Lindoy, L.F.; McNab, H.; Parkin, A.; Parsons, S.; Tasker, P.A.; Wei, G.; White, D.J. Design of base metal extractants. Part 1. Inter-ligand hydrogen bonding in the assembly of pseudo-macrocyclic bis(aminosulfonamidato)M(II) complexes. Dalton Trans. 2006, 2026–2034. [Google Scholar] [CrossRef]
- Plater, M.J.; Harrison, W.T.A. A potential iterative approach to 1,4-dihydro-N-heteroacene arrays. ChemistryOpen 2022, 11, e202100150. [Google Scholar] [CrossRef]
- Plater, M.J.; Harrison, W.T.A. Potential building blocks for 1,4-dihydro-N-heteroacenes. ChemistryOpen 2022, 11, e202200092. [Google Scholar] [CrossRef]
- Tasker, P.; Parkin, A.; Squires, C.; Parsons, S.; Messenger, D. CSD Communication; CCDC: Cambridge, UK, 2005; CCDC 278226. [Google Scholar]
- Basato, M.; Detomi, N.; Meneghetti, M.; Valle, G.; Veronese, A.C. Metal(II) complexes of the carbonylnitronate ligands O2NCHC(O)R− (R = Ph, OMe or OEt) and X-ray structure of (DMANH)[Ni(O2NCHC(O)Ph)3] (DMAN = 1,8-bis(dimethylamino)naphthalene). Inorg. Chim. Acta. 1999, 285, 18–24. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-J.; Yang, S.-J.; Lee, G.H.; Gong, Y.-D. Construction of drug like 2-amido benzo[d]imidazole analogues via desulfurative cyclization of thiourea intermediate resin on solid-phase. ACS Comb. Sci. 2018, 20, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Stetter, H. Über einen fall von sterischer hinderung bei der alkylierung von sulfonamiden aromatischer diamine. Chem. Ber. 1953, 86, 161–167. [Google Scholar] [CrossRef]
- Scherowsky, G. Deprotonierung von diaroyl-und ditosyl-benzimidazolium-salzen in gegenwart elektronen-reicher olefine. Liebigs Ann. Chem. 1970, 739, 45–55. [Google Scholar] [CrossRef]
- Hodgson, H.H.; Whitehurst, J.S. Preparation of 1:5- and 1:8-naphthylenediamine and related compounds. J. Chem. Soc. 1945, 54, 202–204. [Google Scholar] [CrossRef]
- Laha, J.K.; Jethava, K.P.; Dayal, N. Palladium-catalyzed intramolecular oxidative coupling involving double C(sp2)–H bonds for the synthesis of annulated biaryl sultams. J. Org. Chem. 2014, 79, 8010–8019. [Google Scholar] [CrossRef]
- Plater, M.J.; Praveen, M.; Stein, B.K.; Ballantine, J.A. Gas phase argon ion fragmentation of hexafluorotribenzotriphenylene. Tetrahedron Lett. 1996, 37, 7855–7856. [Google Scholar] [CrossRef]
- Eardley-Brunt, A.S.J.; Jones, A.; Mills, T.; Song, L.; Kotronias, R.; Lapolla, P.; Handa, A.; Lee, R.; Channon, K.; de Maria, G.L.; et al. Development of an optimised method for the analysis of human blood plasma samples by atmospheric solids analysis probe mass spectrometry. Int. J. Mass Spec. 2025, 508, 117386. [Google Scholar] [CrossRef]
- Yan, J.; Liu, W.; Cai, J.; Wang, Y.; Li, D.; Hua, H.; Cao, H. Advances in phenazines over the past decade: Review of their pharmacological activities, mechanisms of action, biosynthetic pathways and synthetic strategies. Mar. Drugs 2021, 19, 610. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, W.M.; Milne, B.F.; Wagner, M.; Schumacher, M.; Sandor, P.; Pathom-Aree, W.; Goodfellow, M.; Bull, A.T.; Horikoshi, K.; Ebel, R.; et al. Dermacozines, a new phenazine family from deep-sea dermacocci isolated from a Mariana Trench sediment. Org. Biomol. Chem. 2010, 8, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plater, M.J.; Harrison, W.T.A. Gas Phase Fragmentation of N,N-Ditosyl-2-aminodiphenylamine to Phenazine. Molbank 2025, 2025, M2069. https://doi.org/10.3390/M2069
Plater MJ, Harrison WTA. Gas Phase Fragmentation of N,N-Ditosyl-2-aminodiphenylamine to Phenazine. Molbank. 2025; 2025(4):M2069. https://doi.org/10.3390/M2069
Chicago/Turabian StylePlater, M. John, and William T. A. Harrison. 2025. "Gas Phase Fragmentation of N,N-Ditosyl-2-aminodiphenylamine to Phenazine" Molbank 2025, no. 4: M2069. https://doi.org/10.3390/M2069
APA StylePlater, M. J., & Harrison, W. T. A. (2025). Gas Phase Fragmentation of N,N-Ditosyl-2-aminodiphenylamine to Phenazine. Molbank, 2025(4), M2069. https://doi.org/10.3390/M2069