N-(Benzothiazol-2-yl)-4-((5-chlorobenzoxazol-2-yl)amino)butanamide
Abstract
1. Introduction
2. Results
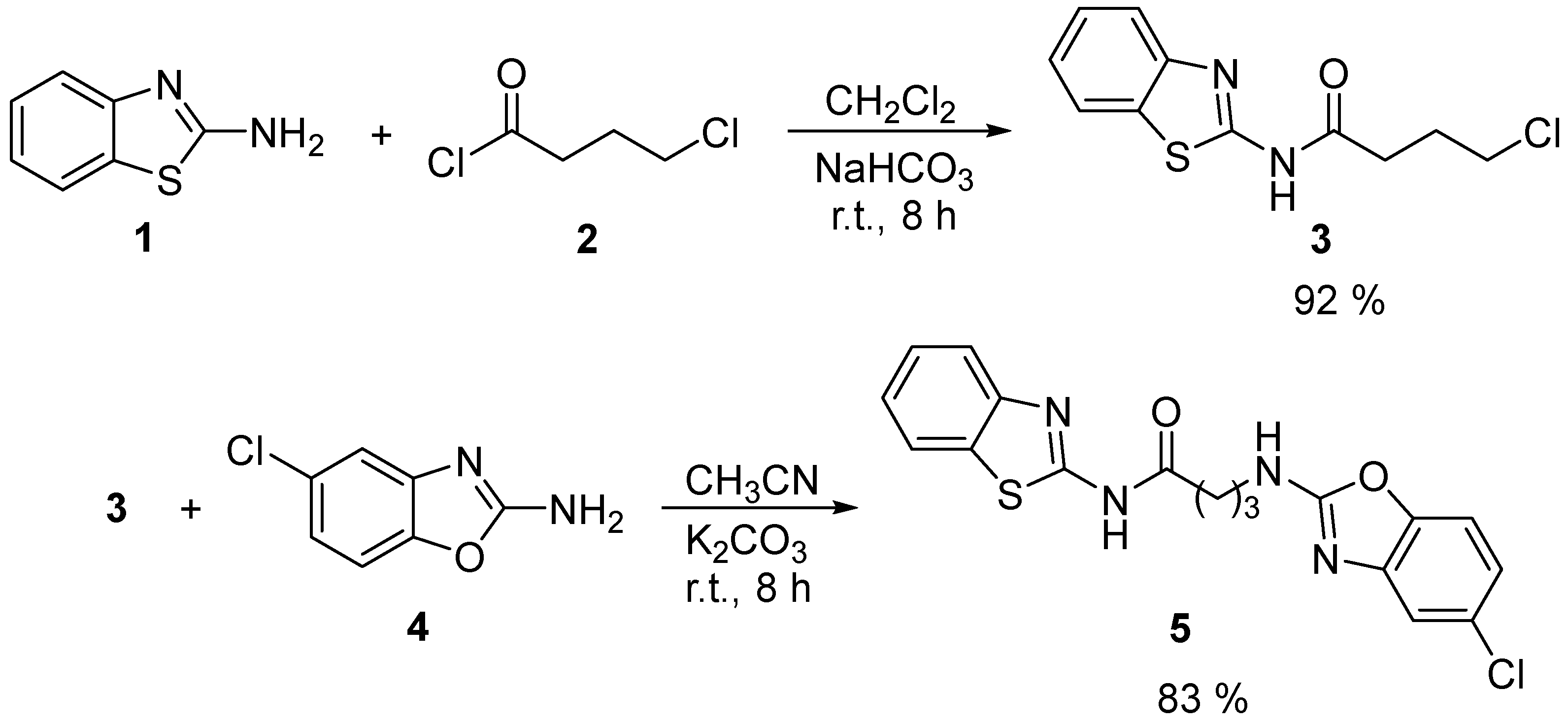
2.1. Synthesis
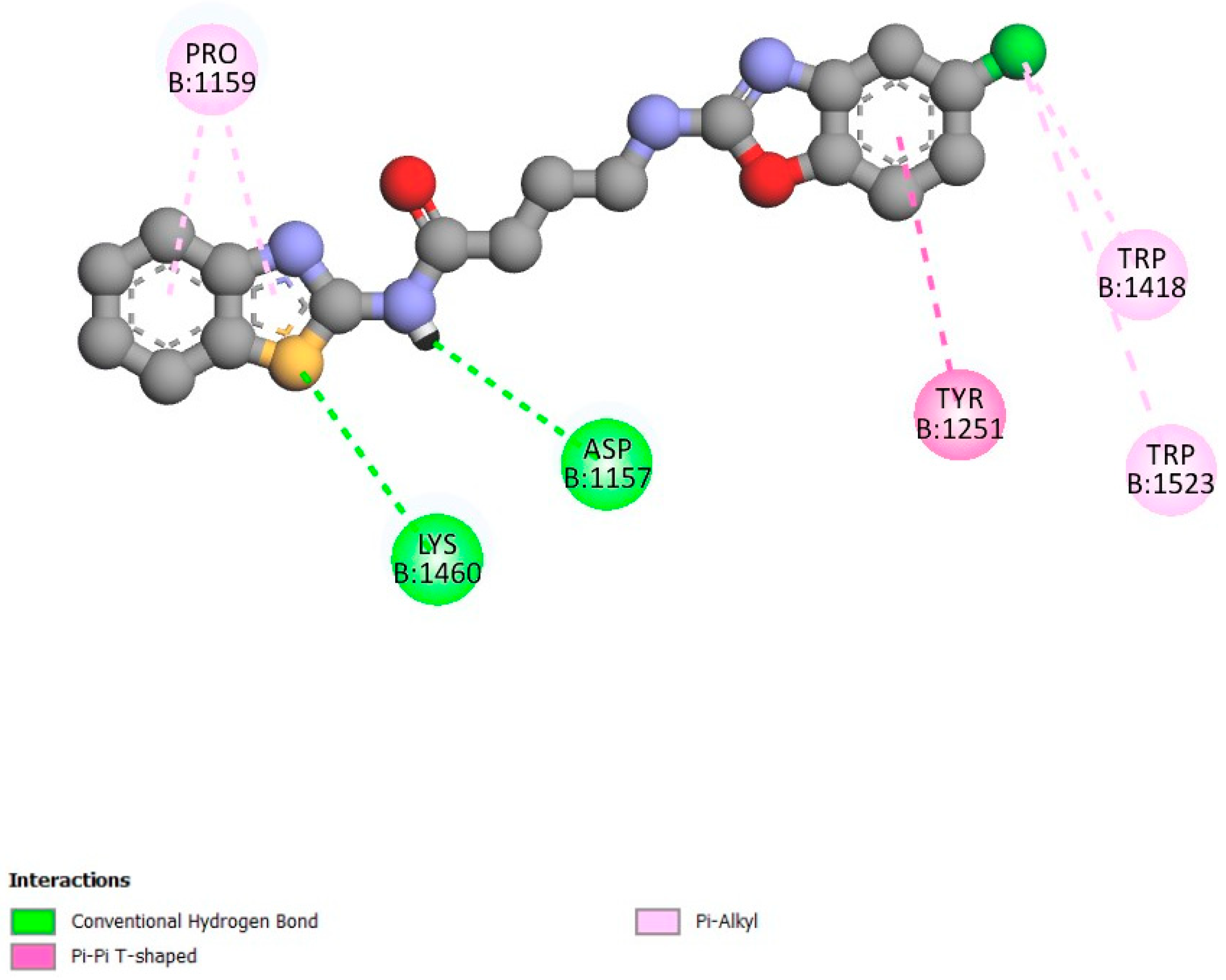
2.2. Molecular Docking Validation
2.3. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. General
4.2. Synthesis of N-(Benzothiazol-2-yl)-4-chlorobutanamide (3)
4.3. Synthesis of N-(Benzothiazol-2-yl)-4-((5-chlorobenzoxazol-2-yl)amino)butanamide (5)
4.4. Validation of the Active Site
4.5. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.B.; Ermolenko, L.; Dean, W.A.; Al-Mourabit, A. Benzazoles from aliphatic amines and o-amino/mercaptan/hidroxyanilines: Elemental sulfur as a highly efficient and traceless oxidizing agent. Org. Lett. 2012, 14, 5948–5951. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, P.; Zodape, S.; Wankhade, A.; Pratap, U. Facile synthesis of benzazoles through biocatalytic cyclization and dehydrogenation employing catalase in water. Enzyme Microb. Technol. 2020, 138, 109562. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Cheng, X.C.; Chen, X.B.; Li, Y.; Zang, L.L.; Duan, Y.Q.; Chen, M.Z.; Yu, P.; Sun, H.; Wang, R.L. Synthesis and biological evaluation of novel N-aryl-ω-(benzoazol-2-yl)-sulfanylalkanamides as dual inhibitors of α-glucosidase and protein tyrosine phosphatase 1B. Chem. Biol. Drug Des. 2018, 92, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, C.; Geronikaki, A.; Hadjipavlou-Litina, D. Synthesis and biological evaluation of new thiazolyl/benzothiazolyl-amides, derivatives of 4-phenyl-piperazine. Il Farmaco 2005, 60, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Azbill, R.D.; Mu, X.; Springer, J.E. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000, 871, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Prabhat, P.; Das, S.K.; Shafaat, K.; Anand, J.P. Evaluation of N-(6-chlorobenzothiazol-2-yl)-2-(substitutedamino)acetamide for its Anti-bacterial Activity. Int. J. Pharm. Sci. Res. 2012, 3, 2669–2674. [Google Scholar]
- Bhausaheb, D.K.; Madhukar, G.V.; Balkrishna, S.S. Synthesis and Evaluation of Antifungal Activity of Benzothiazole Derivatives. Int. J. Pharm. Pharm. Res. 2015, 3, 112–124. [Google Scholar]
- Sun, H.; Saedi, P.; Karuranga, S.; Pinkepank, M.; Orgutsova, K.; Dunca, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. J. Diabetes Res. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.; Kim, D.; Philip, E.; Miyan, Z.; Barykina, I.; Schmidt; Stein, H. A multitational, observational study to investigate the efficacy, safety and tolerability of acarbose as add-on or monotherapy in a range of patiens: The glucoVIP study. Clin. Drug Investig. 2013, 33, 263–274. [Google Scholar] [CrossRef] [PubMed]
- El-Subbagh, H.I.; Hassan, G.S.; El-Azab, A.S.; Abdel-Aziz, A.A.M.; Kadi, A.A.; Al-Obaid, A.M.; Al-Shabanah, O.A.; Sayed-Ahmed, M.M. Synthesis and anticonvulsant activity of some new thiazolo [3,2-a][1,3] diazepine, benzo[d]thiazolo [5,2-a][12,6]diazepine and benzo[d]oxazolo [5,2-a][12,6]diazepine analogues. Eur. J. Med. Chem. 2011, 46, 5567–5572. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Cob-Calan, N.N.; Chi-Uluac, L.A.; Ortiz-Chi, F.; Cerqueda-García, D.; Navarrete-Vázquez, G.; Ruiz-Sánchez, E.; Hernández-Núñez, E. Molecular Docking and Dynamics Simulation of Protein β-Tubulin and Antifungal Cyclic Lipopeptides. Molecules 2019, 24, 3387. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilotzi-Xahuentitla, H.; Canche-Naal, G.d.C.; Ortiz-Andrade, R.R.; Navarrete-Vázquez, G.; Hernández-Núñez, E. N-(Benzothiazol-2-yl)-4-((5-chlorobenzoxazol-2-yl)amino)butanamide. Molbank 2024, 2024, M1854. https://doi.org/10.3390/M1854
Pilotzi-Xahuentitla H, Canche-Naal GdC, Ortiz-Andrade RR, Navarrete-Vázquez G, Hernández-Núñez E. N-(Benzothiazol-2-yl)-4-((5-chlorobenzoxazol-2-yl)amino)butanamide. Molbank. 2024; 2024(3):M1854. https://doi.org/10.3390/M1854
Chicago/Turabian StylePilotzi-Xahuentitla, Hugo, Gabriela del Carmen Canche-Naal, Rolffy Ruben Ortiz-Andrade, Gabriel Navarrete-Vázquez, and Emanuel Hernández-Núñez. 2024. "N-(Benzothiazol-2-yl)-4-((5-chlorobenzoxazol-2-yl)amino)butanamide" Molbank 2024, no. 3: M1854. https://doi.org/10.3390/M1854
APA StylePilotzi-Xahuentitla, H., Canche-Naal, G. d. C., Ortiz-Andrade, R. R., Navarrete-Vázquez, G., & Hernández-Núñez, E. (2024). N-(Benzothiazol-2-yl)-4-((5-chlorobenzoxazol-2-yl)amino)butanamide. Molbank, 2024(3), M1854. https://doi.org/10.3390/M1854








