2α-Methyl-5α-androstan-17β-ol-3-one-17β-heptanoate
Abstract
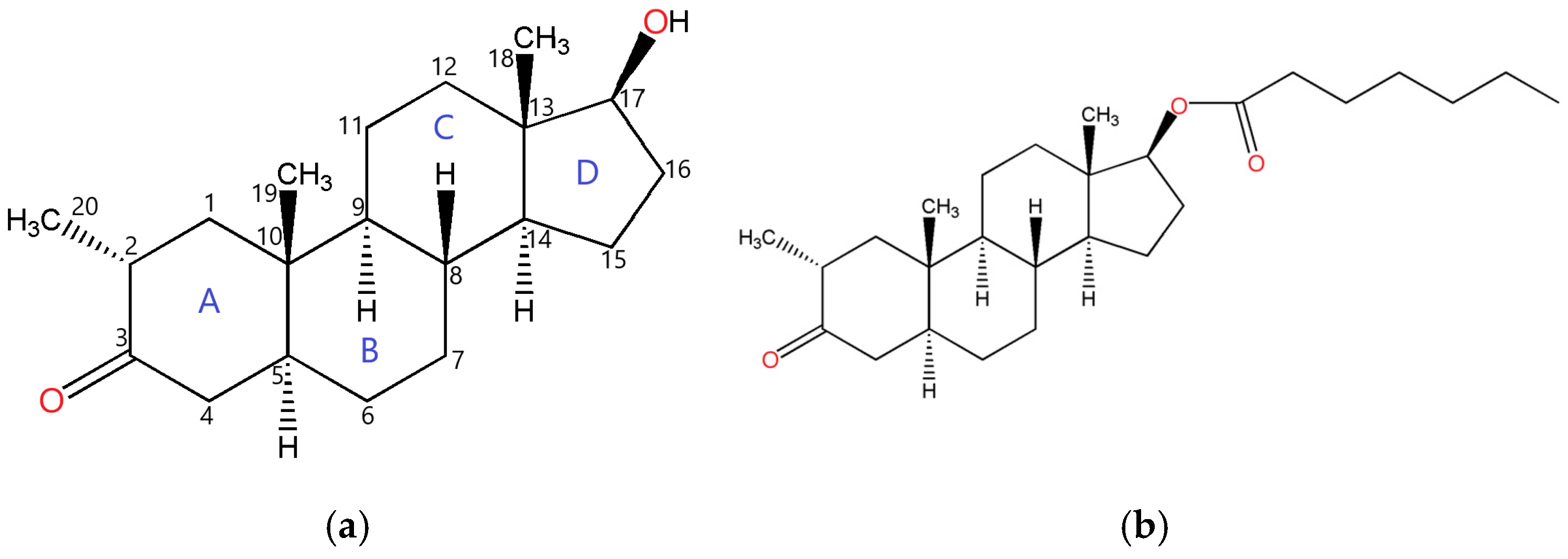
1. Introduction
2. Results
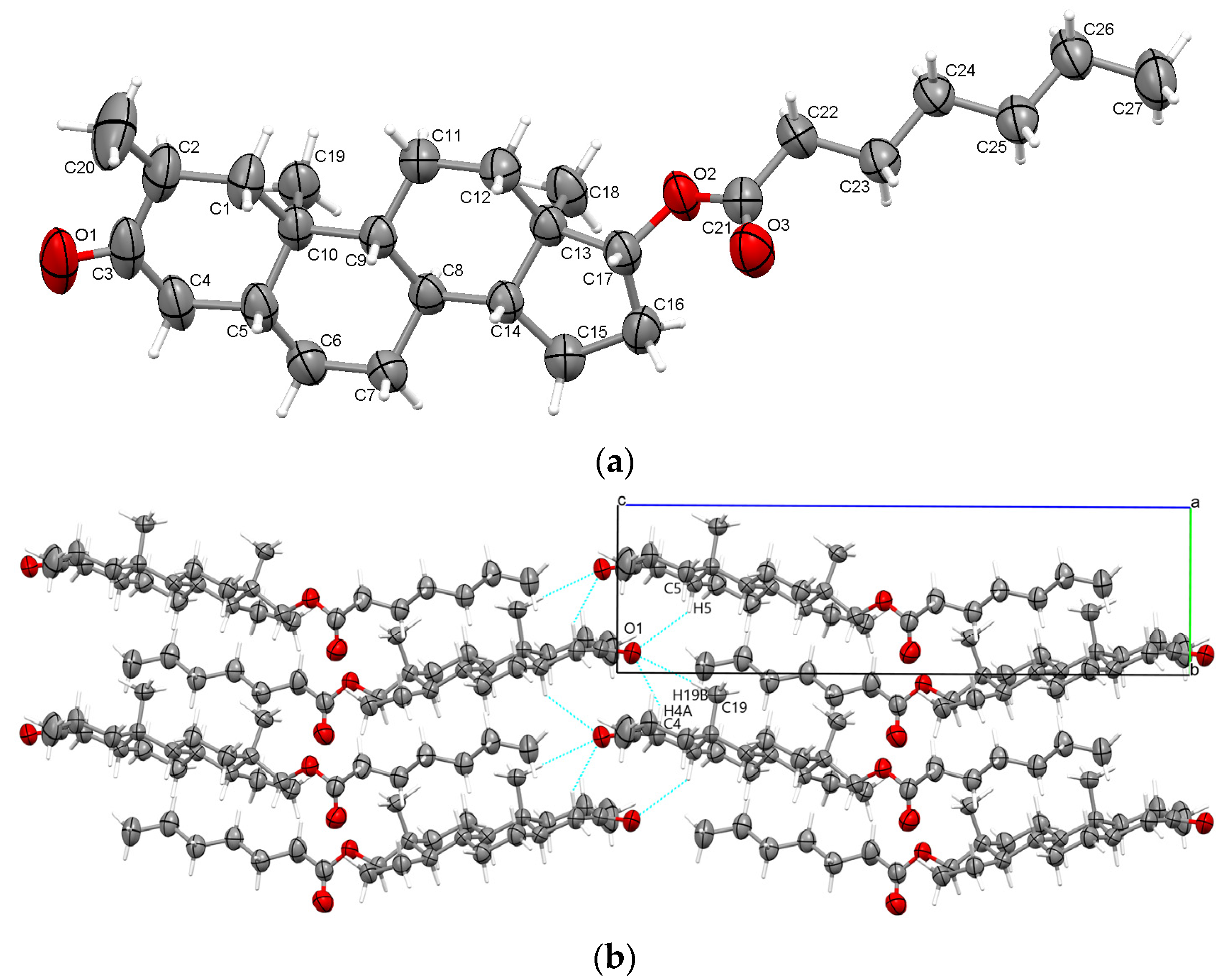
2.1. Crystal Structure Analysis of Drostanolone Enanthate
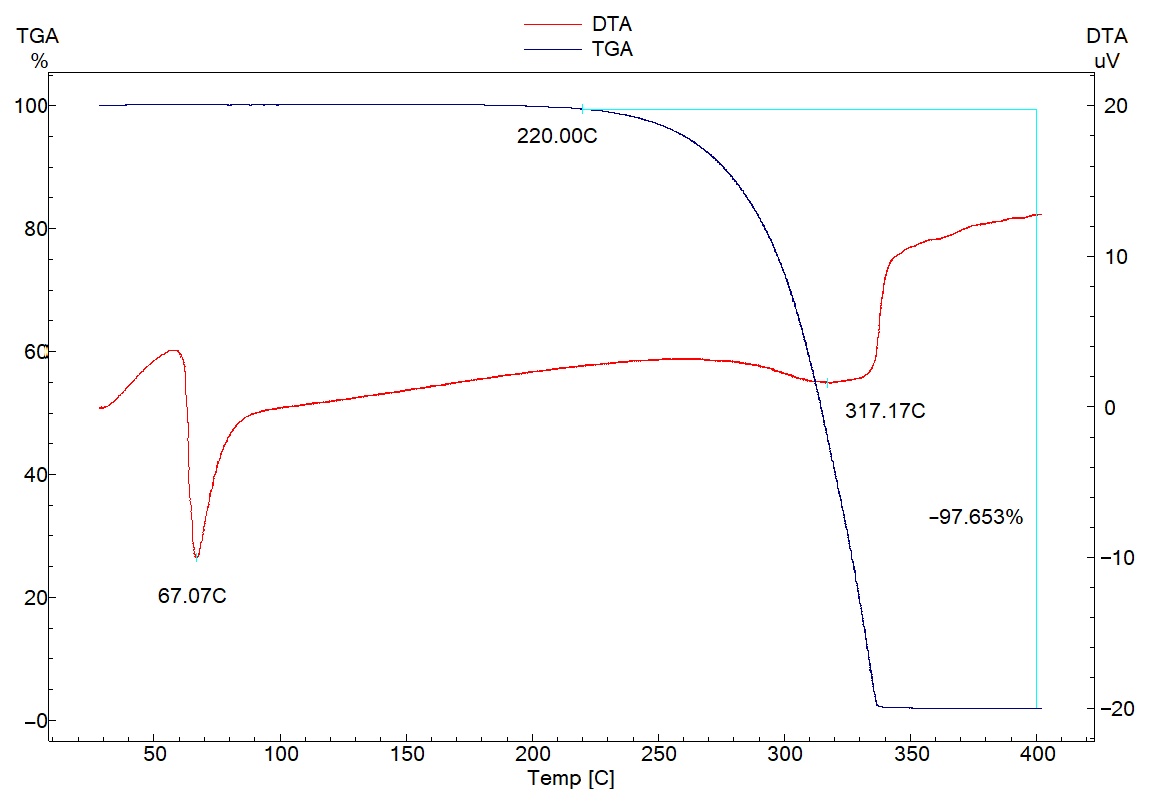
2.2. Thermal DTA/TGA
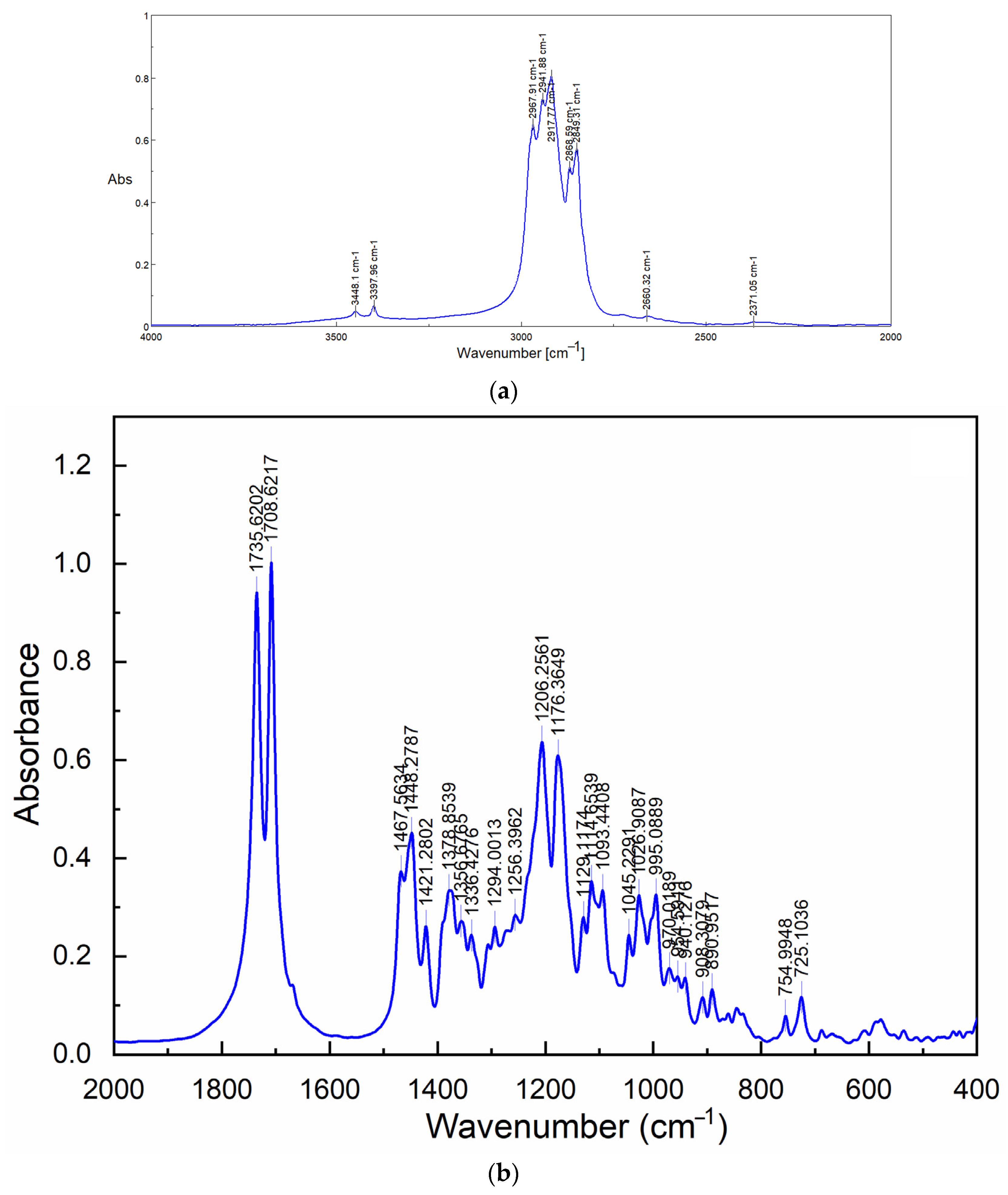
2.3. FT-IR Spectroscopy Results
3. Materials and Methods
3.1. General and Sample Preparations
3.2. X-Ray Diffraction and Structure Refinement
3.3. DTA/TGA Thermal Analysis
3.4. FT-IR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lednicer, D. Steroid Chemistry at a Glance, 1st ed.; Wiley: Chichester, UK, 2010. [Google Scholar]
- Mooradian, A.D.; Morley, J.E.; Korenman, S.G. Biological actions of androgens. Endocr. Rev. 1987, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, N.T. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin. Ther. 2001, 23, 1355–1390. [Google Scholar] [CrossRef] [PubMed]
- Kicman, A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008, 154, 502–521. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A. Longacting steroid preparations. Acta Clin. Belg. 1975, 30, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.S.; Banks, A.J.; Bond, W.H.; Jones, W.G.; Ward, H.W. A comparison of drostanolone propionate (Masteril) and nandrolone decanoate (Deca-durabolin) in the treatment of breast carcinoma. Clin. Oncol. 1976, 2, 203–206. [Google Scholar] [PubMed]
- Turza, A.; Borodi, G.; Muresan-Pop, M.; Ulici, A. Polymorphism and β-cyclodextrin complexation of methyldrostanolone. J. Mol. Struct. 2022, 1250, 131852. [Google Scholar] [CrossRef]
- Borodi, G.; Turza, A.; Bende, A. Exploring the Polymorphism of Drostanolone Propionate. Molecules 2020, 25, 1436. [Google Scholar] [CrossRef] [PubMed]
- Turza, A.; Miclaus, M.O.; Borodi, G. Exploring the crystal and molecular structures of methenolone and drostanolone enanthate. Z. Kristallogr. Cryst. Mater. 2024, 239, 129–138. [Google Scholar] [CrossRef]
- Chistyakov, D.; Sergeev, G. The polymorphism of Drugs: New Approaches to the Synthesis of Nanostructured Polymorphs. Pharmaceutics 2020, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Rendle, D.F.; Trotter, J. Crystal and molecular structure of 17β-hydroxy-17α-methyl-2-oxa-5α -androstan-3-one. J. Chem. Soc. Perkin II 1975, 1361–1365. [Google Scholar] [CrossRef]
- Gaedecki, Z. Structure of 17-α-methyl-testosterone semihydrate C20H30O2·1/2H2O. J. Crystallogr. Spectrosc. Res. 1989, 19, 577–587. [Google Scholar] [CrossRef]
- Gaedecki, Z. Structure of 17β-hydroxy-1,4-androstadien-3-one monohydrate C19H26O2·H2O. J. Crystallogr. Spectrosc. Res. 1989, 19, 933–939. [Google Scholar] [CrossRef]
- Turza, A.; Miclaus, M.O.; Pop, A.; Borodi, G. Crystal and molecular structures of boldenone and four boldenone steroid esters. Z. Kristallogr. Cryst. Mater. 2019, 234, 671–683. [Google Scholar] [CrossRef]
- Precioux, G.; Barrans, Y.; Hospital, M. 17-BETA HYDROXY-4,9,11 ESTRATRIENE-3 ONE,C18H22O2. Cryst. Struct. Commun. 1989, 8, 883–886. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edigington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, J.v.d.S.; Wood, P.A. Mercury CSD 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. 2013, B69, 249–259. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis PRO. Rigaku Oxford Diffraction; CrysAlis PRO: Yarnton, UK, 2015. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
| Identification Code | Drostanolone Enanthate |
|---|---|
| Empirical formula | C27H44O3 |
| Formula weight | 416.62 |
| Temperature/K | 293 (2) |
| Crystal system | monoclinic |
| Space group | P21 |
| a/Å | 9.0864 (5) |
| b/Å | 6.3760 (3) |
| c/Å | 21.8006 (8) |
| α/° | 90 |
| β/° | 90.765 (4) |
| γ/° | 90 |
| Volume/Å3 | 1262.90 (10) |
| Z | 2 |
| ρcalc g/cm3 | 1.096 |
| μ/mm−1 | 0.533 |
| F(000) | 460.0 |
| Radiation | Cu Kα (λ = 1.54184) |
| 2Θ range/° | 8.112 to 142.73 |
| Index ranges | −11 ≤ h ≤ 11, −7 ≤ k ≤ 7, −26 ≤ l ≤ 26 |
| Reflections collected | 16,129 |
| Independent reflections | 4728 [Rint = 0.0462, Rsigma = 0.0403] |
| Data/restraints/parameters | 4728/1/275 |
| Goodness of fit on F2 | 1.025 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0517, wR2 = 0.1226 |
| Final R indexes [all data] | R1 = 0.0758, wR2 = 0.1452 |
| Largest diff. peak/hole/e Å−3 | 0.13/−0.21 |
| Flack’s parameter | 0.15 (18) |
| D-H···A | D-H | H···A | D···A | <(D-H···A) |
|---|---|---|---|---|
| C5-H5···O1 | 1.089 | 2.683 | 3.525 | 133.7 |
| C19-H19B···O1 | 1.089 | 2.603 | 3.688 | 174.2 |
| C4-H4B···O1 | 1.089 | 2.506 | 3.277 | 126.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turza, A.; Muresan-Pop, M.; Miclaus, M.-O.; Borodi, G. 2α-Methyl-5α-androstan-17β-ol-3-one-17β-heptanoate. Molbank 2024, 2024, M1907. https://doi.org/10.3390/M1907
Turza A, Muresan-Pop M, Miclaus M-O, Borodi G. 2α-Methyl-5α-androstan-17β-ol-3-one-17β-heptanoate. Molbank. 2024; 2024(4):M1907. https://doi.org/10.3390/M1907
Chicago/Turabian StyleTurza, Alexandru, Marieta Muresan-Pop, Maria-Olimpia Miclaus, and Gheorghe Borodi. 2024. "2α-Methyl-5α-androstan-17β-ol-3-one-17β-heptanoate" Molbank 2024, no. 4: M1907. https://doi.org/10.3390/M1907
APA StyleTurza, A., Muresan-Pop, M., Miclaus, M.-O., & Borodi, G. (2024). 2α-Methyl-5α-androstan-17β-ol-3-one-17β-heptanoate. Molbank, 2024(4), M1907. https://doi.org/10.3390/M1907








