Synthesis of Diastereomeric 2,6-bis{[3-(2-Hydroxy-5-substitutedbenzyl)octahydro-1H-benzimidazol-1-yl]methyl}-4-substituted Phenols (R = Me, OMe) by Mannich-Type Tandem Reactions
Abstract
1. Introduction
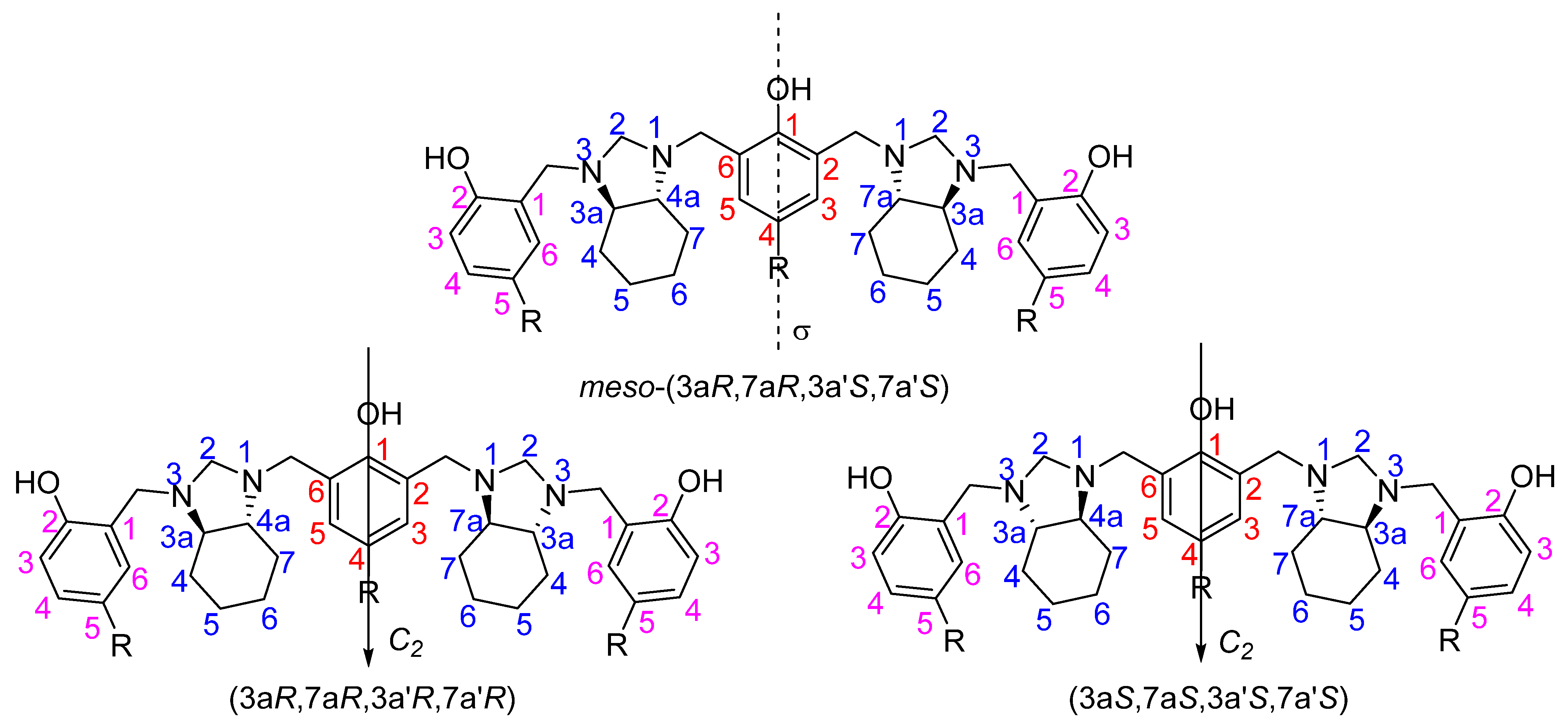
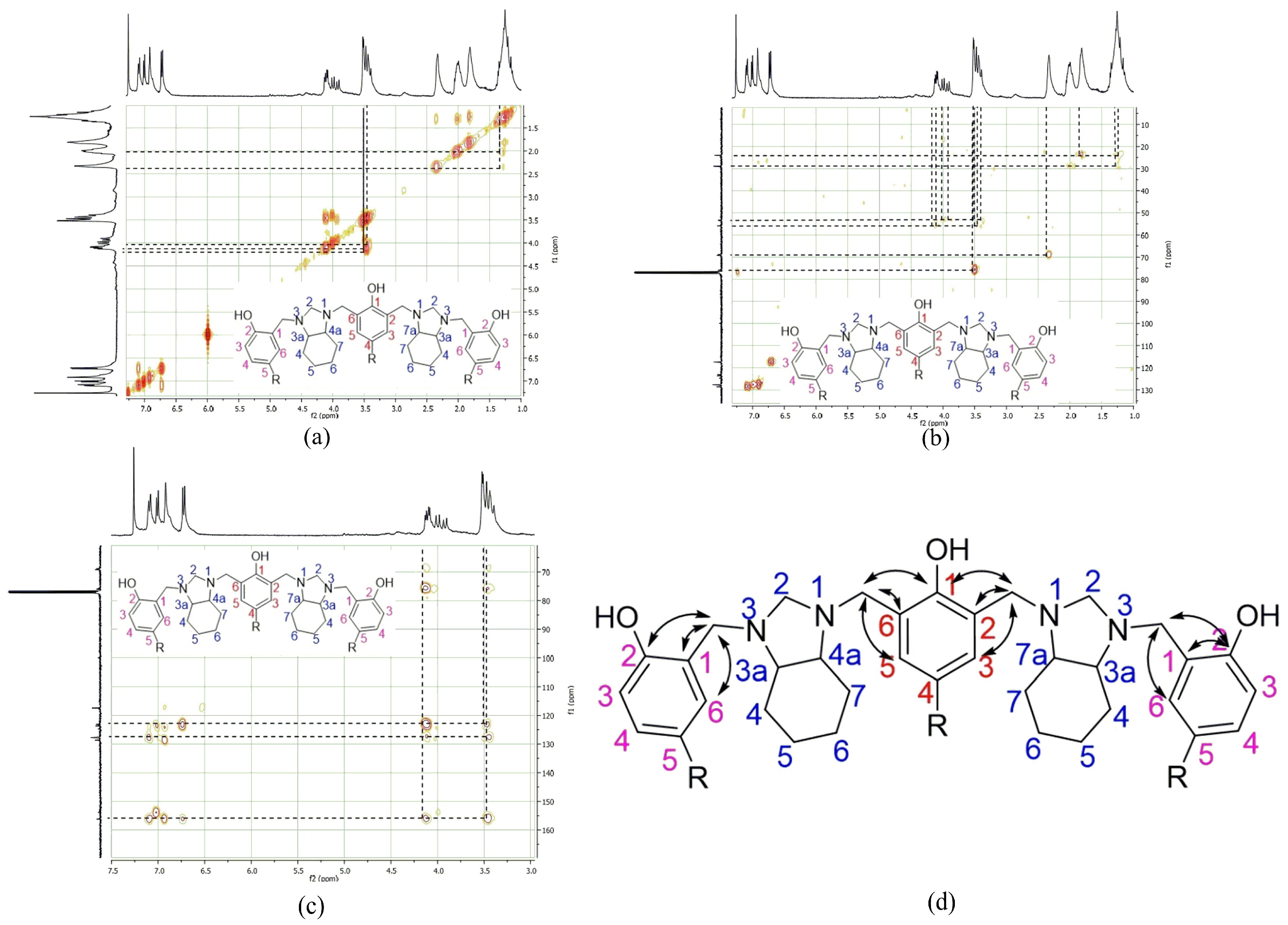
2. Results
3. Materials and Methods
3.1. General
3.2. General Procedure: Reaction between Aminal and p-Substituted Phenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganjoo, R.; Verma, C.; Thakur, A.; AlFantazi, A.; Assad, H.; Sharma, S.; Dubey, S.; Kumar, A. Mannich Bases: Chemical Structure, Chemistry, Coordination Bonding and Application in Aqueous Phase Corrosion Protection. J. Ind. Eng. Chem. 2024, 131, 136–166. [Google Scholar] [CrossRef]
- Bala, S.; Sharma, N.; Kajal, A.; Kamboj, S.; Saini, V. Mannich Bases: An Important Pharmacophore in Present Scenario. Int. J. Med. Chem. 2014, 2014, 191072. [Google Scholar] [CrossRef] [PubMed]
- Roman, G. Mannich Bases in Medicinal Chemistry and Drug Design. Eur. J. Med. Chem. 2015, 89, 743–816. [Google Scholar] [CrossRef] [PubMed]
- Csonka, A.; Varga, B.; Csonka, A.; Molnar, J.; Amaral, L.; Spengler, G. Possible Biological and Clinical Applications of Phenothiazines. Anticancer. Res. 2017, 37, 5983–5993. [Google Scholar]
- Abdula, A.M.; Qarah, A.F.; Alatawi, K.; Qurban, J.; Abualnaja, M.M.; Katuah, H.A.; El-Metwaly, N.M. Design, Synthesis, and Molecular Docking of New Phenothiazine Incorporated N-Mannich Bases as Promising Antimicrobial Agents. Heliyon 2024, 10, e28573. [Google Scholar] [CrossRef]
- Gopi, C.; Dhanaraju, M.D.; Pranusha, K.; Deepan, T.; Magesh, A.; Kavitha, D. Design, Synthesis, Characterization and Antitubercular Activity of Novel Benzimidazole Mannich Base Derivatives: Anti-Tubercular Activity of Novel Benzimidazole Mannich Base Derivatives. Asian J. Chem. 2024, 36, 969–973. [Google Scholar] [CrossRef]
- Dukes, A.O.; Weerawarna, P.M.; Devitt, A.N.; Silverman, R.B. Synthesis of (2R, 4S)-4-Amino-5-Hydroxybicyclo [3.1.1]Heptane-2-Carboxylic Acid via an Asymmetric Intramolecular Mannich Reaction. J. Org. Chem. 2024, 89, 9110–9117. [Google Scholar] [CrossRef]
- Kumar, S.; Arora, D.; Bhardwaj, T.R.; Dhingra, N. Design, Synthesis and Studies on Novel N-Mannich Base Derivatives of Isatin Targeting Dihydrofolate Reductase Receptor. J. Mol. Struct. 2024, 1311, 138385. [Google Scholar] [CrossRef]
- De Kraker, H.; Wang, H.-Y.L.; Arman, H.D.; Renteria, R.N.; Fleischer, C.N.; Messing, R.O.; McHardy, S.F. Asymmetric Synthesis of CIDD-0072424 via an Enantioselective Nitro-Mannich Reaction: A Central Nervous System Penetrant, Selective Small Molecule Inhibitor of Protein Kinase C Epsilon. J. Org. Chem. 2024, 89, 5134–5141. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Su, Y.; Guan, S. Enhancing the Corrosion Inhibition Performance of Mannich Base on Mild Steel in Lactic Acid Solution through Synergistic Effect of Allicin: Experimental and Theoretical Study. J. Mol. Struct. 2024, 1304, 137658. [Google Scholar] [CrossRef]
- Afsah, E.M. Double Mannich Reaction with Ketones as a Route to Heterocyclic Systems: A Mini Review. J. Heterocycl. Chem. 2024, 61, 805–817. [Google Scholar] [CrossRef]
- Singh, R.; Bhasin, G.; Srivastava, R.; Geetanjali, B.S.P. β-Aminocarbonyl Compounds: Chemistry and Biological Activities. Mini-Rev. Org. Chem. 2016, 13, 143–153. [Google Scholar] [CrossRef]
- Šramel, P.; Šebesta, R. Organocatalytic Mannich Type Reactions of Glyoxylate Imines and Related Compounds. Tetrahedron Lett. 2024, 143, 155129. [Google Scholar] [CrossRef]
- Chao, Y.-H.; Jamwal, P.; Ananda Rao, G.; Gurubrahamam, R.; Chen, K. Chiral Spirophosphoric-Acid-Catalyzed Divergent Vinylogous Mannich and Aza -Friedel–Crafts Reactions of 2-Methoxyfuran. Org. Lett. 2024, 26, 4938–4944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhao, Z.; Wei, Z.; Cao, J.; Liang, D.; Lin, Y.; Duan, H. Asymmetric Mannich Reaction of Isatin-Derived Ketimines with α-Fluoroindanones Catalyzed by a Chiral Phase-Transfer Catalyst. J. Org. Chem. 2024, 89, 4474–4483. [Google Scholar] [CrossRef] [PubMed]
- Bessoni Kosctiuk, J.; Ribeiro Neto, M.E.; Alcoforado Pereira, G.; Krieger, N.; Zambelli Mezalira, D.; Pilissão, C. A Multicomponent Mannich Reaction Catalyzed by Hydrolases Immobilized on Titanate Nanotubes. ChemPlusChem 2024, 89, e202300698. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Qu, L.; Chen, Y.; Luo, Z.; Kong, L.; Wang, X. Stereoselective Synthesis of β, Γ-Fused Bicyclic γ-Ureasultams via an Intramolecular Mannich and Aza-Michael Addition Cascade. Chem.—Eur. J. 2024, 30, e202400438. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Kamyab, H.; Chelliapan, S.; Mohammad Amani, A. Light-Induced Access to Gram-Scale Photosynthesis of Polyfunctionalized Dihydro-2-Oxypyrroles: A Recyclable Halide Perovskite Photocatalyst as a Single-Electron Redox Mediator for Radical-Initiated Michael-Mannich Cyclocondensation in Air Atmosphere. Inorg. Chem. Commun. 2024, 167, 112807. [Google Scholar] [CrossRef]
- Rivera, A.; Pacheco, D.J.; Ríos-Motta, J.; Fejfarová, K.; Dušek, M. Synthesis of a New Chiral Cyclic Aminal Derived from Rac-1,2-Propanediamine. Tetrahedron Lett. 2012, 53, 6132–6135. [Google Scholar] [CrossRef]
- Rivera, A.; Quiroga, D.; Ríos-Motta, J.; Eigner, V.; Dušek, M. Single-Step Synthesis of a New Series of Meso Di-Mannich Bases from the Cyclic Aminal (2S,7R,11S,16R)-1,8,10,17-Tetraazapentacyclo [8.8.1.1.8,170.2,7011,16]Icosane and p-Substituted Phenols. Chem. Cent. J. 2013, 7, 100. [Google Scholar] [CrossRef]
- Rivera, A.; Quiroga, D.; Ríos-Motta, J.; Fejfarová, K.; Dušek, M. 4,4′-Dimethoxy-2,2′-{[(3a RS, 7a RS)-2,3,3a,4,5,6,7,7a-Octahydro-1 H -1,3-Benzimidazole-1,3-Diyl]Bis(Methylene)}diphenol. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o2298–o2299. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Quiroga, D.; Ríos-Motta, J.; Carda, J.; Peris, G. Synthesis, Characterization and X-Ray Crystal Structure of the Di-Mannich Base 2,2′-(3aR,7aR/3aS,7aS)-Hexahydro-1H-Benzo[d]Imidazole-1,3(2H)-Diyl)Bis(Methylene)Bis(4-Methylphenol). J. Chem. Crystallogr. 2009, 39, 827–830. [Google Scholar] [CrossRef]
- Rivera, A.; Nerio, L.S.; Quevedo, R. Synthesis of Macrocyclic and Linear Benzylimidazolidine Oligomers from Solvent Free Aromatic Mannich-Type Reaction. Tetrahedron Lett. 2015, 56, 6059–6062. [Google Scholar] [CrossRef]

| Tested Solvent System | Solvent Polarity | Temperature/°C | Reaction Time/h | Recovery of Aminal 1/% | Yield for Compounds 4a–b/% |
|---|---|---|---|---|---|
| Benzene or toluene | Low | 20–40 °C | Up to 40 | >99 | 0 |
| 1,4-Dioxane | Intermediate | 40 °C | 40 | 65 | <2 |
| Ethanol | High | 77 °C | 40 | 0,45 | <2 |
| 1,4-Dioxane/water | Intermediate–high | 90 °C | 40 | 0,1 | 13–15 |
| 1,4-Dioxane/water | Intermediate–high | 94–97 °C | 72 | 0 | 22–27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga, D.; Ríos-Motta, J.; Rivera, A. Synthesis of Diastereomeric 2,6-bis{[3-(2-Hydroxy-5-substitutedbenzyl)octahydro-1H-benzimidazol-1-yl]methyl}-4-substituted Phenols (R = Me, OMe) by Mannich-Type Tandem Reactions. Molbank 2024, 2024, M1876. https://doi.org/10.3390/M1876
Quiroga D, Ríos-Motta J, Rivera A. Synthesis of Diastereomeric 2,6-bis{[3-(2-Hydroxy-5-substitutedbenzyl)octahydro-1H-benzimidazol-1-yl]methyl}-4-substituted Phenols (R = Me, OMe) by Mannich-Type Tandem Reactions. Molbank. 2024; 2024(3):M1876. https://doi.org/10.3390/M1876
Chicago/Turabian StyleQuiroga, Diego, Jaime Ríos-Motta, and Augusto Rivera. 2024. "Synthesis of Diastereomeric 2,6-bis{[3-(2-Hydroxy-5-substitutedbenzyl)octahydro-1H-benzimidazol-1-yl]methyl}-4-substituted Phenols (R = Me, OMe) by Mannich-Type Tandem Reactions" Molbank 2024, no. 3: M1876. https://doi.org/10.3390/M1876
APA StyleQuiroga, D., Ríos-Motta, J., & Rivera, A. (2024). Synthesis of Diastereomeric 2,6-bis{[3-(2-Hydroxy-5-substitutedbenzyl)octahydro-1H-benzimidazol-1-yl]methyl}-4-substituted Phenols (R = Me, OMe) by Mannich-Type Tandem Reactions. Molbank, 2024(3), M1876. https://doi.org/10.3390/M1876







