Synthesis of an Insulated Oligo(phenylene ethynylene) Dimer Through Cyclodextrin-Based [c2]Daisy Chain Rotaxane
Abstract
1. Introduction
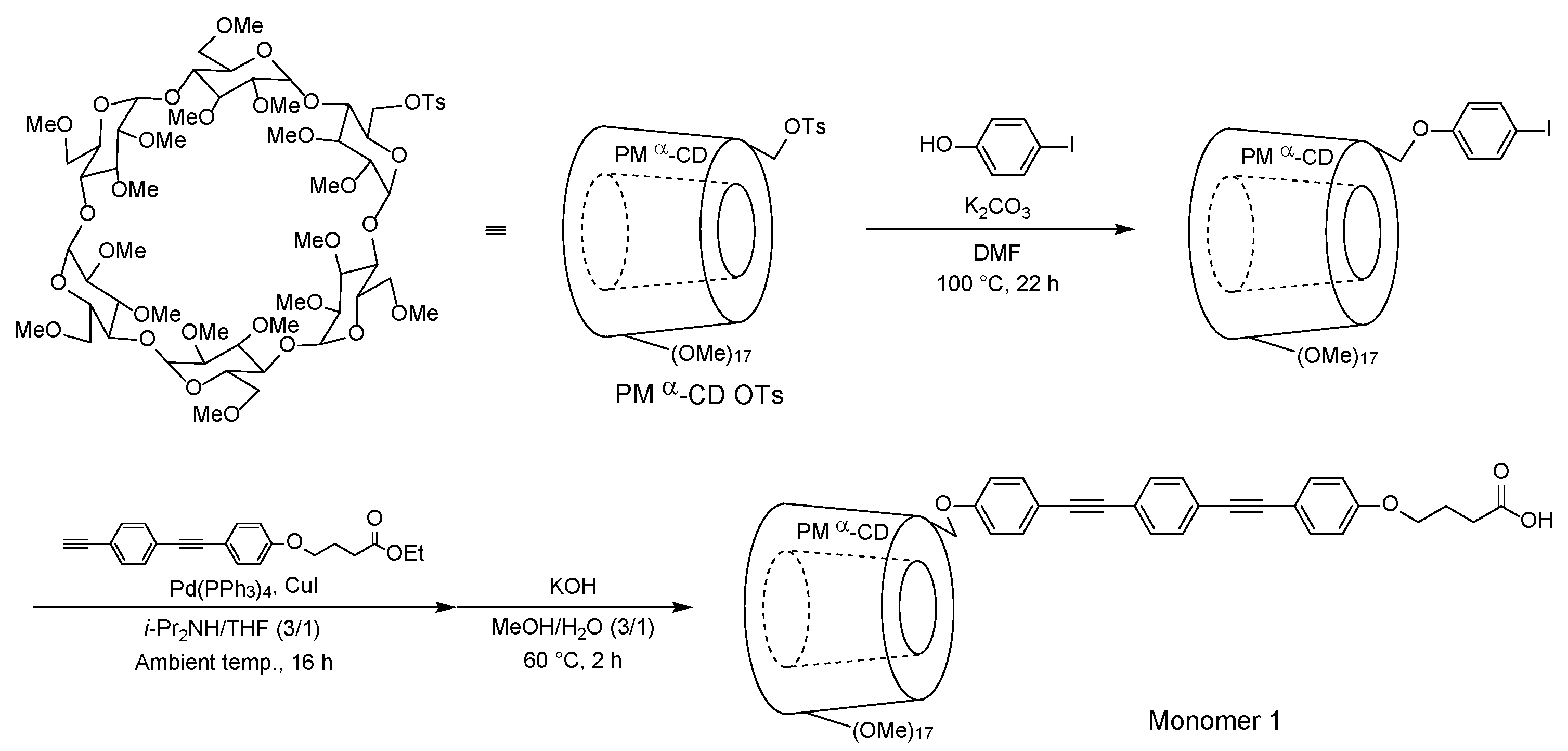
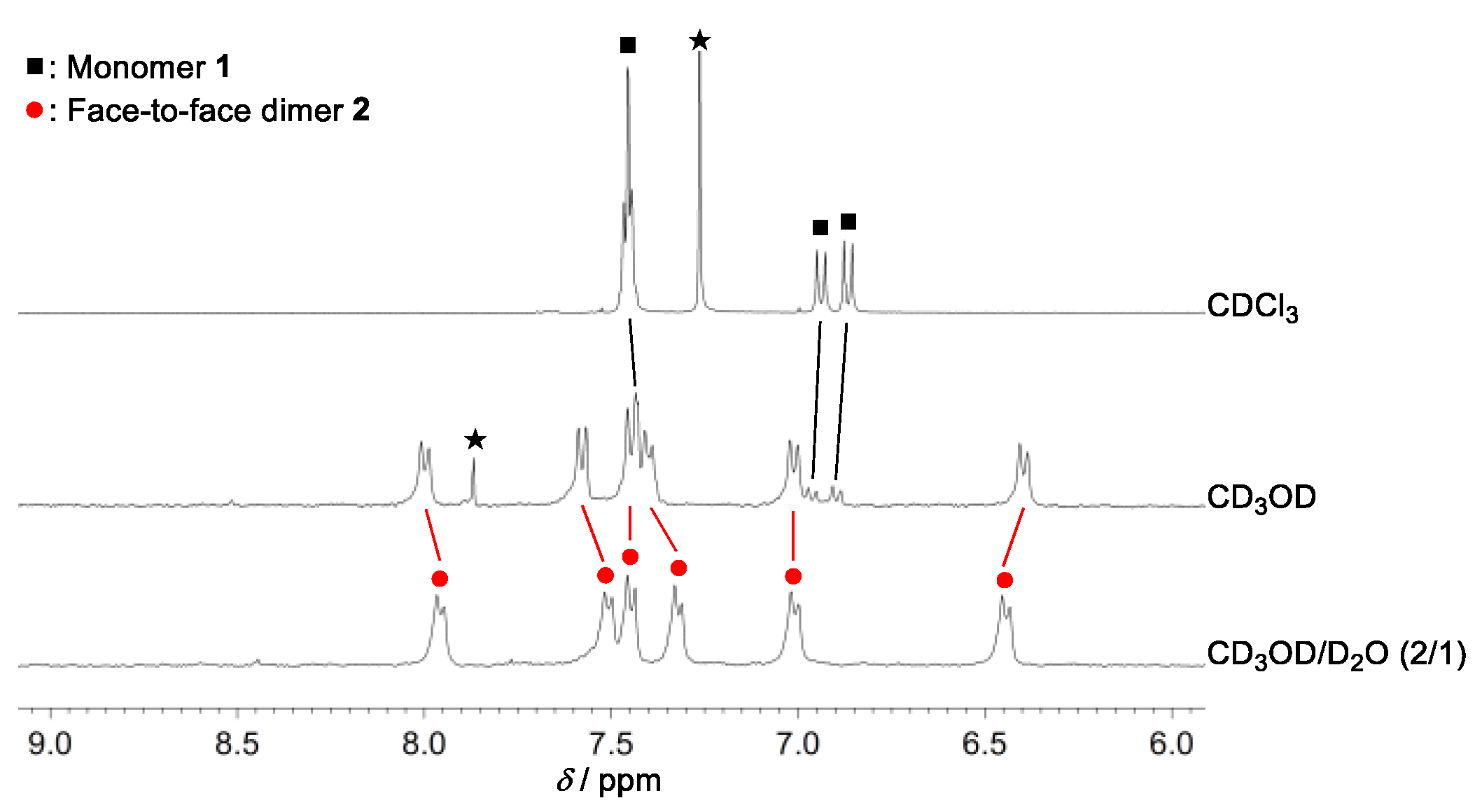
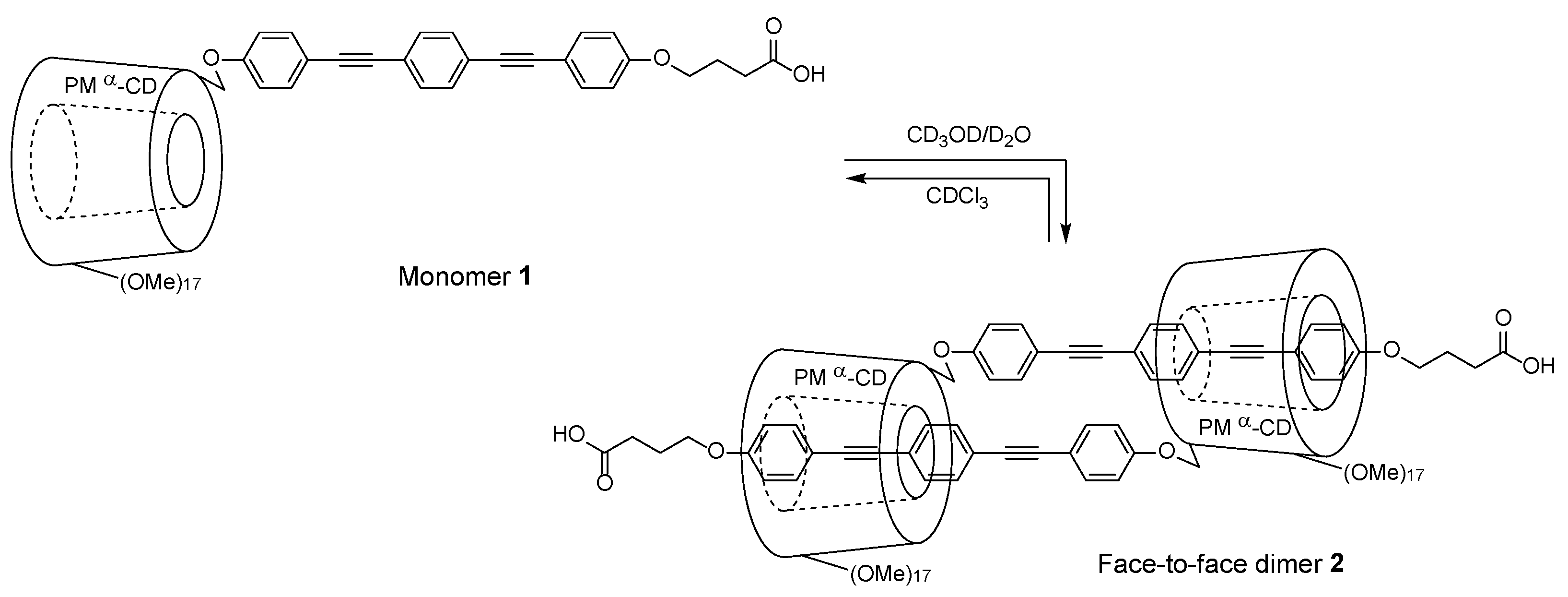
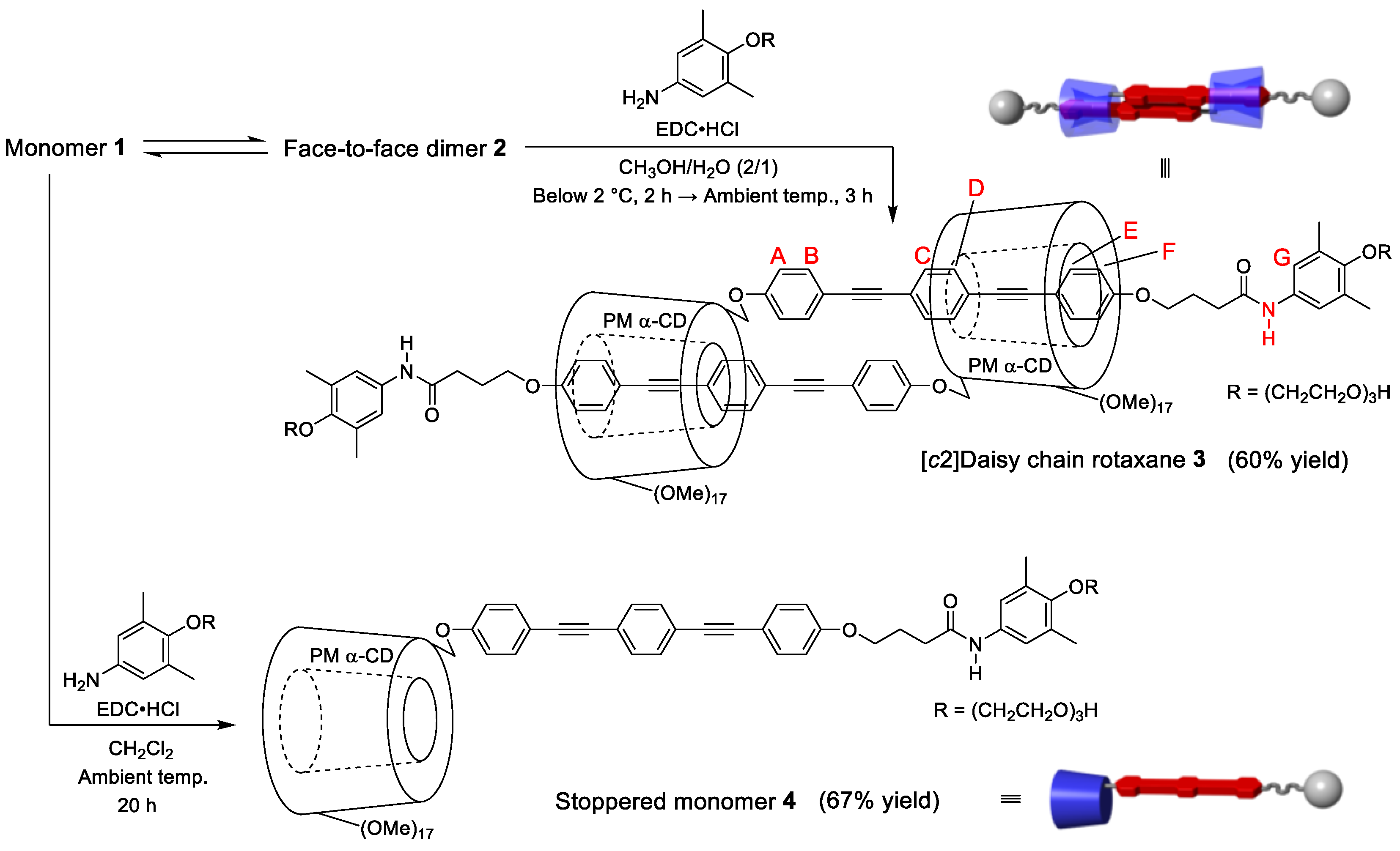
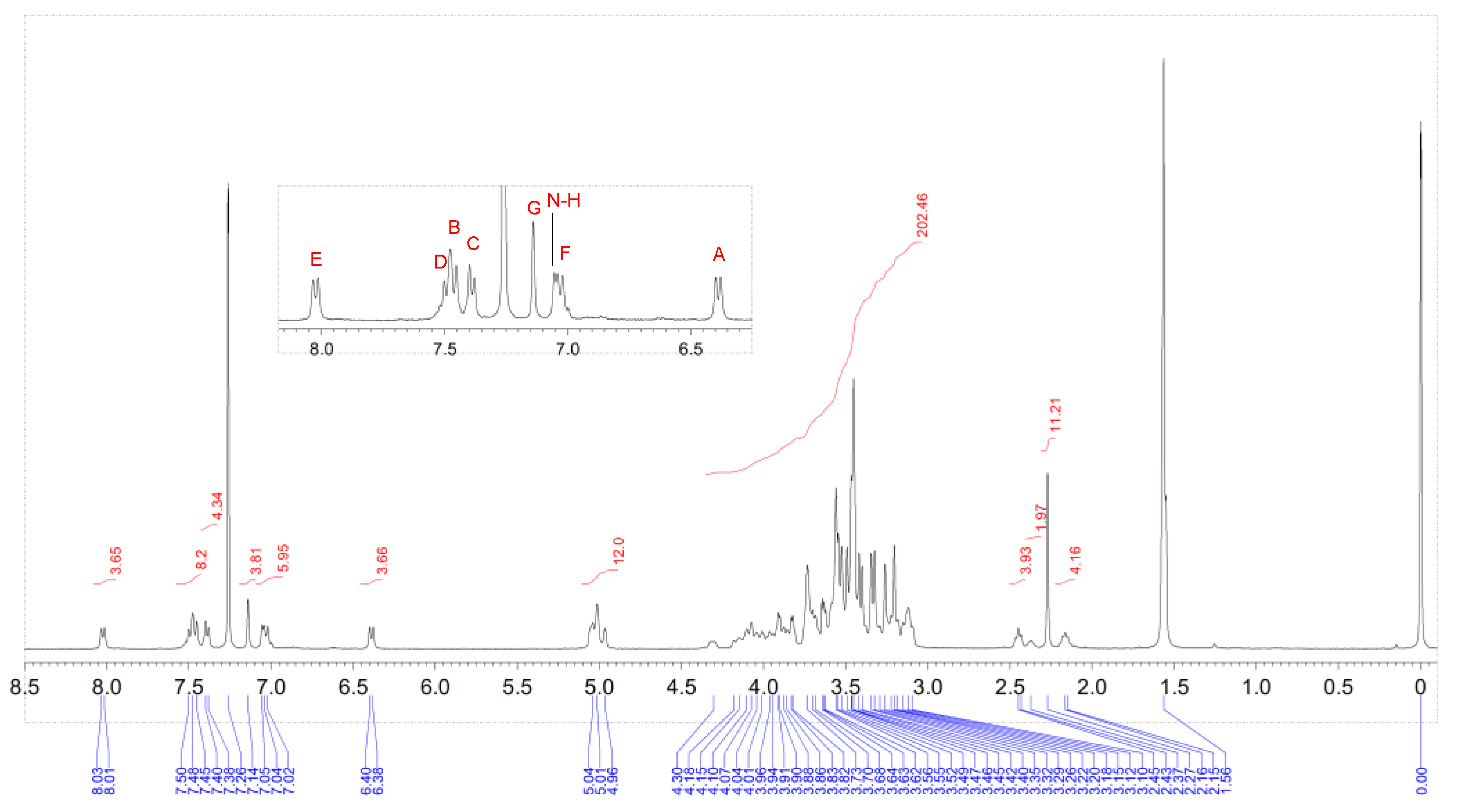
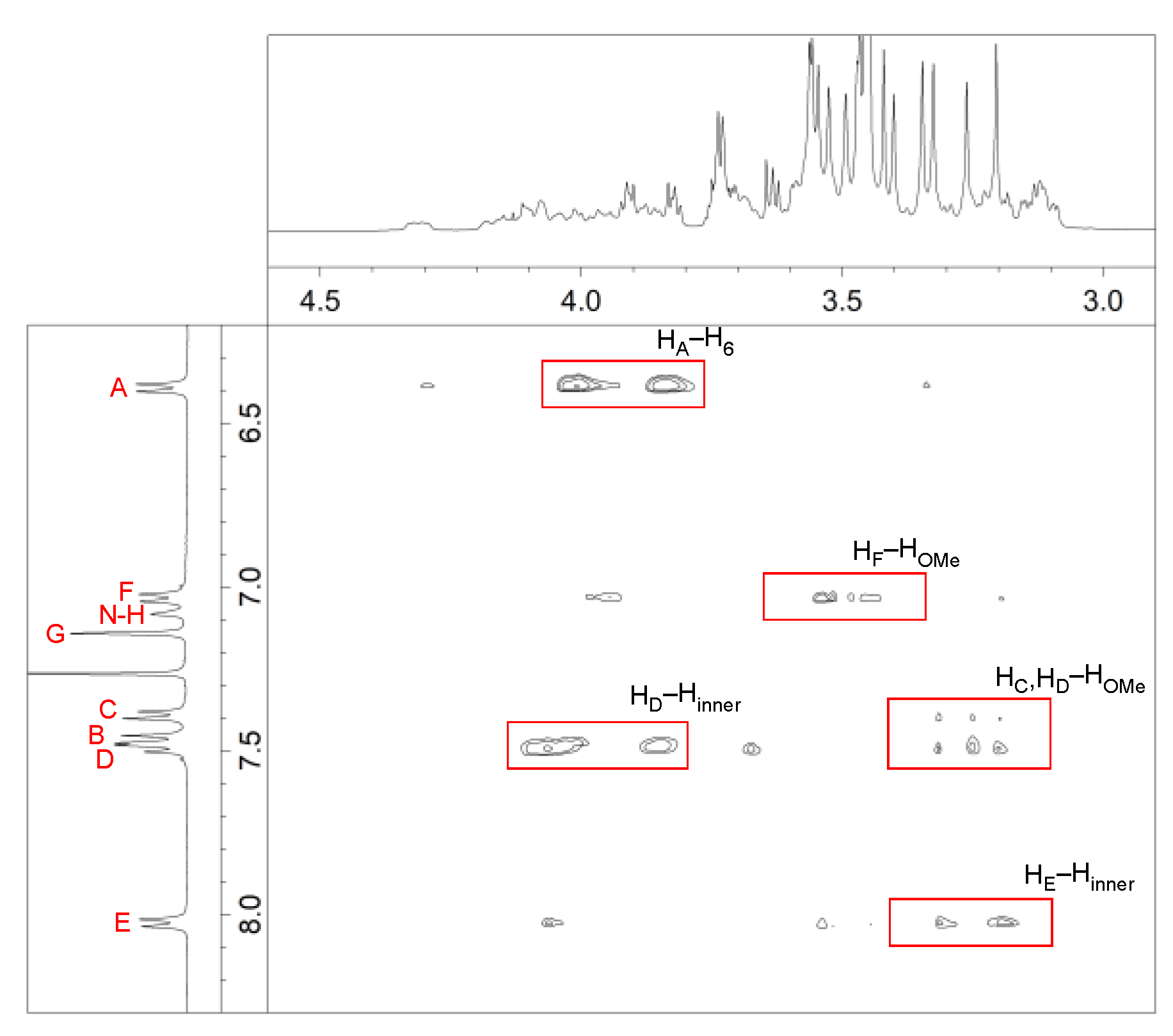
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaya, K.; Khalil, M.; Chi, E.Y. An effective approach to the disinfection of pathogens: Cationic conjugated polyelectrolytes and oligomers. ACS Appl. Bio Mater. 2023, 6, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Seo, S.E.; Park, C.S.; Kim, S.; Lee, S.; Ryu, C.-M.; Yong, D.; Park, Y.M.; Kwon, O.S. Open-bandgap graphene-based field-effect transistor using oligo(phenylene-ethynylene) interfacial chemistry. Angew. Chem. Int. Ed. 2022, 61, e202209726. [Google Scholar] [CrossRef]
- McCuskey, S.R.; Chatsirisupachai, J.; Zeglio, E.; Parlak, O.; Panoy, P.; Herland, A.; Bazan, G.C.; Nguyen, T.-Q. Current progress of interfacing organic semiconducting materials with bacteria. Chem. Rev. 2022, 122, 4791–4825. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pitie, S.; Liu, C.; Zhao, C.; Zhao, C.; Seydou, M.; Dappe, Y.J.; Nichols, R.J.; Yang, L. Asymmetric effect on the length dependence of oligo(phenylene ethynylene)-based molecular junctions. J. Phys. Chem. C 2022, 126, 3635–3645. [Google Scholar] [CrossRef]
- O’Driscoll, L.J.; Bryce, M.R. A review of oligo(arylene ethynylene) derivatives in molecular junctions. Nanoscales 2021, 13, 10668. [Google Scholar] [CrossRef]
- Gangemi, C.M.A.; Barattucci, A.; Bonaccorsi, P.M. A portrait of the OPE as a biological agent. Molecules 2021, 26, 3088. [Google Scholar] [CrossRef]
- Chen, H.; Sangtarash, S.; Li, G.; Gantenbein, M.; Cao, W.; Alqorashi, A.; Liu, J.; Zhang, C.; Zhang, Y.; Chen, L.; et al. Exploring the thermoelectric properties of oligo(phenylene-ethynylene) derivatives. Nanoscale 2020, 12, 15150–15156. [Google Scholar] [CrossRef]
- Wang, B.; Queenan, B.N.; Wang, S.; Nilsson, K.P.R.; Bazan, G.C. Precisely defined conjugated oligoelectrolytes for biosensing and therapeutics. Adv. Mater. 2019, 31, 1806701. [Google Scholar] [CrossRef]
- Whitten, D.G.; Tang, Y.; Zhou, Z.; Yang, J.; Wang, Y.; Hill, E.H.; Pappas, H.C.; Donabedian, P.L.; Chi, E.Y. A retrospective: 10 Years of oligo(phenylene-ethynylene) Electrolytes: Demystifying nanomaterials. Langmuir 2019, 35, 307–325. [Google Scholar] [CrossRef]
- Dubois, V.; Raja, S.N.; Gehring, P.; Caneva, S.; van der Zant, H.S.J.; Niklaus, F.; Stemme, G. Massively parallel fabrication of crack-defined gold break junctions featuring sub-3 nm gaps for molecular devices. Nature Commun. 2018, 9, 3433. [Google Scholar] [CrossRef]
- Miao, R.; Xu, H.; Skripnik, M.; Cui, L.; Wang, K.; Pedersen, K.G.L.; Leijnse, M.; Pauly, F.; Wärnmark, K.; Meyhofer, E.; et al. Influence of quantum interference on the thermoelectric properties on molecular junctions. Nano Lett. 2018, 18, 5666–5672. [Google Scholar] [CrossRef] [PubMed]
- Barattucci, A.; Deni, E.; Bonaccorsi, P.; Ceraolo, M.G.; Papalia, T.; Santoro, A.; Sciortino, M.T.; Puntoriero, F. Oligo(phenylene ethynylene) glucosides: Modulation of cellular uptake capacity preserving light ON. J. Org. Chem. 2014, 79, 5113–5120. [Google Scholar] [CrossRef] [PubMed]
- Wegelin, S.; Meier, M.A.R. Solution self-assembly of branched macromolecules obtained via iterative OPE synthesis and the Passerini three-component reaction. Macromol. Chem. Phys. 2024, 225, 2300337. [Google Scholar] [CrossRef]
- López-Gandul, L.; Morón-Blanco, A.; García, F.; Sánchez, L.L. Supramolecular block copolymers from tricarboxamides. Biasing co-assembly by the incorporation of pyridine rings. Angew. Chem. Int. Ed. 2023, 62, e202308749. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Matsuda, W.; Seki, S. Solid-solid transition of microcrystalline oligo(phenylene ethynylene)s: Impact of crystalline structure on optoelectronic properties. Chem. Phys. Lett. 2022, 801, 139709. [Google Scholar] [CrossRef]
- Graciano, E.E.; Calbo, J.; Buendía, J.; Cerdá, J.; Aragó, J.; Ortí, E.; Sánchez, L. Decoding the consequences of increasing the size of self-assembling tricarboxamides on chiral amplification. J. Am. Chem. Soc. 2019, 141, 7463–7472. [Google Scholar] [CrossRef]
- Chou, S.-Y.; Masai, H.; Tsuda, S.; Terao, J. Synthetic methodology for structurally defined and insulated molecular wires bearing non-centrosymmetric conjugated axle components via iterative intramolecular slippage. Chem. Asian J. 2019, 14, 1667–1671. [Google Scholar] [CrossRef]
- Masai, H.; Fujihara, T.; Tsuji, Y.; Terao, J. Programmed synthesis of molecular wires with fixed insulation and defined length based on oligo(phenylene ethynylene) and permethylated α-cyclodextrins. Chem. Eur. J. 2017, 23, 15073–15079. [Google Scholar] [CrossRef]
- Ahner, J.; Micheel, M.; Enke, M.; Zechel, S.; Schubert, U.S.; Dietzek, B.; Hager, M.D. Directed orientation of oligo(phenylene ethynylene)s using ureas or urethanes in rod-coil copolymers. Macromol. Chem. Phys. 2017, 218, 1700343. [Google Scholar] [CrossRef]
- Poppe, M.; Chen, C.; Liu, F.; Poppe, S.; Tschierske, C. Formation of a cubic liquid crystallin nanostructure with π-conjugated fluorinated rods on the gyroid minimal surface. Chem. Eur. J. 2017, 23, 7196–7200. [Google Scholar] [CrossRef]
- Zhang, S.; Geng, Y.; Fan, Y.; Duan, W.; Deng, K.; Zhao, D.; Zeng, Q. Two-dimensional (2D) self-assembly of oligo(phenylene-ethynylene) molecules and their triangular platinum(II) diamine complexes studied using STM. Phys. Chem. Chem. Phys. 2017, 46, 31284–31289. [Google Scholar] [CrossRef] [PubMed]
- Nuermaimaiti, A.; Bombis, C.; Knudsen, M.M.; Cramer, J.R.; Lægsgaard, E.; Besenbacher, F.; Gotheld, K.V.; Linderoth, T.R. Chiral induction with chiral conformational switches in the limit of low “sergeants to soldiers” ratio. ACS Nano 2014, 8, 8074–8081. [Google Scholar] [CrossRef] [PubMed]
- Jester, S.-S.; Schmitz, D.; Eberhagen, F.; Höger, S. Self-assembled monolayers of clamped oligo(phenylene-ethynylene-butadiynylene)s. Chem. Commun. 2011, 47, 8838–8840. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Kawakita, K.; Yano, Y.; Yasumura, N.; Fujiwara, S.; Nishiyama, Y. Encapsulation of cofacial diarylacetylene dimers using [c2]daisy chain rotaxane strategy. Heterocycles 2023, 106, 2074–2083. [Google Scholar] [CrossRef]
- Tsuda, S.; Komai, Y.; Fujiwara, S.; Nishiyama, Y. Cyclodextrin-based [c2]daisy chain rotaxane insulating two diarylacetylene cores. Chem. Eur. J. 2021, 27, 1966–1969. [Google Scholar] [CrossRef]
- Kaneda, T.; Fujimoto, T.; Goto, J.; Asano, K.; Yasufuku, Y.; Jung, J.H.; Hosono, C.; Sakata, Y. New large-scale preparations of versatile 6-O-monotosyl and 6-monohydroxy permethylated α-, β-, and γ-cyclodextrins. Chem. Lett. 2002, 31, 514–515. [Google Scholar] [CrossRef]
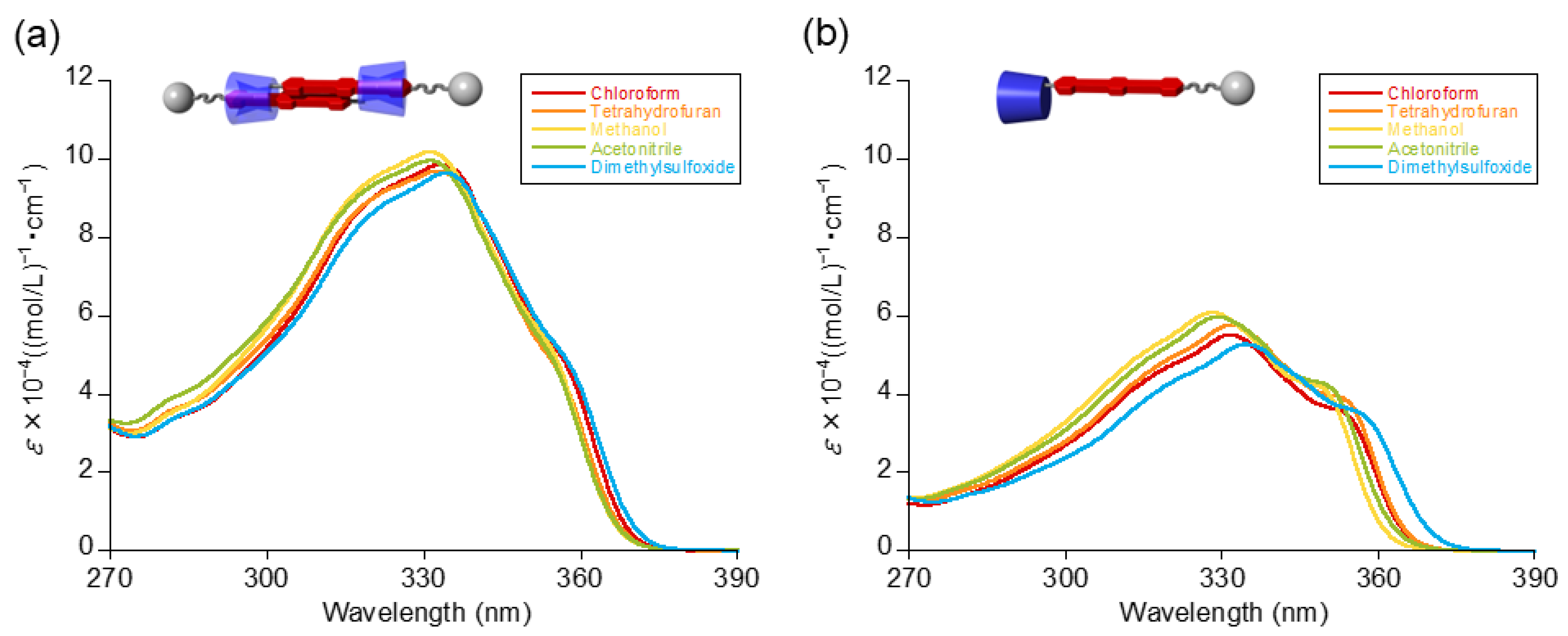
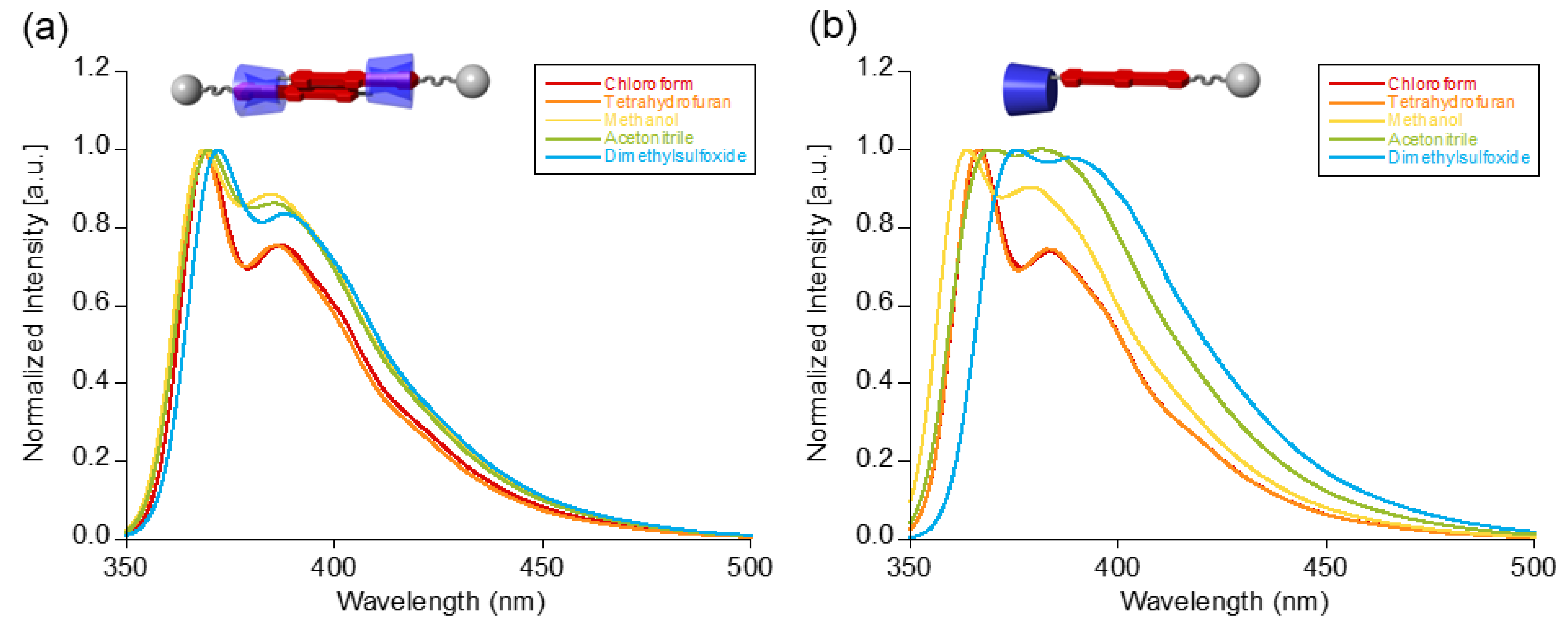
| Solvent | Maximum Absorption Wavelengths (nm) 1 | |
|---|---|---|
| [c2]Daisy Chain Rotaxane 3 2 | Stoppered Monomer 4 3 | |
| Chloroform | 333 (9.87 × 104) | 332 (5.51 × 104) |
| Tatrahydrofuran | 333 (9.70 × 104) | 332 (5.77 × 104) |
| Methanol | 331 (10.2 × 104) | 328 (6.08 × 104) |
| Acetonitrile | 331 (9.97 × 104) | 329 (5.98 × 104) |
| Dimethylsulfoxide | 334 (9.64 × 104) | 335 (5.28 × 104) |
| Solvent | Maximum Emission Wavelengths (nm) 1 | |
|---|---|---|
| [c2]Daisy Chain Rotaxane 3 2 | Stoppered Monomer 4 3 | |
| Chloroform | 369, 387 | 366, 383 |
| Tatrahydrofuran | 368, 386 | 366, 383 |
| Methanol | 369, 385 | 364, 380 |
| Acetonitrile | 370, 385 | 369, 382 |
| Dimethylsulfoxide | 372, 388 | 376, 388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuda, S.; Yasumura, N.; Fujiwara, S.-i.; Nishiyama, Y. Synthesis of an Insulated Oligo(phenylene ethynylene) Dimer Through Cyclodextrin-Based [c2]Daisy Chain Rotaxane. Molbank 2024, 2024, M1906. https://doi.org/10.3390/M1906
Tsuda S, Yasumura N, Fujiwara S-i, Nishiyama Y. Synthesis of an Insulated Oligo(phenylene ethynylene) Dimer Through Cyclodextrin-Based [c2]Daisy Chain Rotaxane. Molbank. 2024; 2024(4):M1906. https://doi.org/10.3390/M1906
Chicago/Turabian StyleTsuda, Susumu, Naoto Yasumura, Shin-ichi Fujiwara, and Yutaka Nishiyama. 2024. "Synthesis of an Insulated Oligo(phenylene ethynylene) Dimer Through Cyclodextrin-Based [c2]Daisy Chain Rotaxane" Molbank 2024, no. 4: M1906. https://doi.org/10.3390/M1906
APA StyleTsuda, S., Yasumura, N., Fujiwara, S.-i., & Nishiyama, Y. (2024). Synthesis of an Insulated Oligo(phenylene ethynylene) Dimer Through Cyclodextrin-Based [c2]Daisy Chain Rotaxane. Molbank, 2024(4), M1906. https://doi.org/10.3390/M1906






