Abstract
The title compound, (E)-5-(3-oxo-3-(3,4,5-trimethoxyphenyl)prop-1-en-1-yl)benzo[d]oxazol-2(3H)-one, was synthesized by the acid- and base-catalyzed aldol condensation of 2-oxo-2,3-dihydrobenzo[d]oxazole-5-carbaldehyde and 3,4,5-trimethoxyacetophenone. The structure of the target compound was confirmed using 1H NMR, 13C NMR, HRMS, and elemental analysis.
1. Introduction
The diverse biological activity and the simple methods for their preparation make chalcones a privileged scaffold in medicinal chemistry [1,2,3]. A large number of natural and synthetic chalcones exhibit anticancer [4,5,6], anti-inflammatory [7], antibacterial [8,9,10], and antioxidant activity [10,11]. An important part in the chalcone molecule is the α,β-unsaturated carbonyl fragment, a Michael acceptor that can covalently interact with the cysteine sulfhydryl groups of protein targets [12,13].
In our previous work, chalcones with a fused oxazole or thiazole motif in ring A showed cytotoxic activity against BV-173, MCF-7, MDA-MB-231, and other cell lines [14,15]. The introduction of the 4-methoxy or 3,4,5-trimetoxy groups in ring B of this series produced chalcones with 4.9–8.3 micromolar range cytotoxicity against BV-173 cells [16]. These results encouraged us to explore placing the azole ring in ring B and the methoxy substituents in ring A of the chalcone scaffold.
The aim of the present work is the synthesis of (E)-5-(3-oxo-3-(3,4,5-trimethoxyphenyl)prop-1-en-1-yl)benzo[d]oxazol-2(3H)-one (3). To our knowledge, this is the first example of a heterocyclic chalcone with an oxazole fused to ring B, a prototype for a new series of chalcones with potentially novel biological properties.
2. Results and Discussion
The synthesis of (E)-5-(3-oxo-3-(3,4,5-trimethoxyphenyl)prop-1-en-1-yl)benzo[d]oxazol-2(3H)-one (3) was performed by the Claisen–Schmidt condensation of 2-oxo-2,3-dihydrobenzo[d]oxazole-5-carbaldehyde (1) and 3,4,5-trimethoxyacetophenone (2) (Scheme 1). The aldehyde 1 was prepared in three steps, as previously described [17]. Briefly, 4-hydroxy-3-nitrobenzaldehyde protected as the 1,3-propanediol acetal was catalytically reduced to the corresponding aniline, cyclized with 1,1′-carbonyldiimidazole (CDI), and deprotected. The resulting aldehyde 1 was condensed with acetophenone 2 under acid- or base-catalyzed conditions. The acid-catalyzed condensation was performed using SOCl2/EtOH, as previously described [18]. The base-catalyzed reaction was carried out in ethanol/aqueous NaOH. Both reactions yielded a single product detectable by thin-layer chromatography. The yields were 85% and 89% for the base-catalyzed and acid-catalyzed reactions, respectively.
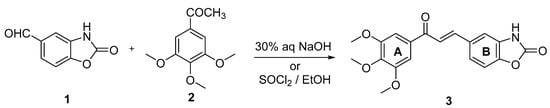
Scheme 1.
Synthesis of (E)-5-(3-oxo-3-(3,4,5-trimethoxyphenyl)prop-1-en-1-yl)benzo[d]oxazol-2(3H)-one (3).
The structure of compound 3 was confirmed by FTIR, 1H and 13C NMR, HRMS, and elemental analysis, and all the data are in agreement with the assumed structure. The 1H NMR spectra are consistent with a pure E configuration, as judged by the vinyl proton coupling constant J = 15.5 Hz. The 13C NMR spectrum displays 16 signals corresponding to the number of carbon atoms in compound 3. All data are available in the Supplementary Material File (Figures S1–S4).
3. Materials and Methods
3.1. General
All chemicals were purchased from Acros Organics (Geel, Belgium) and Fisher Scientific GmbH (Schwerte, Germany). Reactions and purity of the final compound were monitored by thin-layer chromatography (TLC) on silica gel plates Kieselgel 60 F254 (Merck, Darmstadt, Germany), using toluene/chloroform/ethyl acetate (3:1:1 v/v) as eluent.
Melting points were determined on a Boetius hot-stage microscope (Carl Zeiss Jena, Germany). Infrared spectra (FTIR) were acquired on a Shimadzu FTIR 8400S Fourier transform infrared spectrophotometer (Duisburg, Germany) in nujol. NMR spectra were recorded in DMSO-d6 on a Bruker Avance III HD 500 (Bruker BioSpin GmbH, Rheinstetten, Germany), operating at 500 MHz for 1H and at 125.8 MHz for 13C. Chemical shifts are reported in parts per million (ppm) and were referenced to the residual solvent peaks (DMSO-d6): 2.50 and 39.52 ppm for 1H and 13C NMR, respectively. Coupling constants (J) were measured in hertz (Hz). High-resolution mass spectra (HRMS) were obtained with an Orbitrap Exploris 120 Mass Spectrometer (Thermo Fisher Scientific GmbH, Bremen, Germany). The elemental analysis was carried out on a VARIO EL III Elemental Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany) and the results for C, H, and N were within ± 0.4% of the theoretical values.
3.2. Synthesis of (E)-5-(3-Oxo-3-(3,4,5-trimethoxyphenyl)prop-1-en-1-yl)benzo[d]oxazol-2(3H)-one (3)
3.2.1. Base-Catalyzed Aldol Condensation
To a suspension of 2-oxo-2,3-dihydrobenzo[d]oxazole-5-carbaldehyde (0.82 g, 5 mmol) and 3,4,5-trimethoxyacetophenone (1.05 g, 5 mmol) in ethanol (25 mL), 30% aq. NaOH (5 mL) was added. The obtained yellow mixture was stirred for 24 h at room temperature to yield a precipitate. The mixture was poured in 30 mL water, warmed, and acidified with 10% HCl. The crystalline product 3 was filtered, washed with cold ethanol and water to neutrality, and dried. Yield: 85% (1.51 g).
3.2.2. Acid-Catalyzed Aldol Condensation
To an ice-cool suspension of 2-oxo-2,3-dihydrobenzo[d]oxazole-5-carbaldehyde (0.82 g, 5 mmol) and 3,4,5-trimethoxyacetophenone (1.05 g, 5 mmol) in absolute ethanol (25 mL), SOCl2 (3.5 mL) was added slowly. The obtained mixture was stirred for 12 h at room temperature. Water (50 mL) was added and the suspension was heated to boiling. After being cooled to room temperature, the crystalline product 3 was filtered, washed with water, and dried. Yield: 89% (1.58 g).
Light yellow crystals, m.p.: 228–230 °C (ethanol). IR (nujol): 3227 (N-H), 1812 (C=O), 1779 (C=O), 1652 (C=C), 1571 (C=C), 1129 (O-C-O), 902 (C-H) cm−1. 1H NMR (500 MHz, DMSO-d6): δ (ppm) 3.76 (s, 3H, OCH3), 3.90 (s, 6H, OCH3), 7.37 (d, 1H, arom. H, J = 8.2 Hz), 7.43 (s, 2H, arom. H), 7.66 (m, 2H, arom. H), 7.77 (d, 1H, =CHCO, J = 15.5 Hz), 7.91 (d, 1H, ArCH=, J = 15.5 Hz), 11.89 (br.s., 1H, NH). 13C NMR (125.8 MHz, DMSO-d6): δ (ppm) 56.2, 60.2, 106.2, 109.4, 109.8, 121.3, 124.2, 130.9, 131.2, 133.1, 142.0, 143.8, 145.1, 152.9, 154.5, 187.9. HRMS (ESI): Found 356.1132. Calcd. for C19H17NO6: 356.1134 [M + H]+. Anal. calcd. for C19H17NO6 (355.35): C, 64.22; H, 4.82; N 3.94. Found: C, 64.39; H, 4.82; N, 3.63.
Supplementary Materials
Figure S1: FT-IR spectra of compound 3, Figure S2: 1H NMR spectrum of compound 3; Figure S3: 13C NMR spectrum of compound 3; Figure S4: HRMS of compound 3.
Author Contributions
Conceptualization, O.I.P. and Y.B.I.; methodology, Y.B.I. and F.E.S.; writing—original draft preparation, Y.B.I., F.E.S. and O.I.P.; writing—review and editing, Y.B.I. and O.I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors are grateful for the financing provided by the European Union-NextGenerationEU through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0008, and for the administrative and technical support of the Faculty of Chemistry and Pharmacy, Sofia University St. Kliment Ohridski.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Mihis, A.G. Two Important Anticancer Mechanisms of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2022, 23, 11595. [Google Scholar] [CrossRef] [PubMed]
- Bilginer, S.; Gul, H.I.; Erdal, F.S.; Sakagami, H.; Gulcin, I. New halogenated chalcones with cytotoxic and carbonic anhydrase inhibitory properties: 6-(3-Halogenated phenyl-2-propen-1-oyl)-2(3H)-benzoxazolones. Arch. Pharm. 2020, 353, e1900384. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, Y.; Momekov, G.; Petrov, O.; Karaivanova, M.; Kalcheva, V. Cytotoxic Mannich bases of 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolones. Eur. J. Med. Chem. 2007, 42, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives. Curr. Top. Med. Chem. 2017, 17, 3146–3169. [Google Scholar] [CrossRef] [PubMed]
- Lagu, S.B.; Yejella, R.P.; Bhandare, R.R.; Shaik, A.B. Design, Synthesis, and Antibacterial and Antifungal Activities of Novel Trifluoromethyl and Trifluoromethoxy Substituted Chalcone Derivatives. Pharmaceuticals 2020, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.J.; Bird, S.J.; Gowland, P.; Collins, M.; Cassella, J.P. Ferrocenyl chalcone derivatives as possible antimicrobial agents. J. Antibiot. 2020, 73, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Okolo, E.N.; Ugwu, D.I.; Ezema, B.E.; Ndefo, J.C.; Eze, F.U.; Ezema, C.G.; Ezugwu, J.A.; Ujam, O.T. New chalcone derivatives as potential antimicrobial and antioxidant agent. Sci. Rep. 2021, 11, 21781. [Google Scholar] [CrossRef] [PubMed]
- Kudličková, Z.; Michalková, R.; Salayová, A.; Ksiažek, M.; Vilková, M.; Bekešová, S.; Mojžiš, J. Design, Synthesis, and Evaluation of Novel Indole Hybrid Chalcones and Their Antiproliferative and Antioxidant Activity. Molecules 2023, 28, 6583. [Google Scholar] [CrossRef] [PubMed]
- Pati, H.N.; Das, U.; Sharma, R.K.; Dimmock, J.R. Cytotoxic Thiol Alkylators. Mini-Rev. Med. Chem. 2007, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Petrov, O.I.; Ivanova, Y.B.; Gerova, M.S.; Momekov, G.T. Synthesis and Cytotoxicity of New Mannich Bases of 6-[3-(3,4,5-Trimetoxyphenyl)-2-propenoyl]-2(3H)-Benzoxazolone. Lett. Drug Des. Discov. 2020, 17, 512–517. [Google Scholar] [CrossRef]
- Ivanova, Y.; Momekov, G.; Petrov, O. Synthesis of novel substituted 1,3-diarylpropenone derivatives and their in vitro cytotoxic activity. Lett. Drug Des. Discov. 2009, 6, 353–357. [Google Scholar] [CrossRef]
- Ivanova, Y.B.; Gerova, M.S.; Momekov, G.T.; Petrov, O.I. Synthetic chalcones of 2(3H)-benzothiazolone with potential cytotoxic activity. C. R. Acad. Bulg. Sci. 2007, 60, 641–644. [Google Scholar]
- Ivanova, Y.B.; Momekov, G.T.; Petrov, O.I. New heterocyclic chalcones. Part 6. Synthesis and cytotoxic activities of 5- or 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolones. Heterocycl. Commun. 2013, 19, 23–28. [Google Scholar] [CrossRef]
- Blum, G.; Gazit, A.; Levitzki, A. Development of New Insulin-like Growth Factor-1 Receptor Kinase Inhibitors Using Catechol Mimics. J. Biol. Chem. 2003, 278, 40442–40454. [Google Scholar] [CrossRef] [PubMed]
- Petrov, O.; Ivanova, Y.; Gerova, M. SOCl2/EtOH: Catalytic system for synthesis of chalcones. Catal. Commun. 2008, 9, 315–316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).