Abstract
The Vilsmeier–Haack reaction is a convenient method for the formylation of electron-rich aromatic compounds. However, this interaction sometimes gives specific side products. We report the isolation and structural characterization of a novel compound (Z)-1-(3,5-dichloro-2-pyrrolylidene)-N,N-dimethylmethanamine obtained as a side product during the formylation of 2-chloropyrrole. The product was characterized using NMR spectroscopy and X-ray crystallography.
1. Introduction
The Vilsmeier–Haack reaction (VHR) plays a crucial role in modern organic synthesis [1,2]. It facilitates the introduction of a formyl group into the core of electron-rich aromatic molecules such as phenols, pyrrole [3], pyrazoles [4] and other [5]. Additionally, Vilsmeier reagent (VR) could interact with nonaromatic compound like alkenes, ketones, aldehydes and heterocycles [6]. Formylpyrroles are important intermediates in the synthesis of pyrrole-containing molecules, e.g., difluoroboron dyes like BODIPY, BOPHY, etc. [7]. Using halogenated 2-formylpyrroles enables the preparation of halogenated pyrrole-containing intermediates. Commonly, halogenated 2-formylpyrroles could be obtained by formylation of 2-halopyrroles with VR [8]. However, yields of this reaction are often low. It is well known that VHR can lead to the formation of different unexpected products, depending on substrate and reaction conditions. Herein, we report the isolation and characterization of (Z)-1-(3,5-dichloro-2-pyrrolylidene)-N,N-dimethylmethanamine from the reaction mixture of 2-chloropyrrole VHR.
2. Results
2.1. Synthesis of (Z)-1-(3,5-Dichloro-2-pyrrolylidene)-N,N-dimethylmethanamine
Typical procedures for 5-chloro-2-formylpyrrole preparation include chlorination of pyrrole and further VHR without purification of 2-chloropyrrole. The reaction yielded a complex mixture of products, from which (Z)-1-(3,5-dichloro-2-pyrrolylidene)-N,N-dimethylmethanamine was isolated by flash-chromatography with a moderate yield (Scheme 1). 5-Chloro-2-formylpyrrole and 1-(3-chloro-2-pyrrolylidene)-N,N-dimethylmethanamine were also identified by GC-MS in the crude products mixture as main components. However, only 5-chloro-2-formylpyrrole was isolated by chromatography.
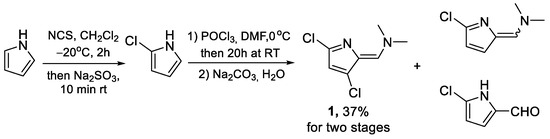
Scheme 1.
Reaction of 2-chloropyrrole with Vilsmeier–Haack reagent.
The obtained product was characterized using 1H and 13C NMR spectroscopy, HRMS and X-ray crystallography. In 1H NMR spectrum singlet signals of methyl groups were observed at 3.36 and 3.72 ppm, singlet signals were also observed at 6.21 (=CHNMe2) and 7.22 ppm (CH of pyrrole fragment). In 13C NMR, all signals of alkene fragments are observed at 113–145 ppm for heterocyclic and vinylidene carbons, and methyl groups gave signals at 40.5 and 47.5 ppm.
A plausible mechanism of formation of product 1 is shown in Scheme 2. We propose that the reaction of 2-chloropyrrole with VR leads to the formation of cation 2, which could undergo alkaline hydrolysis to yield 2-formyl-5-chloropyrrole. Apparently, due to the presence of the electron-withdrawing chlorine atom at position 5, cation 2 has sufficient electrophilicity to attach a chloride anion and form compound 3 or its isomer. Oxidation of compound 3 with atmospheric oxygen during work-up process leads to the isolated product 1.
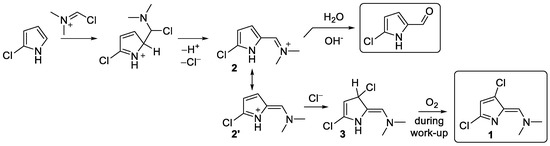
Scheme 2.
Proposed mechanism of formation of product 1.
2.2. XRD Studies
Single crystal X-ray studies were performed. The obtained substance in crystal has monoclinic P21/n space group with two molecules in the asymmetric unit M1 and M2. The structure of M1 and M2 with corresponding labeling scheme is shown in Figure 1. The conformation of molecules is planar with intramolecular C-H···N interactions (C6-H···N2 (dH···N2 = 2.20 Å) and C13-H···N4 (dH···N4 = 2.22 Å)).
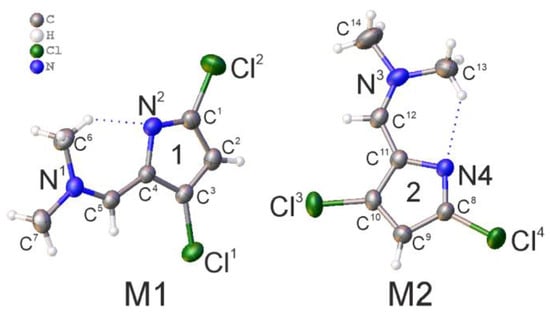
Figure 1.
Molecular structure, atom and cycle numbering of product 1 with anisotropic displacement ellipsoids drawn at 50% probability level.
M1 molecules form dimer connected by C6-H···Cl1 and C7-H···π1 interactions (π1 is N2-C1-C2-C3-C4 ring), and dimers are packing into the chain along the a-axis (Figure 2a,b). M2 molecules also form dimers and chains along the a-axis, but intermolecular interactions are absent. Chains M1 and M2 are connected to each other by C2-H···Cl3 interactions (Figure 2c).
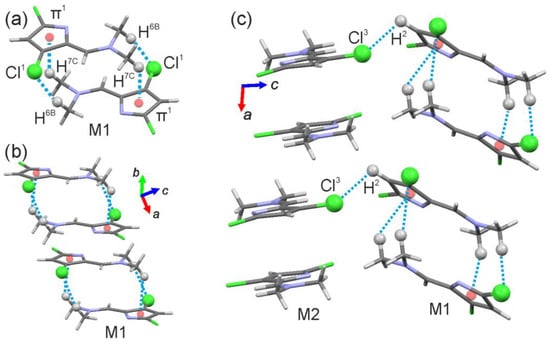
Figure 2.
Fragments of product 1 crystal structure forming (a) dimer between M1 molecules connected by C6-H···Cl1 and C7-H···π1 interactions; (b) chain of M1 molecules along a-axis; and (c) intermolecular interactions between M1 and M2 along a-axis. Dashed blue lines represent noncovalent interactions. The arrows indicate the orientation of the crystallographic axes.
3. Materials and Methods
All chemicals were purchased from commercial sources and used without additional purification unless otherwise noted. The progress of reactions was monitored by thin-layer chromatography (TLC) on Sorbfil Silica 60 F254 on aluminum sheets with UV visualization. Column chromatography was performed by using 100–200 mesh silica gel. NMR spectra were recorded on Bruker Avance-400 (400.13 MHz for 1H, 100.62 MHz for 13C) spectrometer; chemical shifts of 1H and 13C{1H} are provided in ppm, with solvent signals serving as the internal standard (1H = 7.24 ppm and 13C = 77.16 ppm for CDCl3). The masses of molecular ions were determined by high-resolution mass spectrometry (HRMS) by means of a DFS Thermo Scientific instrument (EI, 70 eV). X-ray diffraction data for compound was collected at 296 K using a Bruker KAPPA APEX II diffractometer with graphite monochromated MoKα radiation. Absorption corrections were applied using SADABS [9]. The structure was solved by SHELXT [10]. Refinement was carried out by the full-matrix least-squares technique with SHELXL [10] using OLEX2 [11] software. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were calculated at idealized positions according to the riding model.
Synthesis of 2-chloro-1H-pyrrole.
A mass of 5.00 g (75 mmol) of pyrrole was added to 200 mL of dry CH2Cl2. The solution was cooled to −20 °C. Then, a 10.46 g (78 mmol) of NCS was added to the cooled mixture. The obtained mixture was stirred for 10 min and was kept at the temperature −20 °C for 2 h. A mass of 9.87 g (78 mmol) of Na2SO3 was added, and the resulting mixture was stirred for 10 min. After that, 150 mL water and 100 mL CH2Cl2 were added, the organic layer was separated and the aqueous layer was extracted with CH2Cl2 (3 × 50 mL). The combined organic phase was washed with water (3 × 100 mL) and dried over Na2SO4. Evaporation under reduced pressure at room temperature gave 4.80 g of crude 2-chloro-1H-pyrrole. The product was used without purification.
1H NMR (δ, 400.13 MHz, CDCl3): 6.11 (m, 1H, NH-CH=CH), 6.21 (t, 1H, J = 3.4 Hz, NH-CCl=CH), 6.66 (m, 1H, NH-CH=CH), 8.08 (s, 1H, NH).
Synthesis of (Z)-1-(3,5-dichloro-2H-pyrrol-2-ylidene)-N,N-dimethylmethanamine (1) (Supplementary Materials).
To an ice-cooled solution of 4.86 mL (52 mmol) POCl3, 4.03 mL (52 mmol) DMF was added dropwise in an Ar atmosphere. After the addition, 70 mL of dry CH2Cl2 was added, then a solution of 4.80 g (47 mmol) 2-chloro-1H-pyrrole in 50 mL dry CH2Cl2 was added dropwise in 30 min while cooling to 0 °C in an Ar atmosphere. The resulting mixture was stirred for 20 h at RT. After that, a solution of 25 g Na2CO3 in 100 mL water was added, and the mixture was stirred for 50 min. Then, the organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2 × 50 mL). The combined organic phase was washed with water (3 × 100 mL), dried over Na2SO4, filtered and evaporated under reduced pressure. The crude product was purified by column chromatography on silica gel, with EtOAc/Hex (1:1) as eluent, Rf = 0.48. Light yellow solid. Yield = 2.27 g (16% based on pyrrole). M. p.: 69–71 °C.
1H NMR (δ, 400.13 MHz, CDCl3): 3.36 (s, 3H, Me1), 3.72 (s, 3H, Me2), 6.21 (s, 1H, Cy C-H), 7.22 (s, 1H, -C=CH-NMe2).
13C NMR (δ, 100.6 MHz, CDCl3): 40.5, 47.5, 113.1, 126.3, 131.7, 144.2, 144.4.
HRMS (EI, m/z): calculated for C7H835Cl2N2—190.0059, found—190.0063.
Next, 2-chloro-5-formylpyrrole was eluted, 1.37 g (14% based on pyrrole), colorless crystals.
1H NMR (δ, 400.13 MHz, CDCl3): 6.20 (d, J = 4.0 Hz, 1H), 6.93 (d, J = 4.0, Hz, 1H), 9.35 (s, 1H), 10.86 (br. s, 1 H, NH). NMR spectrum identical with [8,12].
X-ray structure determination: crystal data for C7H8Cl2N2, M = 191.05 g mol–1, colorless elongated prism, crystal dimensions 1.00 × 0.62 × 0.18 mm, monoclinic, space group P21/n, a = 7.3574(3), b = 15.7753(6), c = 15.4311(6) Å, β = 100.599(2)°, V = 1760.46(12) Å3, Z/Z’ = 8/2, Dcalc = 1.442 g cm–3, R1 = 0.037, wR2 = 0.109, Rint = 0.036, S = 1.05. Data have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2360994.
Supplementary Materials
The following supporting information can be downloaded, Figure S1: 1H NMR spectrum of compound 1; Figure S2: 13C NMR spectrum of compound 1.
Author Contributions
Conceptualization, A.V.; methodology, A.V.; investigation, D.R. and A.S.; writing—original draft preparation, A.V., D.R. and A.S.; writing—review and editing, A.V.; supervision, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Novosibirsk Institute of Organic Chemistry, programs № 1021052605821-9 and № 122040800263-6 (X-ray studies).
Data Availability Statement
The X-ray data are available in a publicly accessible repository.
Acknowledgments
The authors thank the Multi-Access Chemical Center at the Novosibirsk Institute of Organic Chemistry for the NMR and HRMS studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Neena, N.; Chaudhri, V.; Singh, F.V.; China, H.; Dohi, T. Synthetic Utility of the Vilsmeier–Haack Reagent in Organic Synthesis. Synlett 2023, 34, 777–792. [Google Scholar]
- Su, W.; Weng, Y.; Jiang, L.; Yang, Y.; Zhao, L.; Chen, Z.; Li, Z.; Li, J. Recent Progress in the Use of Vilsmeier-Type Reagents. Org. Prep. Proced. Int. 2010, 42, 503–555. [Google Scholar] [CrossRef]
- 2-Pyrrolealdehyde. Org. Synth. 1956, 36, 74. [CrossRef]
- Badalyan, K.S.; Akopyan, A.E.; Attaryan, H.S.; Asratyan, G.V. Vilsmeier-Haack formylation of 1H-pyrazoles. Russ. J. Gen. Chem. 2014, 84, 793–795. [Google Scholar] [CrossRef]
- Chahal, M.; Dhillon, S.; Rani, P.; Kumari, G.; Aneja, D.K.; Kinger, M. Unravelling the Synthetic and Therapeutic Aspects of Five, Six and Fused Heterocycles Using Vilsmeier–Haack Reagent. RSC Adv. 2023, 13, 26604–26629. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Stanforth, S.P. The Vilsmeier reaction of non-aromatic compounds. In Organic Reactions; Jones, G., Stanforth, S.P., Eds.; John Wiley: New York, NY, USA, 2000; Volume 56, p. 355. [Google Scholar]
- Yu, C.; Hao, E.; Sun, Y.; Jiao, L. Recent Advances in Highly Fluorescent Hydrazine-Inserted Pyrrole-Based Diboron-Anchoring Fluorophores: Synthesis and Properties. Synlett 2023, 35, 37–54. [Google Scholar] [CrossRef]
- Cordell, G.A. 2-Halopyrroles. Synthesis and chemistry. J. Org. Chem. 1975, 40, 3161–3169. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Area Detector Adsorption Correction; Institute for Inorganic Chemistry, University of Goettingen: Goettingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Leen, V.; Leemans, T.; Boens, N.; Dehaen, W. 2- and 3-Monohalogenated BODIPY Dyes and Their Functionalized Analogues: Synthesis and Spectroscopy. Eur. J. Org. Chem. 2011, 23, 4386–4396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).