2,2,3,3-Tetrafluoropropyl 4-azido-2,3,5,6-Tetrafluorobenzoate
Abstract
1. Introduction
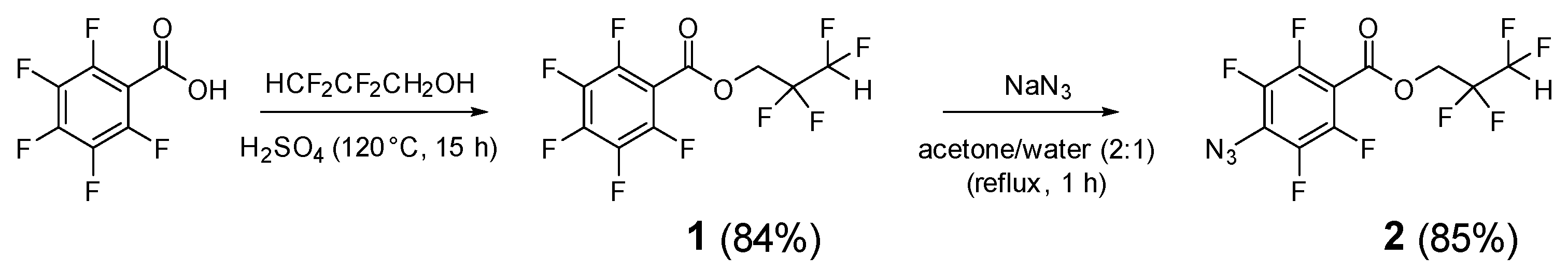
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic Azides: An Exploding Diversity of a Unique Class of Compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef]
- Geng, J.; Zhang, Y.; Gao, Q.; Neumann, K.; Dong, H.; Porter, H.; Potter, M.; Ren, H.; Argyle, D.; Bradley, M. Switching on prodrugs using radiotherapy. Nat. Chem. 2021, 13, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.R.; Keana, J.F.W. Rapid and Selective Reduction of Functionalized Fluoroaryl Azides to the Corresponding Anilines with Stannous Chloride Dihydrate. Synth. Commun. 1993, 23, 357–360. [Google Scholar] [CrossRef]
- Xie, S.; Fukumoto, R.; Ramström, O.; Yan, M. Anilide Formation from Thioacids and Perfluoroaryl Azides. J. Org. Chem. 2015, 80, 4392–4397. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.M.; Hamilton, M.; Keen, B.; Despain, M.; Day, J.; Weaver, J.D. A Selective Single Step Amidation of Polyfluoroarenes. J. Fluor. Chem. 2021, 248, 109821. [Google Scholar] [CrossRef]
- Laev, S.S.; Evtefeev, V.U.; Shteingarts, V.D. A new approach to polyfluoroaromatic amines with an unsubstituted position ortho to the amino group. J. Fluor. Chem. 2001, 110, 43–46. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Y.; Ramström, O.; Yan, M. Base-catalyzed synthesis of aryl amides from aryl azides and aldehydes. Chem. Sci. 2016, 7, 713–718. [Google Scholar] [CrossRef]
- Hamada, M.; Yoshida, S. Conditional fluorescent changes of azaylides with fluorescent chromophores. Chem. Lett. 2025, 54, upaf033. [Google Scholar] [CrossRef]
- Cao, L.; Liu, C.; Tang, X.; Yin, X.; Zhang, B. Highly selective synthesis of 1-polyfluoroaryl-1,2,3-triazoles via a one-pot three-component reaction. Tetrahedron Lett. 2014, 55, 5033–5037. [Google Scholar] [CrossRef]
- Nizam, M.; Çakır Çanak, T.; Serhatlı, İ.E. Fabrication of Fluorine and Nitrogen-Based Flame Retardants Containing Rigid Polyurethane Foam with Improved Hydrophobicity and Flame Retardancy. ACS Omega 2025, 10, 17847–17858. [Google Scholar] [CrossRef]
- Riddhidev, B.; Endri, K.; Sabitri, L.; Kotsull, N.; Nishanth, K.; Dragan, I.; James, S.; William, T.; Viranga, T.L. Rational design of metabolically stable HDAC inhibitors: An overhaul of trifluoromethyl ketones. Eur. J. Med. Chem. 2022, 244, 114807. [Google Scholar] [CrossRef]
- Maugeri, L.; Asencio-Hernández, J.; Lébl, T.; Cordes, D.B.; Slawin, A.M.Z.; Delsuc, M.-A.; Philp, D. Neutral iodotriazoles as scaffolds for stable halogen-bonded assemblies in solution. Chem. Sci. 2016, 7, 6422–6428. [Google Scholar] [CrossRef]
- Ostrovskii, V.A.; Trifonov, R.E. Fluorinated Triazoles and Tetrazoles. In Fluorine in Heterocyclic Chemistry Volume 1: 5-Membered Heterocycles and Macrocycles; Nenajdenko, V., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 459–513. [Google Scholar] [CrossRef]
- Siegmann, K.; Inauen, J.; Villamaina, D.; Winkler, M. Photografting of perfluoroalkanes onto polyethylene surfaces via azide/nitrene chemistry. Appl. Surf. Sci. 2017, 396, 672–680. [Google Scholar] [CrossRef]
- Abdollahi, M.F.; Welsh, E.N.; Shayan, M.; Olivier, A.; Wilson-Faubert, N.; Werner-Zwanziger, U.; Nazemi, A.; Laventure, A.; Chitnis, S.S. Poly(hydrazinophosphine diazide)s (PHPDs): Hybrid Organic–Inorganic Polymers via Polycondensation between PN Cages and Organic Diazides. J. Am. Chem. Soc. 2025, 147, 9229–9241. [Google Scholar] [CrossRef]
- Liao, Y.; Xiang, H.; Hu, T.; Saparbaev, A.; Zheng, X.; Wan, M.; Wu, J.; Xie, Y.; Hu, S.; Xiao, Q.; et al. Dual Liquid Rubber Matrix Based Highly Efficient and Mechanically Robust Layer-by-Layer Organic Solar Cells. SusMat 2025, 5, e70005. [Google Scholar] [CrossRef]
- Tan, Z.-S.; Jamal, Z.; Teo, D.W.Y.; Ko, H.-C.; Seah, Z.-L.; Phua, H.-Y.; Ho, P.K.H.; Png, R.-Q.; Chua, L.-L. Optimization of fluorinated phenyl azides as universal photocrosslinkers for semiconducting polymers. Nat. Commun. 2024, 15, 6354. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.Y.; Kamarulzaman, S.; Rasyiddin, R.; Sim, S.Y.X.; Seah, G.E.K.K.; Gan, A.W.; Li, Z.; Png, Z.M.; Goh, S.S. Dynamic crosslinking of thermoplastics via perfluorophenyl nitrene C–H insertion to form recyclable thermosets. Chem 2025, 11, 102479. [Google Scholar] [CrossRef]
- Wang, K.; Gladysz, J.A. Azide- and Fluorine-Containing Polystyrenes as Potential “Phosphine Sponges” Based upon Staudinger Reactions: Application to the Phase Transfer Activation of Grubbs’ Catalyst. Macromolecules 2022, 55, 8883–8891. [Google Scholar] [CrossRef]
- Cen, J.-H.; Xie, Q.-H.; Guo, G.-H.; Gao, L.-J.; Liao, Y.-H.; Zhong, X.-P.; Liu, H.-Y. Azide-modified corrole phosphorus complexes for endoplasmic reticulum-targeted fluorescence bioimaging and effective cancer photodynamic therapy. Eur. J. Med. Chem. 2024, 265, 116102. [Google Scholar] [CrossRef]
- Kascheeva, S.S.; Lastovka, A.V.; Vinogradov, A.S.; Parkhomenko, D.A. 1H,1H,7H-Dodecafluoroheptyl Pentafluorobenzoate. Molbank 2025, 2025, M2053. [Google Scholar] [CrossRef]
- Deng, T.; Mazumdar, W.; Yoshinaga, Y.; Patel, P.B.; Malo, D.; Malo, T.; Wink, D.J.; Driver, T.G. Rh2(II)-Catalyzed Intermolecular N-Aryl Aziridination of Olefins Using Nonactivated N Atom Precursors. J. Am. Chem. Soc. 2021, 143, 19149–19159. [Google Scholar] [CrossRef]
- Senaweera, S.M.; Singh, A.; Weaver, J.D. Photocatalytic Hydrodefluorination: Facile Access to Partially Fluorinated Aromatics. J. Am. Chem. Soc. 2014, 136, 3002–3005. [Google Scholar] [CrossRef]
- Tian, X.; Li, W.; Li, F.; Cai, M.; Si, Y.; Tang, H.; Li, H.; Zhang, H. Direct Photopatterning of Zeolitic Imidazolate Frameworks via Photoinduced Fluorination. Angew. Chem. Int. Ed. 2025, 64, e202500476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kascheeva, S.S.; Lastovka, A.V.; Vinogradov, A.S.; Mezhenkova, T.V.; Parkhomenko, D.A. 2,2,3,3-Tetrafluoropropyl 4-azido-2,3,5,6-Tetrafluorobenzoate. Molbank 2025, 2025, M2070. https://doi.org/10.3390/M2070
Kascheeva SS, Lastovka AV, Vinogradov AS, Mezhenkova TV, Parkhomenko DA. 2,2,3,3-Tetrafluoropropyl 4-azido-2,3,5,6-Tetrafluorobenzoate. Molbank. 2025; 2025(4):M2070. https://doi.org/10.3390/M2070
Chicago/Turabian StyleKascheeva, Sofia S., Anastasiya V. Lastovka, Andrey S. Vinogradov, Tatyana V. Mezhenkova, and Dmitriy A. Parkhomenko. 2025. "2,2,3,3-Tetrafluoropropyl 4-azido-2,3,5,6-Tetrafluorobenzoate" Molbank 2025, no. 4: M2070. https://doi.org/10.3390/M2070
APA StyleKascheeva, S. S., Lastovka, A. V., Vinogradov, A. S., Mezhenkova, T. V., & Parkhomenko, D. A. (2025). 2,2,3,3-Tetrafluoropropyl 4-azido-2,3,5,6-Tetrafluorobenzoate. Molbank, 2025(4), M2070. https://doi.org/10.3390/M2070






