Synthesis and Characterization of Novel Pyridinium Salts of (E)-2-(Pyridin-4-ylmethylene)hydrazine-1-carboximidamide
Abstract
1. Introduction
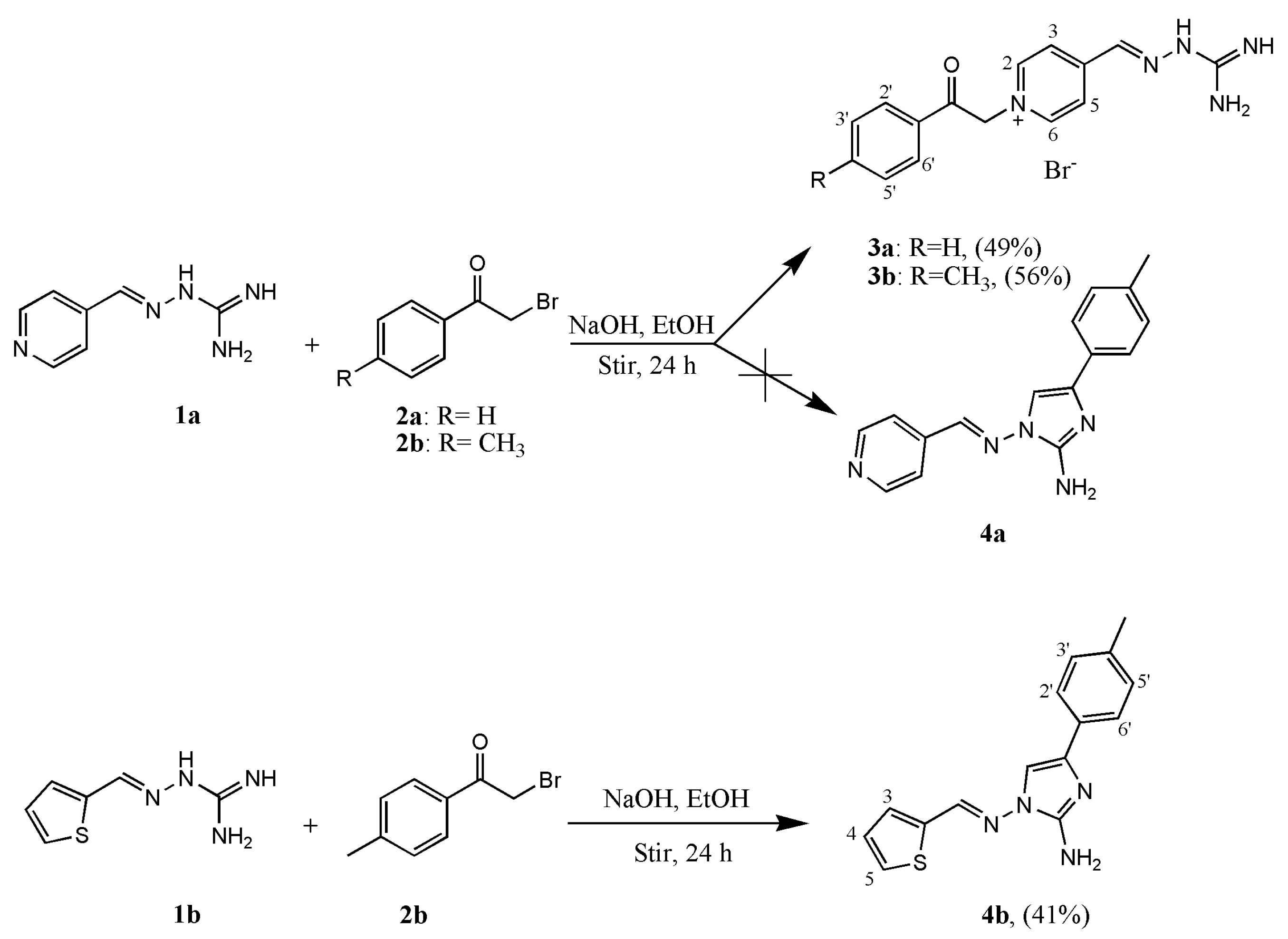
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. (E)-4-((2-Carbamimidoylhydrazineylidene)methyl)-1-(2-oxo-2-phenylethyl)pyridin-1-ium bromide (3a)
3.2.2. (E)-4-((2-Carbamimidoylhydrazineylidene)methyl)-1-(2-oxo-2-(p-tolyl)ethyl)pyridin-1-ium bromide (3b)
3.2.3. (E)-1-((Thiophen-2-ylmethylene)amino)-4-(p-tolyl)-1H-imidazol-2-amine (4b)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javahershenas, R. Application of phenacyl bromide analogs as a versatile organic intermediate for the synthesis of heterocyclic compounds via multicomponent reactions. Mol. Divers. 2023, 27, 2399–2430. [Google Scholar] [CrossRef] [PubMed]
- Vekariya, R.H.; Patel, K.D.; Prajapati, N.P.; Patel, H.D. Phenacyl bromide: A versatile organic intermediate for the synthesis of heterocyclic compounds. Synth. Commun. 2018, 48, 1505–1533. [Google Scholar] [CrossRef]
- Sanaeishoar, H.; Nazarpour, R.; Mohave, F. Novel one-pot pseudo four component reaction: Expeditious synthesis of functionalized imidazo [1,2-a]pyridines. RSC Adv. 2015, 5, 68571–68578. [Google Scholar] [CrossRef]
- Li, B.; Chiu, C.K.F.; Hank, R.F.; Murry, J.; Roth, J.; Tobiassen, H. An Optimized Process for Formation of 2,4-Disubstituted Imidazoles from Condensation of Amidines and α-Haloketones. Org. Process. Res. Dev. 2002, 6, 682–683. [Google Scholar] [CrossRef]
- Vieira, M.M.; Dalberto, B.T.; Coelho, F.L.; Schneider, P.H. Ultrasound-promoted regioselective synthesis of chalcogeno-indolizines by a stepwise 1,3-dipolar cycloaddition. Ultrason. Sonochem. 2020, 68, 105228. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-builla, J.; Novella, J.L.; Gálvez, E.; Smith, P.; Florencio, F.; Garciá-Blanco, S.; Bellanato, J.; Santos, M. Synthesis and structural study on α-substituted-1-styrylpyridinium salts: Reinvestigation of krohnke condensation. Tetrahedron 1986, 42, 699–708. [Google Scholar] [CrossRef]
- Kong, M.; Zhou, X.; Chen, Q.; Zhang, F.; Zhao, Y. Efficient synthesis of novel indolizine C-nucleoside analogues via coupling of sugar alkynes, pyridines and α-bromo carbonyl compounds in one pot. Carbohydr. Res. 2021, 505, 108337. [Google Scholar] [CrossRef]
- Bora, U.; Saikia, A.; Boruah, R.C. A Novel Microwave-Mediated One-Pot Synthesis of Indolizines via a Three-Component Reaction. Org. Lett. 2003, 5, 435–438. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; He, Y.; Guo, S.; Zhang, X. Synthesis of 1,2,3-Trisubstituted Indolizines, Pyrrolo[1,2-a]quinolines, and Pyrrolo[2,1-a]isoquinolines from 1,2-Allenyl Ketones. Eur. J. Org. Chem. 2014, 2014, 713–717. [Google Scholar] [CrossRef]
- Amidi, S.; Esfahanizadeh, M.; Tabib, K.; Soleimani, Z.; Kobarfard, F. Rational Design and Synthesis of 1-(Arylideneamino)-4-aryl-1 H -imidazole-2-amine Derivatives as Antiplatelet Agents. ChemMedChem 2017, 12, 962–971. [Google Scholar] [CrossRef]
- Li, W.T.; Hwang, D.R.; Song, J.S.; Chen, C.P.; Chuu, J.J.; Hu, C.B.; Lin, H.L.; Huang, C.L.; Huang, C.Y.; Tseng, H.Y.; et al. Synthesis and Biological Activities of 2-Amino-1-arylidenamino Imidazoles as Orally Active Anticancer Agents. J. Med. Chem. 2010, 53, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Hayman, R.A.; Gray, O.D. Imidazole, a new natural product from the leguminosae. Phytochemistry. 1987, 26, 3247–3248. [Google Scholar] [CrossRef]
- Ali, I.; Lone, M.N.; Aboul-Enein, H.Y. Imidazoles as potential anticancer agents. Medchemcomm 2017, 8, 1742–1773. [Google Scholar] [CrossRef] [PubMed]
- Bayar, İ.; Akkoç, S. Antibacterial Activities of Imidazole-Based Compounds (A Review). Russ. J. Org. Chem. 2024, 59, S7–S19. [Google Scholar] [CrossRef]
- Sadeghian, S.; Bekhradi, F.; Mansouri, F.; Razmi, R.; Mansouri, S.; Poustforoosh, A.; Khabnadideh, S.; Zomorodian, K.; Zareshahrabadi, Z.; Rezaei, Z. Imidazole Derivatives as Novel and Potent Antifungal Agents: Synthesis, Biological Evaluation, Molecular Docking Study, Molecular Dynamic Simulation and ADME Prediction. J. Mol. Struct. 2023, 1302, 137447. [Google Scholar] [CrossRef]
- Sharma, P.; LaRosa, C.; Antwi, J.; Govindarajan, R.; Werbovetz, K.A. Imidazoles as Potential Anticancer Agents: An Update on Recent Studies. Molecules 2021, 26, 4213. [Google Scholar] [CrossRef]
- Sharma, D.; Narasimhan, B.; Kumar, P.; Judge, V.; Narang, R.; De Clercq, E.; Balzarini, J. Synthesis, antimicrobial and antiviral evaluation of substituted imidazole derivatives. Eur. J. Med. Chem. 2009, 44, 2347–2353. [Google Scholar] [CrossRef]
- Carini, D.J.; Duncia, J.V.; Aldrich, P.E.; Chiu, A.T.; Johnson, A.L.; Pierce, M.E.; Price, W.A.; Santella, J.B., 3rd; Wells, G.J.; Wexler, R.R.; et al. Nonpeptide angiotensin II receptor antagonists: The discovery of a series of N-(biphenylylmethyl)imidazoles as potent, orally active antihypertensives. J. Med. Chem. 1991, 34, 2525–2547. [Google Scholar]
- Rehse, K.; Steege, J. Synthesis and antiplatelet activity of new imidazole-4-carboxylic acid derivatives. Arch. Der Pharm. Int. J. Pharm. Med. Chem. 2005, 338, 539–547. [Google Scholar] [CrossRef]
- Györgydeák, Z.; Szabó, G.; Holzer, W. 2-Amino-4-aryl-1-arylideneaminoimidazoles and Acylation Products: A Multinuclear (1H, 13C, 15N) NMR Study. Monatsh. Chem. 2004, 135, 173–184. [Google Scholar]
- Krimer, M.Z.; Makaev, F.Z.; Styngach, E.P.; Koretskii, A.G.; Pogrebnoi, S.I.; Kochug, A.I. Synthesis of substituted 2-amino-1-arylidenaminoimidazoles and 1-arylidenaminoimidazo[1,2-a]imidazoles. Chem. Heterocycl. Compd. 1996, 32, 1035–1039. [Google Scholar] [CrossRef]
- Weinmann, H.; Harre, M.; Koenig, K.; Merten, E.; Tilstam, U. Efficient and environmentally friendly synthesis of 2-amino-imidazole. Tetrahedron Lett. 2002, 43, 593–595. [Google Scholar] [CrossRef]
- Ghanbarimasir, Z.; Bekhradnia, A.; Morteza-Semnani, K.; Rafiei, A.; Razzaghi-Asl, N.; Kardan, M. Design, synthesis, biological assessment and molecular docking studies of new 2-aminoimidazole-quinoxaline hybrids as potential anticancer agents. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2018, 194, 21–35. [Google Scholar]
- Holzer, W.; Györgydeák, Z. On the structure of guanylhydrazones derived from aromatic aldehydes. Monatsh. Chem. 1992, 123, 1163–1173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataie Alani, F.; Ahmadian, F.; Houshdar Tehrani, A.; Amidi, S. Synthesis and Characterization of Novel Pyridinium Salts of (E)-2-(Pyridin-4-ylmethylene)hydrazine-1-carboximidamide. Molbank 2025, 2025, M2068. https://doi.org/10.3390/M2068
Ataie Alani F, Ahmadian F, Houshdar Tehrani A, Amidi S. Synthesis and Characterization of Novel Pyridinium Salts of (E)-2-(Pyridin-4-ylmethylene)hydrazine-1-carboximidamide. Molbank. 2025; 2025(4):M2068. https://doi.org/10.3390/M2068
Chicago/Turabian StyleAtaie Alani, Fatemeh, Fatemeh Ahmadian, Alireza Houshdar Tehrani, and Salimeh Amidi. 2025. "Synthesis and Characterization of Novel Pyridinium Salts of (E)-2-(Pyridin-4-ylmethylene)hydrazine-1-carboximidamide" Molbank 2025, no. 4: M2068. https://doi.org/10.3390/M2068
APA StyleAtaie Alani, F., Ahmadian, F., Houshdar Tehrani, A., & Amidi, S. (2025). Synthesis and Characterization of Novel Pyridinium Salts of (E)-2-(Pyridin-4-ylmethylene)hydrazine-1-carboximidamide. Molbank, 2025(4), M2068. https://doi.org/10.3390/M2068





