Ethyl 4-Ene-4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pentanoate
Abstract
1. Introduction
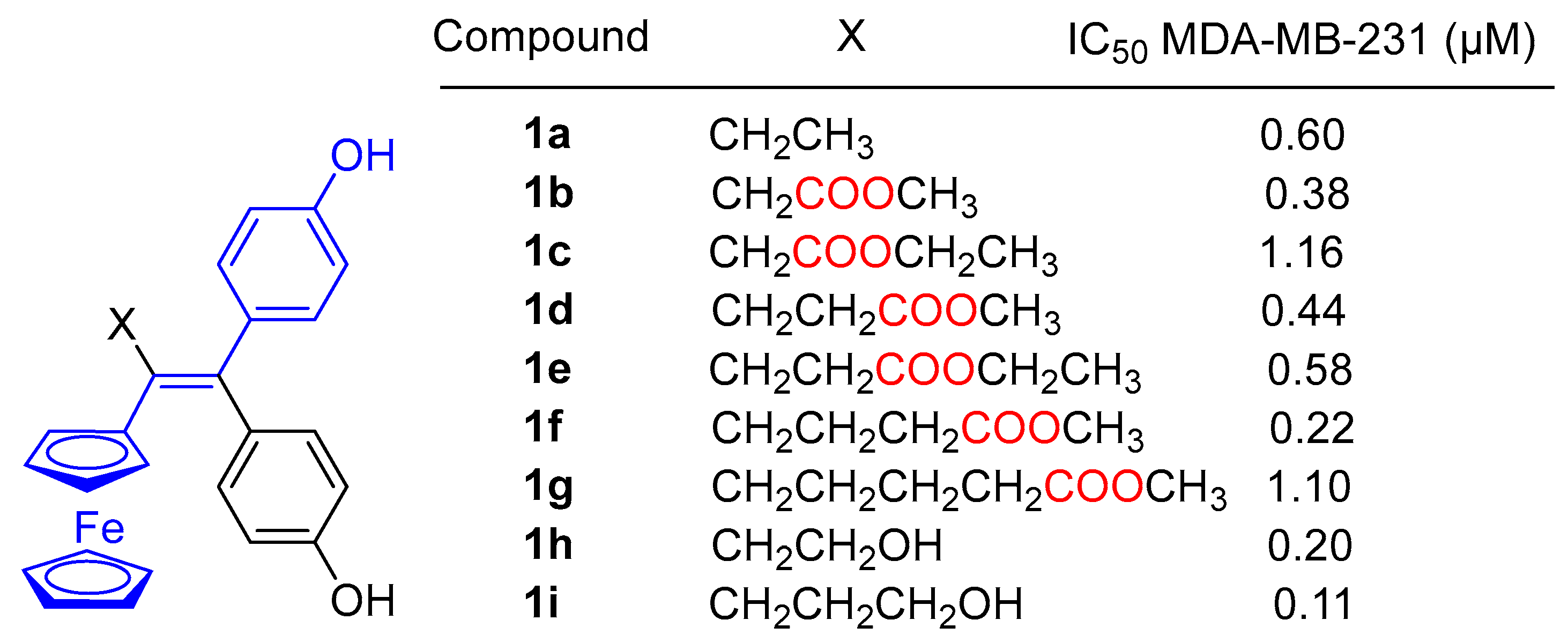
2. Results and Discussion
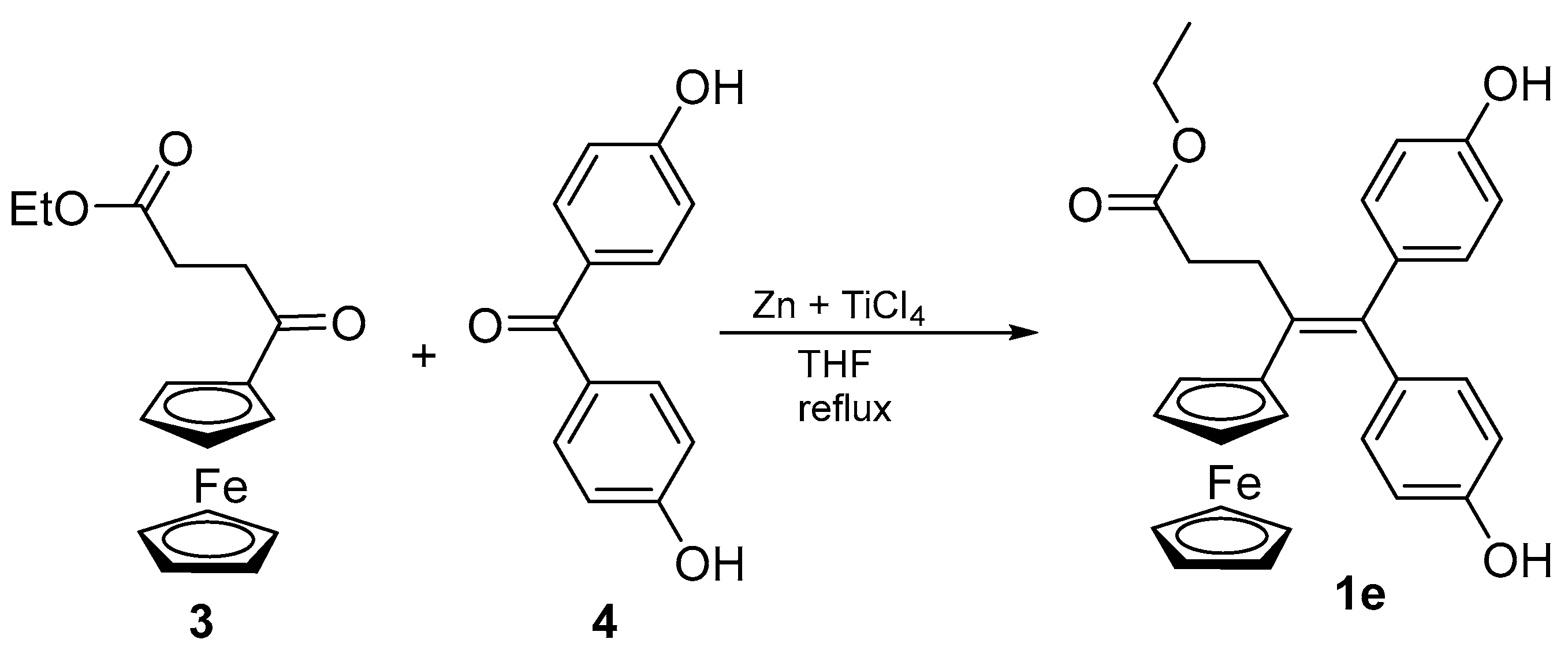
2.1. Synthesis of 1e
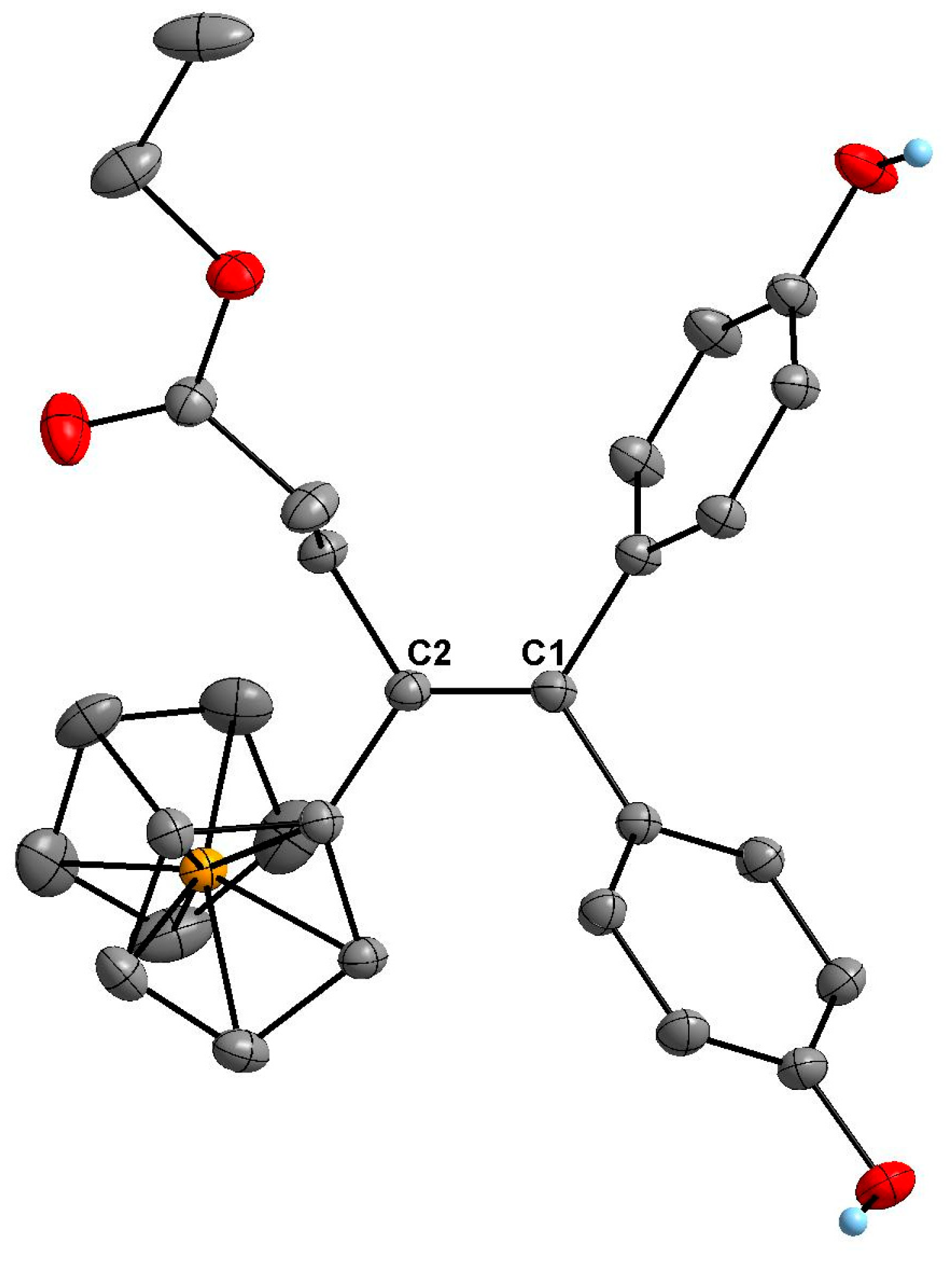
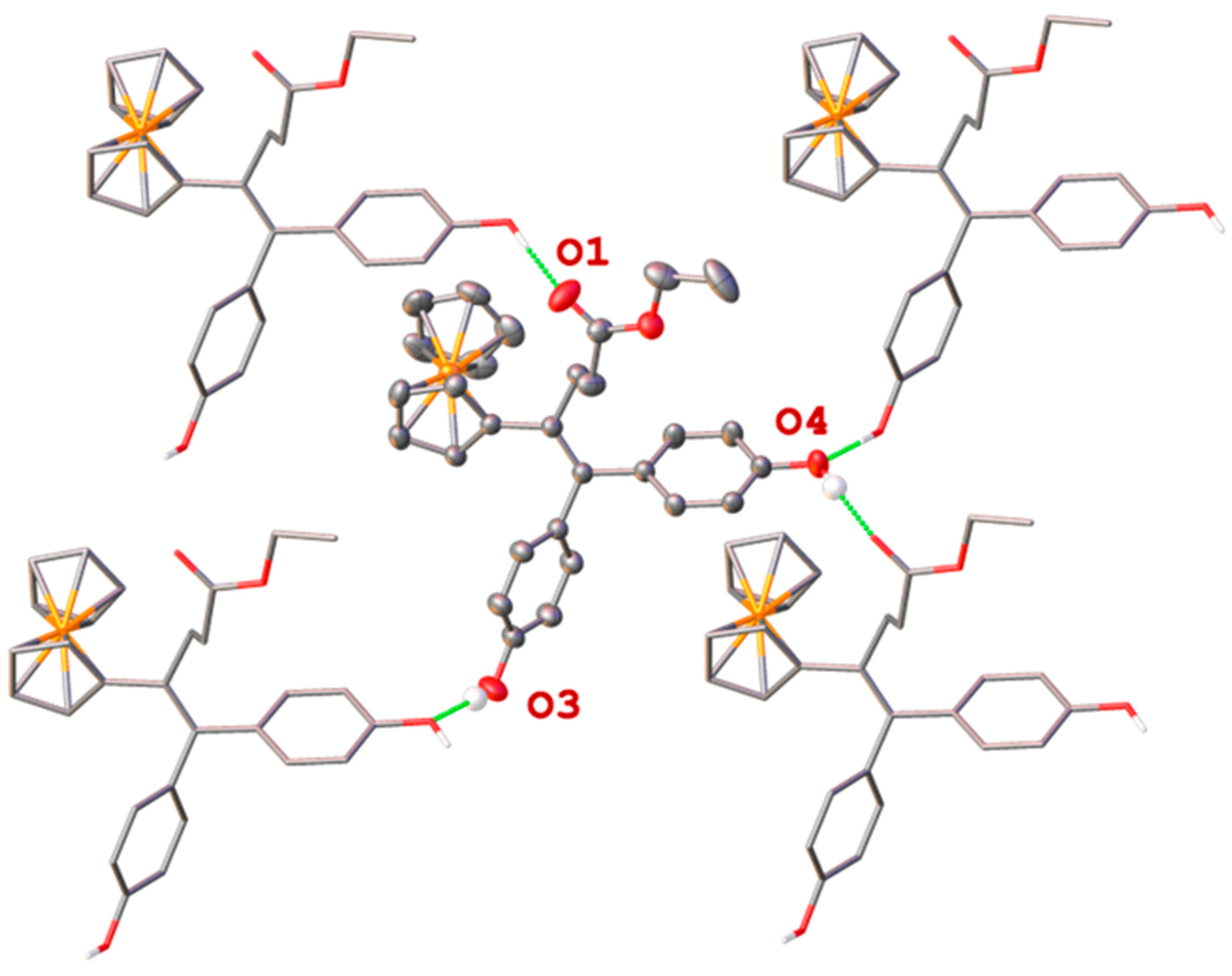
2.2. X-Ray Crystal Structure Determination of 1e
3. Materials and Methods
3.1. General Procedure
3.2. Synthesis of Ethyl 4-En-4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pentanoate (1e)
3.3. X-Ray Diffraction Experiment
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anti-cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K. Recent developments in the chemistry of ferrocenyl secondary natural product conjugates. Coord. Chem. Rev. 2018, 366, 91–108. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-Appended Pharmacophores: An exciting approach for modulating biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef] [PubMed]
- Sijongesonke, P.; Aderibigbe, B.A. Ferrocene-Based Compounds with Antimalaria/Anticancer Activity. Molecules 2019, 24, 3604. [Google Scholar] [CrossRef]
- Wang, R.; Chen, H.; Yan, W.; Zheng, M.; Zhang, T.; Zhang, Y. Ferrocene-containing hybrids as potential anticancer agents: Current developments, mechanisms of action and structure-activity relationships. Eur. J. Med. Chem. 2020, 190, 112109. [Google Scholar] [CrossRef]
- Sharma, B.; Kumar, V. Has Ferrocene Really Delivered Its Role in Accentuating the Bioactivity of Organic Scaffolds? J. Med. Chem. 2021, 64, 16865–16921. [Google Scholar] [CrossRef]
- Yong, J.; Lu, C.; Yang, M.; Wu, X. New Ferrocene Formates Bearing Isoxazole Moieties: Synthesis, Characterization, X-ray Crystallography, and Preliminarily Cytotoxicity against A549, HCT116, and MCF-7 Cell Lines. Curr. Pharm. Des. 2022, 28, 2835–2841. [Google Scholar] [CrossRef]
- Snegur, L.V. Modern Trends in Bio-Organometallic Ferrocene Chemistry. Inorganics 2022, 10, 226. [Google Scholar] [CrossRef]
- Cybulski, M.; Michalak, O.; Buchowicz, W.; Mazur, M. Ansa–Ferrocene Derivatives as Potential Therapeutics. Molecules 2024, 29, 4903. [Google Scholar] [CrossRef]
- Tomar, V.; Kumar, P.; Sharma, D.; Joshi, R.K.; Nemiwal, M. Anticancer potential of ferrocene-containing derivatives: Current and future prospective. J. Mol. Struct. 2025, 1319, 139589. [Google Scholar] [CrossRef]
- Richard, M.-A.; Hamels, D.; Pigeon, P.; Top, S.; Dansette, P.M.; Lee, H.Z.S.; Vessières, A.; Mansuy, D.; Jaouen, G. Oxidative metabolism of ferrocene analogues of Tamoxifen: Characterization and antiproliferative activities of the metabolites. ChemMedChem 2015, 10, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Richard, M.A.; Top, S.; Dansette, P.M.; Pigeon, P.; Vessières, A.; Mansuy, D.; Jaouen, G. Ferrocenyl quinone methide−thiol adducts as new antiproliferative agents: Synthesis, metabolic formation from ferrociphenols, and oxidative transformation. Angew. Chem. Int. Ed. 2016, 55, 10431–10434. [Google Scholar] [CrossRef] [PubMed]
- Tonolo, F.; Salmain, M.; Scalcon, V.; Top, S.; Pigeon, P.; Folda, A.; Caron, B.; McGlinchey, M.J.; Toillon, R.-A.; Bindoli, A.; et al. Small Structural Differences Between Two Ferrocenyl Diphenols Determine Large Discrepancies of Reactivity and Biological Effects. ChemMedChem 2019, 14, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Vessières, A.; Top, S.; Pigeon, P.; Hillard, E.A.; Boubeker, L.; Spera, D.; Jaouen, G. Modification of the estrogenic properties of diphenols by the incorporation of ferrocene. Generation of antiproliferative effects in vitro. J. Med. Chem. 2005, 48, 3937–3940. [Google Scholar] [CrossRef]
- Wang, H.; Fan, X.; Xie, P.-P.; Yang, S.; Pigeon, P.; Xiong, Y.; Gai, S.; Qi, X.; Wang, J.; Zhang, Q.; et al. Deciphering the Diversified Metabolic Behavior of Hydroxyalkyl Ferrocidiphenols as Anticancer Complexes. J. Med. Chem. 2023, 67, 1209–1224. [Google Scholar] [CrossRef]
- Pigeon, P.; Görmen, M.; Kowalski, K.; Müller-Bunz, H.; McGlinchey, M.J.; Top, S.; Jaouen, G. Atypical McMurry Cross-Coupling Reactions Leading to a New Series of Potent Antiproliferative Compounds Bearing the Key [Ferrocenyl-Ene-Phenol] Motif. Molecules 2014, 19, 10350–10369. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Jaouen, G. Organometallic Antitumor Compounds: Ferrocifens as Precursors to Quinone Methides. Angew. Chem. Ed. Int. 2015, 54, 10230–10233. [Google Scholar] [CrossRef]
- Neuse, E.W.; Crossland, R.K. Metallocene polymers. XXVII. 1,3-Terferrocenyl. J. Organomet. Chem. 1972, 43, 385–392. [Google Scholar] [CrossRef]
- Forte, J.; Pigeon, P. CSD Communication; CCDC: Cambridge, UK, 2025. [Google Scholar] [CrossRef]
- Bruker. APEX/SAINT; Bruker AXS Inc.: Madison, WI, USA, 2012; Available online: https://www.bruker.com/en/products-and-solutions/diffractometers-and-x-ray-microscopes/single-crystal-x-ray-diffractometers/sc-xrd-software/apex.html (accessed on 26 June 2023).
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Kleemiss, F.; Dolomanov, O.V.; Bodensteiner, M.; Peyerimhoff, N.; Midgley, M.; Bourhis, L.J.; Genoni, A.; Malaspina, L.A.; Jayatilaka, D.; Spencer, J.L.; et al. Accurate crystal structures and chemical properties from NoSpherA2. Chem. Sci. 2021, 12, 1675–1692. [Google Scholar] [CrossRef]
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| CCDC deposit number | 2426552 | Crystal size (mm3) | 0.23 × 0.08 × 0.07 |
| Empirical formula a | C29H28FeO4 | Wavelength λ (Å) | 1.54178 |
| Moiety formula | C29H28FeO4 | 2ϴ range (°) | 7.2–133.98 |
| Formula weight (g/mol) | 496.388 | Miller indices ranges | −10 ≤ h ≤ 11, −13 ≤ k ≤ 13, −15 ≤ l ≤ 15 |
| Temperature (K) | 200 | ||
| Crystal system | Triclinic | ||
| Space group | P | Measured reflections | 18,999 |
| a (Å) | 9.5599(3) | Unique reflections | 4413 |
| b (Å) | 11.2459(4) | Rint/Rsigma | 0.0437/0.0332 |
| c (Å) | 13.3652(4) | Reflections [I ≥ 2σ(I)] | 3843 |
| α (°) | 67.299(2) | Restraints | 2 |
| β (°) | 87.272(2) | Parameters | 419 |
| γ (°) | 70.734(2) | Goodness of fit F2 | 1.010 |
| Volume (Å3) | 1246.08(8) | Final R indices bc [all data] | R1 = 0.0338, wR2 = 0.0591 |
| Z | 2 | ||
| ρcalc (g/cm3) | 1.323 | Final R indices bc [I ≥ 2σ(I)] | R1 = 0.0267, wR2 = 0.0569 |
| Absorption coefficient μ (mm−1) | 5.110 (CuKα) | ||
| F(000) | 519.6 | Largest diff. peak/hole (e/Å3) | 0.22/−0.20 |
| D-H…A | D-H (Å) | H…A (Å) | D…A (Å) | D-H…A (°) |
|---|---|---|---|---|
| O3-H3…O4i | 0.96(4) | 1.86(4) | 2.808(2) | 172(4) |
| O4-H4…O1ii | 0.92(4) | 1.78(4) | 2.684(2) | 170(4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forté, J.; Pigeon, P. Ethyl 4-Ene-4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pentanoate. Molbank 2025, 2025, M1980. https://doi.org/10.3390/M1980
Forté J, Pigeon P. Ethyl 4-Ene-4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pentanoate. Molbank. 2025; 2025(1):M1980. https://doi.org/10.3390/M1980
Chicago/Turabian StyleForté, Jérémy, and Pascal Pigeon. 2025. "Ethyl 4-Ene-4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pentanoate" Molbank 2025, no. 1: M1980. https://doi.org/10.3390/M1980
APA StyleForté, J., & Pigeon, P. (2025). Ethyl 4-Ene-4-ferrocenyl-5,5-bis-(4-hydroxyphenyl)-pentanoate. Molbank, 2025(1), M1980. https://doi.org/10.3390/M1980






