Abstract
The unusual reactivity of key enamine intermediates led to the formation of 3-methylamino-2-(2-nitrobenzoyl)-4H-naphthalen-1-one as an unexpected product in an attempted synthesis of the P. aeruginosa metabolite 2-benzyl-4(1H)-quinolone. Although the synthesis of the natural product has not been successful, this methodology allows for the easy preparation of novel derivatives carrying a carboxamide moiety at the C3 position.
1. Introduction
2-Alkyl-4-(1H)-quinolones are an important class of bacterial secondary metabolites produced by various Pseudomonas and Burkholderia species [1]. While some of them, especially those that are oxidized at the quinolone nitrogen, act as antibiotics and directly suppress competing microorganisms [2], others have the more intricate function of chemical signals, coordinating the group behavior of their producers [3]. Such cell population density-dependent regulation is known as “quorum sensing” and is a matter of great research interest, as it may hold the key to novel antibacterial therapies [4,5]. Since the pioneering works of Wells et al., who for the first time isolated alkylquinolones from cultures of Pseudomonas aeruginosa and determined their structure by synthesis, [6,7] many other bacterial 2-alkyl-4(1H)-quinolones with various substituents in the heterocyclic ring have been discovered and synthesized [1,8]. The structural diversity of this class of natural compounds is impressive, but most often, at the C2 position of the quinolone core, they feature linear carbon chains of varying lengths and degrees of unsaturation. 2-Benzyl-4(1H)-quinolone (Figure 1) is one of the more unusual representatives of this class, which was isolated from P. aeruginosa BD06-03 by Clark et al. in 2020 [9] and synthesized later by the same research group [10].
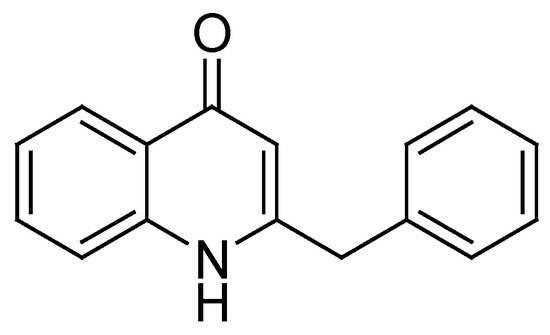
Figure 1.
Structure of 2-benzyl-4(1H)-quinolone.
2. Results
We considered the P. aeruginosa metabolite 2-benzyl-4(1H)-quinolone to be an interesting target and a good testing ground for our recently published method for the preparation of 2-alkyl-4(1H)-quinolones (Scheme 1) [11]. At the core of this method are intermediates III, which are readily prepared from the corresponding β-keto amides I. Compounds III are susceptible to decarbamoylation upon heating in H3PO4, which we have observed in a number of examples to give the corresponding β-enaminoketones IV in high yields [11,12]. Finally, the reduction of the nitro group in IV is followed by spontaneous cyclization to the corresponding 2-alkyl-4(1H)-quinolone V, with the elimination of an amine (R3-NH2). The type of the R3 substituent is not of decisive importance to this method, so it is most convenient to use an inexpensive lower aliphatic amine in this auxiliary role.
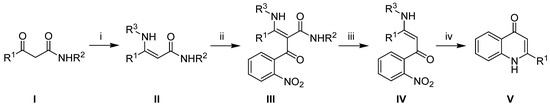
Scheme 1.
General method for the synthesis of 2-alkyl-4(1H)-quinolones (V): (i) R3NH2, CH2Cl2, Na2SO4, 24 h r.t.; (ii) NMM (1 equiv.), DMAP (0.2 equiv), 2-nitrobenzoyl chloride (1 equiv.), CH2Cl2, 2h, r.t.; (iii) H3PO4, 60 °C, 1–2 h; (iv) Zn/AcOH or H2, Pd/C.
With this in mind, three different γ-phenyl β-keto amides 1a–c were prepared [13] and condensed with methylamine to provide the corresponding β-enamino amides 2a–c in quantitative yields (Scheme 2). The acylation of 2a–c with 2-nitrobenzoyl chloride proceeded smoothly and resulted in the corresponding intermediates 3a–c with 65–74% yield.
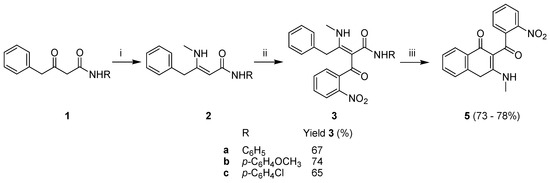
Scheme 2.
Reagents and conditions: (i) CH3NH2 (40% aq., 1.05–1.15 equiv.), CH2Cl2, Na2SO4, 24 h r.t.; (ii) NMM (1 equiv.), DMAP (0.2 equiv), 2-nitrobenzoyl chloride (1 equiv.), CH2Cl2, 2 h, r.t.; (iii) H3PO4, 60 °C, 2 h.
In the next stage, however, the attempted decarbamoylation of 3 in neat H3PO4 at 60 °C did not give the expected result with any of the intermediates 3a–c. In all three cases, cyclization to the same product 5 was observed, with the elimination of the corresponding amine (RNH2) and no trace of the expected product 4 (Scheme 3). The yields of the unexpected product 3-methylamino-2-(2-nitrobenzoyl)-4H-naphthalen-1-one 5 were consistently found to be within the range of 73–78%, regardless of the R substituent. The structure of 5 was elucidated on the basis of NMR and MS data (see Supplementary Materials) and could be a result of either an intramolecular Friedel–Crafts reaction or a 6π–electrocyclic process, occurring in a tautomer of 3, possessing the prerequisite conjugated π system. Interestingly, the protonated molecular ion of 5 was observed as the base peak in the mass spectra of intermediates 3a and 3c, with abundance exceeding that of the actual [M+H]+ ions of 3. The base peak for intermediate 3b corresponded to its own [M+H]+, but the protonated molecular ion of 5 was still present with high abundancy (50%), indicating that the cyclization depicted in Scheme 3 is also a favored process under the conditions of positive electrospray ionization.
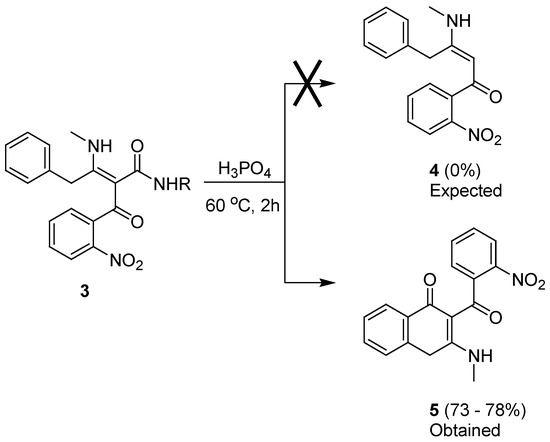
Scheme 3.
Expected and observed reactions of intermediates 3 in H3PO4.
Even though this unexpected reactivity of the benzyl-substituted intermediates 3 did not allow us to accomplish the planned synthesis of 2-benzyl-4(1H)-quinolone, we were still in the position to prepare derivatives of this natural product, carrying a carboxamide moiety at the C3 position of the quinolone core. This was easily achieved by the reduction of the nitro group in intermediates 3 with Zn in AcOH/CH2Cl2, which was followed by spontaneous cyclization to carboxamide derivatives 6 with 71–74% yield (Scheme 4).
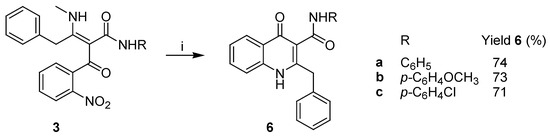
Scheme 4.
Preparation of 3-carboxamide derivatives of 2-benzyl-4(1H)-quinolone (6). Reagents and conditions: (i) Zn/AcOH/CH2Cl2, r.t., 24 h.
In conclusion, we encountered an unexpected reactivity of benzyl-substituted intermediates 3, which limits the scope of a previously published methodology [11]. The methodology, however, still allowed 3-carboxamide derivatives of 2-benzyl-4(1H)-quinolone to be obtained in high yields. The unexpected product 3-methylamino-2-(2-nitrobenzoyl)-4H-naphthalen-1-one (5) was also obtained in a good yield, and probably other 3-alkylamino analogs of 5 are within reach by this route.
3. Materials and Methods
Compounds 1 were prepared according to our previously published method [13]. All other reagents and solvents were purchased from Sigma-Aldrich, Darmstadt, Germany, and were used as supplied. NMR spectra were generated on a Bruker NEO 400 (400/100 MHz 1H/13C) spectrometer. Chemical shifts (δ, ppm) were downfield from TMS. TLC was performed on aluminum-backed Silica gel 60 sheets (Merck, Darmstadt, Germany) with KMnO4 staining. Melting point measurements were carried out in capillary tubes on KRÜSS M5000 automatic mp meter and were not corrected. Mass spectral measurements were performed on a Thermo Fisher Scientific Q Exactive Plus high-resolution mass spectrometer with a heated electrospray ionization source (HESI-II). Petrol refers to the fraction 40–60 °C, NMM refers to N-methylmorpholine, and DMAP refers to 4-dimethylaminopyridine.
Synthesis of β-enaminoamides 2, general procedure: Methylamine (40% aq. solution, 0.45 mL, 5.2 mmol) was added to a solution of the corresponding β-keto amide 1 (5 mmol) in CH2Cl2 (15–20 mL). The mixture was magnetically stirred in a tightly closed vial for 4 h at r.t.; then, anhydrous sodium sulfate was added, and stirring was continued overnight at r.t. Then, the sulfate was filtered off, and the solvent was evaporated under reduced pressure. Compounds 2 crystallized upon trituration with a small volume of diethyl ether and were used in the next step without further purification.
3-Methylamino-4-phenylbut-2-enoic acid phenylamide (2a): m.p. 123–124 °C; 1H-NMR (400 MHz, CDCl3, δ ppm, J Hz): 2.83 (d, J = 5.3, 3H), 3.57 (s, 2H), 4.41 (s, 1H), 6.71 (br s, 1H), 7.03 (m, 1H), 7.27–7.31 (m, 5H), 7.36 (m, 2H), 7.47 (m, 2H), 9.24 (br s, 1H); 13C-NMR (100 MHz, CDCl3, δ ppm): 29.53, 38.74, 86.19, 119.61, 122.82, 126.79, 128.72, 128.84, 136.51, 139.23, 162.60, 169.16; HRMS m/z (ES+): calcd. for C17H19N2O+ [M+H]+ 267.1492, found 267.1490.
3-Methylamino-4-phenylbut-2-enoic acid 4-methoxyphenylamide (2b): m.p. 119–120 °C; 1H-NMR (400 MHz, CDCl3, δ ppm, J Hz): 2.82 (d, J = 5.3, 3H), 3.56 (s, 2H), 3.79 (s, 3H), 4.38 (s, 1H), 6.60 (br s, 1H), 6.84 (m, 2H), 7.28 (m, 3H), 7.35 (m, 4H), 9.20 (br s, 1H); 13C-NMR (100 MHz, CDCl3, δ ppm): 29.51, 38.76, 55.49, 85.91, 114.07, 121.91, 126.74, 128.69, 129.56, 132.22, 136.61, 155.69, 162.21, 169.27; HRMS m/z (ES+): calcd. for C18H21N2O2+ [M+H]+ 297.1598, found 297.1594.
3-Methylamino-4-phenylbut-2-enoic acid 4-chlorophenylamide (2c): m.p. 110–111 °C; 1H-NMR (400 MHz, CDCl3, δ ppm, J Hz): 2.83 (d, J = 5.3, 3H), 3.57 (s, 2H), 4.37 (s, 1H), 6.70 (br s, 1H), 7.23 (m, 2H), 7.28 (m, 3H), 7.35 (m, 2H), 7.41 (m, 2H), 9.21 (br s, 1H); 13C-NMR (100 MHz, CDCl3, δ ppm): 29.55, 38.70, 85.97, 120.59, 126.85, 127.52, 128.72, 128.75, 128.78, 136.34, 137.87, 163.02, 168.94; HRMS m/z (ES+): calcd. for C17H18ClN2O+ [M+H]+ 301.1102, found 301.1107.
Synthesis of α-(2-nitrobenzoyl)-β-enaminoamides 3, general procedure [11]: 2-nitrobenzoyl chloride (0.13 mL, 1 mmol) was slowly added to a magnetically stirred solution containing the corresponding enaminoamide 2 (1 mmol), 4-methylmorpholine (0.11 mL, 1 mmol) and DMAP (24 mg, 0.2 mmol) in CH2Cl2 (20 mL). The mixture was left to stir for 2 h at r.t., and then it was transferred to a separatory funnel with an additional 30 mL of CH2Cl2 and washed with dilute aqueous (20:1) HCl. The aqueous layer was extracted again with 25 mL of CH2Cl2, the combined organic layers were dried with anhydrous sodium sulfate, the drying agent was removed by filtration, and the solvent was distilled off. The residue was purified by column chromatography on silica gel, using Et2O–petrol 1:1 as the eluent.
3-Methylamino-2-(2-nitrobenzoyl)-4-phenylbut-2-enoic acid phenylamide (3a): m.p. 125–126 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 2.91 (d, J = 5.3, 3H), 3.93 (s, 2H), 6.93 (m, 1H), 7.14 (m, 2H), 7.25 (m, 3H), 7.37 (m, 4H), 7.50 (m, 1H), 7.59 (m, 1H), 7.69 (m, 1H), 8.00 (m 1H), 9.79 (br s, 1H), 11.49 (q, J = 5.3, 1H); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 30.48, 35.68, 109.11, 119.71, 123.76, 124.31, 127.20, 128.88, 129.00, 129.12, 129.94, 134.09, 135.94, 137.75, 139.32, 146.34, 167.20, 167.36, 186.55; HRMS m/z (ES+): calcd. for C24H22N3O4+ [M+H]+ 416.1605, found 416.1605.
3-Methylamino-2-(2-nitrobenzoyl)-4-phenylbut-2-enoic acid 4-methoxyphenylamide (3b): m.p. 157–158 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 2.91 (d, J = 5.3, 3H), 3.64 (s, 3H), 3.91 (s, 2H), 6.71 (m, 2H), 7.09 (m, 2H), 7.26 (m, 1H), 7.35 (m, 2H), 7.40 (m, 2H), 7.52 (m, 1H), 7.58 (m, 1H), 7.69 (m, 1H), 8.00 (m, 1H), 9.61 (br s, 1H), 11.46 (q, J = 5.3, 1H); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 30.45, 35.64, 55.55, 114.02, 121.36, 124.29, 124.50, 127.18, 128.92, 129.02, 129.10, 129.44, 129.92, 131.12, 132.42, 133.98, 134.04, 135.99, 137.77, 146.33, 155.78, 166.77, 167.28, 186.48; HRMS m/z (ES+): calcd. for C25H24N3O5+ [M+H]+ 446.1710, found 446.1702.
3-Methylamino-2-(2-nitrobenzoyl)-4-phenylbut-2-enoic acid 4-chlorophenylamide (3c): m.p. 156–157 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 2.92 (d, J = 5.3, 3H), 3.91 (s, 2H), 7.19 (m, 2H), 7.25 (m, 3H), 7.35 (m, 4H), 7.50, (m, 1H), 7.57 (m, 1H), 7.69 (m, 1H), 7.99 (dd, J = 8.2, J = 1.0, 1H), 9.94 (br s, 1H), 11.48 (q, J = 5.3, 1H); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 30.51, 35.66, 109.00, 121.07, 124.32, 127.22, 127.29, 128.80, 128.89, 128.98, 129.12, 129.99, 134.11, 135.87, 137.63, 138.29, 146.27, 167.32, 167.40, 186.52; calcd. for C24H21ClN3O4+ [M+H]+ 450.1215, found 450.1220.
Synthesis of 3-methylamino-2-(2-nitrobenzoyl)-4H-naphthalen-1-one (5): Intermediate 3a, 3b, or 3c (1 mmol) was mixed with H3PO4 (4–5 g) in a glass vial. The mixture was heated to 60 °C and was magnetically stirred in the course of 2 h at this temperature. After the completion of the reaction, the vial was cooled to r.t. with tap water, and the contents were rinsed and poured into a separatory funnel with 50–70 mL of water. The product was extracted in CH2Cl2 (2×40 mL), the combined organic layers were dried (Na2SO4), and the solvent was removed under reduced pressure. Trituration and washing with a small volume of diethyl ether gave practically clean product 5 as a white solid: m.p. 174–175 °C; 1H-NMR (400 MHz, CDCl3, δ ppm, J Hz): 3.20 (d, J = 5.3, 3H, CH3), 4.00 (s, 2H, CH2), 7.19 (dd, J = 7.6, J = 1.2, 1H, C17-H), 7.28 (m, 1H, C6-H), 7.31 (m, 1H, C8-H), 7.45 (m, 1H, C7-H), 7.49 (m, 1H, C15-H), 7.65 (td, J = 7.5, J = 1.2, 1H, C16-H), 8.00 (dd, J = 7.9, J = 1.0, 1H, C9-H), 8.21 (dd, J = 8.3, J = 0.9, 1H, C14-H), 12.20 (br s, 1H, NH); 13C-NMR (100 MHz, CDCl3, δ ppm): 30.14 (CH3), 30.37 (CH2, C4), 107.17 (C2), 123.93 (C14), 126.23 (C17), 126.90 (C9), 127.21 (C6), 127.51 (C8), 127.96 (C15), 131.84 (C7), 133.34 (C5), 134.11 (C16), 141.32 (C12), 144.94 (C13), 171.00 (C3), 181.11 (C1), 194.74 (C11); see Figure 2 for atom numbering; HRMS m/z (ES+): calcd. for C18H15N2O4+ [M+H]+ 323.1026, found 323.1025.
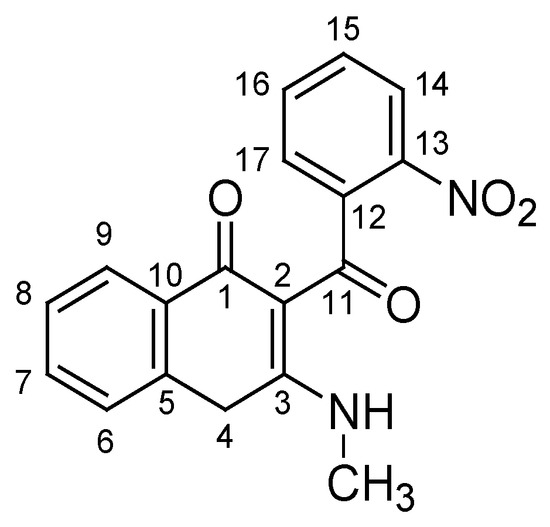
Figure 2.
Structure of 3-methylamino-2-(2-nitrobenzoyl)-4H-naphthalen-1-one (5), with C atoms numbered for NMR assignment.
Synthesis of 2-Benzyl-4-quinolone-3-carboxamides 6, general procedure [11]: Zinc powder (1–1.5 g) was added to the corresponding nitro-intermediate 3 (1 mmol), dissolved in a mixture of CH2Cl2 (30 mL) and acetic acid (4 mL). The mixture was magnetically stirred for 24 h at r.t., and then the solids were filtered off with suction and rinsed thoroughly with CH2Cl2. The dichloromethane filtrate was transferred to a separatory funnel and extracted with water (50 mL) and then with a saturated aqueous solution of NaHCO3 (25 mL). The organic phase was dried with anhydrous sodium sulfate, the drying agent was filtered off, and the solvent was removed under reduced pressure. The crude products were purified by column chromatography on silica gel with Et2O as the eluent, increasing polarity to Et2O:MeOH 20:1 where necessary.
2-Benzyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid phenylamide (6a): m.p. 269–270 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 4.66 (s, 2H), 7.06 (m, 1H), 7.20 (m, 1H), 7.27–7.35 (m, 6H), 7.47 (m, 1H), 7.68 (m, 3H), 7.77 (m, 1H), 8.26 (m, 1H), 12.23 (s, 1H), 12.41 (br s, 1H); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 38.28, 112.99, 118.84, 120.16, 123.63, 125.13, 125.29, 125.96, 126.90, 128.83, 128.88, 129.27, 133.33, 138.39, 139.00, 139.64, 156.15, 164.45, 176.93; HRMS m/z (ES+): calcd. for C23H19N2O2+ [M+H]+ 355.1441, found 355.1436.
2-Benzyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4-methoxyphenylamide (6b): m.p. 240–241 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 3.74 (s, 3H), 4.67 (s, 2H), 6.91 (m, 2H), 7.20 (m, 1H), 7.30 (m, 4H), 7.46 (m, 1H), 7.60 (m, 2H), 7.69 (m, 1H), 7.76 (m, 1H), 8.25 (dd, J = 9.4, J = 1.3, 1H), 12.07 (s, 1H), 12.36 (br s, 1H); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 38.21, 55.63, 113.11, 114.39, 118.78, 121.59, 125.05, 125.27, 125.94, 126.88, 128.86, 132.84, 133.26, 138.45, 138.96, 155.64, 155.94, 164.01, 176.88; HRMS m/z (ES+): calcd. for C24H21N2O3+ [M+H]+ 385.1547, found 385.1550.
2-Benzyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4-chlorophenylamide (6c): m.p. 260–261 °C; 1H-NMR (70 °C, 400 MHz, DMSO-d6, δ ppm, J Hz): 4.70 (s, 1H), 7.20 (m, 1H), 7.25–7.37 (m, 6H), 7.47 (m, 1H), 7.70 (m, 3H), 7.76 (m, 1H), 8.28 (dd, J = 8.1, J = 1.1, 1H), 12.33 (s, 1H); 13C-NMR (70 °C, 100 MHz, DMSO-d6, δ ppm): 38.37, 112.54, 118.82, 121.89, 125.13, 125.40, 125.99, 126.83, 127.25, 128.81, 129.05, 133.26, 138.35, 138.60, 139.02, 156.62, 164.57, 177.13; HRMS m/z (ES+): calcd. for C23H18ClN2O2+ [M+H]+ 389.1051, found 389.1046.
Supplementary Materials
The following supporting information can be downloaded at S1.PDF—processed NMR and MS spectra, IR.ZIP—infrared spectra.
Author Contributions
Conceptualization and methodology, P.A.; investigation, Y.M.-S.; mass spectrometry, P.N.; writing, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bulgarian National Science Fund under grant number KP-06-N59/14 and by the European Union—NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project DUECOS BG-RRP-2.004-0001-C01.
Data Availability Statement
The data presented in this study are available in this article and the Supplementary Materials. The raw NMR data of all compounds are available for download at https://zenodo.org/doi/10.5281/zenodo.12733642 (accessed on 28 August 2024). Further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saalim, M.; Villegas-Moreno, J.; Clark, B.R. Bacterial alkyl-4-quinolones: Discovery, structural diversity and biological properties. Molecules 2020, 25, 5689. [Google Scholar] [CrossRef] [PubMed]
- Szamosvári, D.; Böttcher, T. 4-Quinolone N-Oxides as Bacterial Weapons. Synlett 2018, 29, 542–547. [Google Scholar] [CrossRef]
- Huse, H.; Whiteley, M. 4-Quinolones: Smart Phones of the Microbial World. Chem. Rev. 2011, 111, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Empting, M. Targeting the Pseudomonas quinolone signal quorum sensing system for the discovery of novel anti-infective pathoblockers. Beilstein J. Org. Chem. 2018, 14, 2627–2645. [Google Scholar] [CrossRef] [PubMed]
- Muimhneacháin, E.Ó.; Reen, F.J.; O’Gara, F.; McGlacken, G.P. Analogues of Pseudomonas aeruginosa signaling molecules to tackle infections. Org. Biomol. Chem. 2018, 16, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Hays, E.E.; Wells, I.C.; Katzman, P.A.; Cain, C.K.; Jacobs, F.A.; Thayer, S.A.; Doisy, E.A.; Gaby, W.L.; Roberts, E.C.; Muir, R.D.; et al. Antibiotic substances produced by Pseudomonas aeruginosa. J. Biol. Chem. 1945, 159, 725–750. [Google Scholar] [CrossRef]
- Wells, I.C. Antibiotic substances produced by Pseudomonas aeruginosa. Syntheses of Pyo 1b, Pyo 1c, and Pyo III. J. Biol. Chem. 1952, 196, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.N.; Szamosvári, D.; Böttcher, T. Synthesis of bacterial 2-alkyl-4(1H)-quinolone derivatives. Arkivoc 2021, part ix, 218–239. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Saalim, M.; Wei, G.; Zaleta-Pinet, D.A.; Clark, B.R. Isolation of 2-Alkyl-4-quinolones with Unusual Side Chains from a Chinese Pseudomonas aeruginosa Isolate. J. Nat. Prod. 2020, 83, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Clark, B.R. Synthesis of Natural and Unnatural Quinolones Inhibiting the Growth and Motility of Bacteria. J. Nat. Prod. 2020, 83, 3181–3190. [Google Scholar] [CrossRef] [PubMed]
- Mollova-Sapundzhieva, Y.; Angelov, P.; Georgiev, D.; Yanev, P. Synthetic approach to 2-alkyl-4-quinolones and 2-alkyl-4-quinolone-3-carboxamides based on common β-keto amide precursors. Beilstein J. Org. Chem. 2023, 19, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Venkov, A.P.; Angelov, P.A. Synthesis of Unsymmetrical β-Enamino Ketones. Synthesis 2003, 14, 2221–2225. [Google Scholar] [CrossRef]
- Angelov, P. Enamine-Based Domino Strategy for C-Acylation/Deacetylation of Acetoacetamides: A Practical Synthesis of β-Keto Amides. Synlett 2010, 8, 1273–1275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).