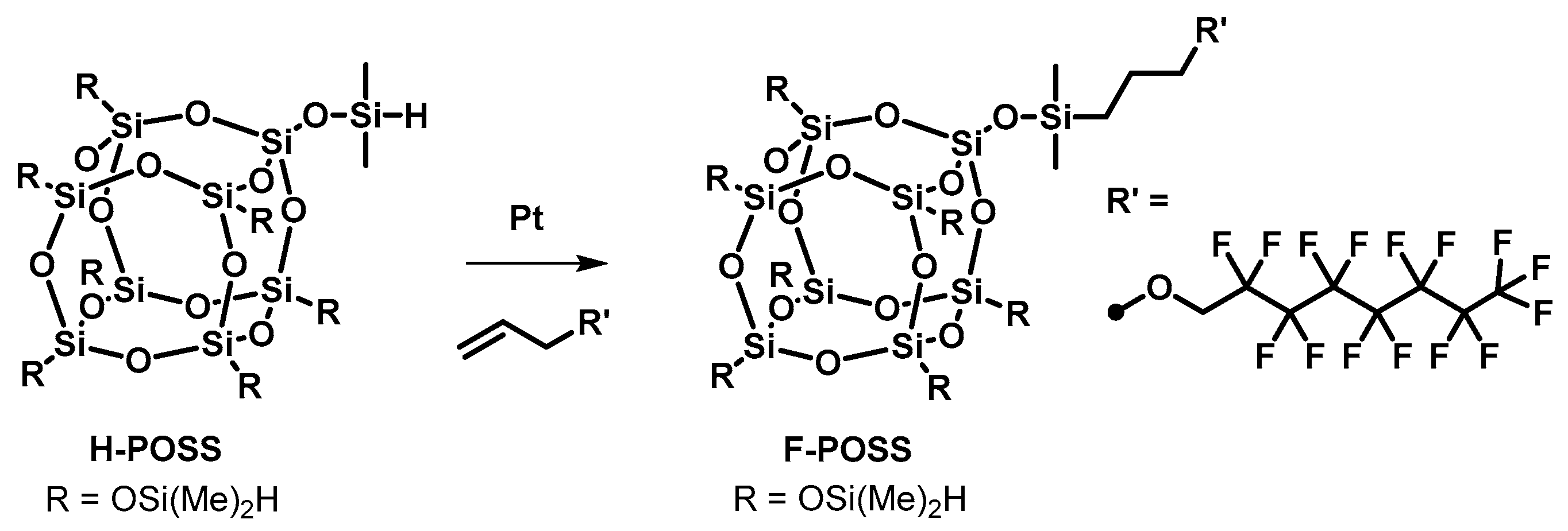
1-((Dimethyl(3-((2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl)oxy)propyl)silyl)oxy)-3,5,7,9,11,13,15-heptakis((dimethylsilyl)oxy)-octasilsesquioxane
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
1-((Dimethyl(3-((2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooc-tyl)oxy)propyl)silyl)oxy)-3,5,7,9,11,13,15-heptakis((dimethylsilyl)oxy)-octasilsesquioxane (F-POSS)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U., Jr. Polyhedral Oligomeric Silsesquioxane (POSS) Polymers and Copolymers: A Review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Cordes, D.; Lickiss, P.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Lichtenhan, J.D.; Pielichowski, K.; Blanco, I. POSS-Based Polymers. Polymers 2019, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of Hybrid Organic–Inorganic Nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Phillips, S.; Haddad, T.; Tomczak, S. Developments in nanoscience: Polyhedral oligomeric silsesquioxane (POSS)-polymers. Cur. Opin. Solid State Mater. Sci. 2004, 8, 21–29. [Google Scholar] [CrossRef]
- Walczak, M.; Franczyk, A.; Dutkiewicz, M.; Marciniec, B. Synthesis of Bifunctional Silsesquioxanes (RSiMe2O)∼4(R′SiMe2O)∼4Si8O12 via Hydrosilylation of Alkenes. Organometallics 2019, 38, 3018–3024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliaccio, A.C.H.; Kelley, A.R.; Iacono, S.T. 1-((Dimethyl(3-((2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl)oxy)propyl)silyl)oxy)-3,5,7,9,11,13,15-heptakis((dimethylsilyl)oxy)-octasilsesquioxane. Molbank 2025, 2025, M2062. https://doi.org/10.3390/M2062
Migliaccio ACH, Kelley AR, Iacono ST. 1-((Dimethyl(3-((2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl)oxy)propyl)silyl)oxy)-3,5,7,9,11,13,15-heptakis((dimethylsilyl)oxy)-octasilsesquioxane. Molbank. 2025; 2025(3):M2062. https://doi.org/10.3390/M2062
Chicago/Turabian StyleMigliaccio, Analise C. H., Andrea R. Kelley, and Scott T. Iacono. 2025. "1-((Dimethyl(3-((2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl)oxy)propyl)silyl)oxy)-3,5,7,9,11,13,15-heptakis((dimethylsilyl)oxy)-octasilsesquioxane" Molbank 2025, no. 3: M2062. https://doi.org/10.3390/M2062
APA StyleMigliaccio, A. C. H., Kelley, A. R., & Iacono, S. T. (2025). 1-((Dimethyl(3-((2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl)oxy)propyl)silyl)oxy)-3,5,7,9,11,13,15-heptakis((dimethylsilyl)oxy)-octasilsesquioxane. Molbank, 2025(3), M2062. https://doi.org/10.3390/M2062





