Abstract
A new oxylipin (1) was isolated from cyanobacteria collected at Tokyo Bay, Japan. The structure of 1 was elucidated based on spectroscopic data including 1D and 2D NMR, as well as high-resolution mass spectrometry. The structure of 1 was elucidated to be (S)-2-hydroxy-3-(((2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)propyl (E)-8-hydroperoxyhexadec-6-enoate.
1. Introduction
Oxylipins are a class of oxygenated natural products formed from fatty acids by a pathway involving at least one step of dioxygen (O2)-dependent oxidation [1,2]. It has been revealed that oxylipins have physiologically significant activities in many organisms [3,4]. Recently, our research group has found new oxylipins from the Okinawan cyanobacteria [5,6]. In the present study, further research on the cyanobacteria collected from Tokyo Bay led to the isolation of a new oxylipin ester, (S)-2-hydroxy-3-(((2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)propyl (E)-8-hydroperoxyhexadec-6-enoate (1; Figure 1).

Figure 1.
Structure of compound (1).
2. Results and Discussion
An HR-ESI-MS analysis of compound (1) showed an [M − H]− ion peak at m/z 521.2950(calcd. for C25H45O11, 521.2967), indicating a molecular formula of C25H46O11 with three degrees of unsaturation. The 1H and 13C-NMR spectra indicated that compound (1) was an oxidized derivative of monogalactosyl monoacylglycerol (MGMG). In the 13C NMR spectrum, 11 oxygen-bearing carbons including a carbonyl carbon were observed. Overall, 6 of the 11 oxygen-bearing carbons were assigned to a galactose unit (Table 1. δC 62.4, 69.9, 72.5, 74.7, 76.3, 105.2) and a glycerol unit (δC 66.1, 69.3, 72.1). The remaining two 13C signals (δC 86.6, 173.7) were supposed to belong to a fatty acid moiety.

Table 1.
1H (600 MHz) and 13C NMR (150 MHz) spectroscopic data for compound (1) in acetone-d6.
The glycerol part—H2-1 (δH 4.12) −H-2 (δH 3.94) −H2-3 (δH 3.66 and 3.82)—and the galactose part—H-1′ (δH 4.26) −H-2′ (δH 3.53) − H-3′ (δH 3.51) −H-4′ (δH 3.88) −H-5′ (δH 3.55) −H2-6′ (δH 3.76)—were assigned by the detailed analysis of COSY correlations (Figure 2). The 3JH-1′,H-2′ (7.2 Hz) and 3JH-3′,H-4′ (2.8 Hz) confirmed that the sugar was β-galactose. In the fatty acid moiety of 1, the existence of a hydroperoxy group (-OOH) was speculated from unusual oxymethine signals H-8″ (δH 4.19) and C-8″ (δC 86.6), together with the molecular formula of C25H46O11. A hydroperoxy proton at δH 10.31 was also observed [7]. The position of the double bond and the -OOH group was elucidated by the COSY and TOCSY experiments (Figure 2). The COSY correlations of H2-2″ (δH 2.33) − H2-3″ (δH 1.62) −H2-4″ (δH 1.44) −H2-5″ (δH 2.07) −H-6″ (δH 5.68) indicated that the double bond was positioned at Δ6″. The olefin protons in 1 were observed at δH 5.68 (H-6″) and δH 5.41 (H-7″). The proton coupling constant of 15.4 Hz between H-6″ and H-7″ and the NOE correlation H-6″/H-8″ confirmed the E configuration of the double bond Δ6″. Furthermore, the COSY correlation between olefin proton H-7″ (δH 5.41) and an oxy-proton H-8″ (δH 4.19) indicated that the hydroperoxy group was substituted on C-8″. These results, along with the HMBC correlation of H-2″ (δH 2.33)/C-1″ (δC 173.7), led to the determination of the fatty acid unit as (E)-8-hydroperoxyhexadec-6-enoic acid. Moreover, HMBC correlations from H2-3 (δH 3.66 and 3.82) to C-1′ (δC 105.2) and H2-1 (δH 4.12) to C-1″ (δC 173.7) confirmed an ether linkage between C-3 and C-1′ and an ester linkage between C-1 and C-1″, respectively.

Figure 2.
Key COSY and TOCSY (bold line) and HMBC (arrow, H to C) correlations for compound (1).
The configuration at C-2 (δC 69.3) of glycerol in 1 was determined on the basis of the specific rotation of galactosylglycerol [8]. Galactosylglycerol (2, Figure 3) obtained by methanolysis of 1 showed a negative specific rotation ([α]D27 −8.16 (c 0.49, MeOH)), indicating that the configuration at C-2 in galactosylglycerol is R [8]. Absolute configuration of the hydroperoxy group at C-8″ could not be determined in this study.
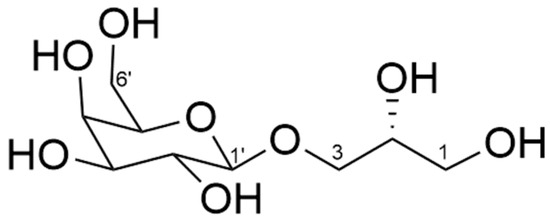
Figure 3.
Structure of galactosylglycerol (2).
Compound (1) is a novel esterified oxylipin which clearly originated from monogalactosylmonoacylglycerol (MGMG).
3. Materials and Methods
3.1. General Procedure and Method
The HR ESI-MS was measured with a micrOTOF-QII (Bruker, Billerica, MA, USA), a quadrupole time-of-flight mass spectrometer (QTOFMS), along with a high-performance liquid chromatograph of UltiMate 3000 Series (Thermo Fisher Scientific, Waltham, MA, USA). The NMR spectra were recorded on a Bruker AVANCE III 600 spectrometer (Bruker Biospin AG, Fällanden, Switzerland) at 300 K and referenced to residual solvent signals (δH 2.05, δC 29.84 for acetone-d6). Optical rotations were measured using a JASCO P-2100 (JASCO Co., Tokyo, Japan) with a 10 mm long cell. Bioassay results were recorded on a Model 550 microplate reader (Bio-Rad Co., Hercules, CA, USA).
3.2. Biological Materials
Cyanobacteria were collected at Tokyo Bay (35.58 N, 139.77 E), Japan, in 2016. The 16S rRNA sequence amplicon analysis (Bioengineering Lab. Co., Ltd., Tokyo, Japan) was used to identify the cyanobacteria sample. The results revealed that the cyanobacterium belonged to Oscillatoriales. A voucher specimen (20160725-a) was deposited at the Tokyo University of Marine Science and Technology.
3.3. Isolation of Compound (1)
The frozen algal sample (wet weight: 5.6 kg) was extracted with methanol (MeOH) thrice. After the elimination of MeOH under reduced pressure, the extracts were partitioned between ethyl acetate (EtOAc) and H2O. After evaporation of EtOAc, the extract was fractionated by MPLC using ODS gel (Cosmosil 75C18-OPN, Nacalai Tesque Inc., Kyoto, Japan) with stepwise elution with 50%, 70%, 90%, and 100% MeOH [size of the column, 40 × 180 mm; each eluant volume, 700 mL]. The 90% MeOH eluate was subjected to an ODS column (Cosmosil 5C18-AR-II, 20 × 250 mm, Nacalai Tesque Inc., Kyoto, Japan) with 80% MeOH for 10 min, followed by a gradient elution from 80% to 100% MeOH for 50 min [flow rate, 4.0 mL/min; detection at 254 nm]. The fraction was further purified by an ODS column (Cosmosil 5C18-AR-II, 10 × 250 mm, Nacalai Tesque Inc., Kyoto, Japan) with 80% MeOH for 10 min, then a gradient elution from 80% to 100% MeOH for 50 min [flow rate, 2.0 mL/min; detection at 210 nm]. The final purification was performed via recycle HPLC using the reversed-phase column (CAPCELLPAK, 10 × 250 mm, Nacalai Tesque Inc., Kyoto, Japan) with 75% Acetonitrile (MeCN) [flow rate, 2.0 mL/min; detection at 210 nm]. Compound 1 (3.88 mg) was isolated at a retention time of 8.7 min. Compound (1); white solid, [α −4.17 (c 0.72, MeOH).
3.4. Preparation of Compound (2)
A solution of triethylamine/MeOH (1:4) was added to the compound (1, 1.08 mg) and the mixture was stirred at 20 °C for 46 h [9]. The reaction mixture was partitioned between hexane and 80% MeOH. Compound (2, 0.4 mg) was obtained in the aq. MeOH layer.
Supplementary Materials
The following supporting information can be downloaded online: Figure S1: HR-ESI-MS of compound (1) (negative); Figure S2: 1H-NMR spectrum of compound (1) (acetone-d6, 600 MHz); Figure S3: 13C-NMR spectrum of compound (1) (acetone-d6, 150 MHz); Figure S4: 1H-1H COSY spectrum of compound (1) (acetone-d6, 600 MHz); Figure S5: HSQC spectrum of compound (1) (acetone-d6, 600 MHz); Figure S6: HMBC spectrum of compound (1) (acetone-d6, 600 MHz); Figure S7: NOESY spectrum of compound (1) (acetone-d6, 600 MHz).
Author Contributions
Conceptualisation, H.N. and M.S.; isolation, M.H.; structure analysis, B.Z., M.H., R.K. and M.S.; MS analysis, H.U.; writing—original draft preparation, M.S., B.Z. and R.K.; writing—review and editing, H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the JSPS KAKENHI (grant number 22K05817 for H. Nagai; grant numbers 23H01962 and 24K08613 for M.S.) and JST SPRING (grant number JPMJSP2147 for B.Z.).
Data Availability Statement
The spectroscopic data presented in this study are available as Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Parchem, K.; Letsiou, S.; Petan, T.; Oskolkova, O.; Medina, I.; Kuda, O.; O’Donnell, V.B.; Nicolaou, A.; Fedorova, M.; Bochkov, V.; et al. Oxylipin profiling for clinical research: Current status and future perspectives. Prog. Lipid Res. 2024, 95, 101276. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.L.; Richel, D.J.; Ritsema, T.; Peppelenbosch, M.P.; Versteeg, H.H. Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 2004, 36, 1187–1205. [Google Scholar] [CrossRef] [PubMed]
- A Howe, G.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Kanda, N.; Zhang, B.T.; Kamio, M.; Uchida, H.; Sugahara, K.; Nagai, H.; Satake, M. Okeanic acid–A, a trihydroxy fatty acid from the Okinawan cyanobacterium Okeania hirsuta. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Zhang, B.-T.; Uchida, H.; Kamio, M.; Nagai, H.; Satake, M. (10E,15Z)-12-(Dimethylsulfonio)-9,13-dihydroxyoctadeca-10,15-dienoate. Molbank 2024, 2024, M1784. [Google Scholar] [CrossRef]
- Smith, M.W.; Brown, R.; Smullin, S.; Eager, J. Photosensitized peroxidation of lipids: An Experiment Using 1H-NMR. J. Chem. Edu. 1997, 74, 1471–1473. [Google Scholar] [CrossRef]
- Kikuchi, H.; Tsukitani, Y.; Manda, T.; Fujii, T.; Nakanishi, H.; Kobayashi, M.; Kitagawa, I. Marine Natural Products. X. Pharmacological Active Glycolipids from the Okinawan Marine Sponge Phyllospongia foliascens (Pallas). Chem. Pharm. Bull. 1982, 30, 3544–3547. [Google Scholar] [CrossRef]
- Han, C.; Yamano, Y.; Kakiuchi, F.; Nakamura, K.; Uemura, D. Grubbs carbene complex-catalyzed cleavage of allyl vic-diols to aldehydes with a co-oxidant application to the selective cleavage of huge marine molecules. Tetrahedron 2011, 67, 9622–9626. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).