Abstract
The compound 1-(3-chlorophenyl)-3-(6-((1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)amino)hexyl)thiourea was synthesized for the first time from 6-((1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)amino)hexan-1-amine and 3-chlorophenylisothiocyanate in DMF with a 60% yield. It was characterized by 1H, 13C{1H} NMR, FT-IR, MS, and elemental analysis.
1. Introduction
Previously, we have observed synthesis and inhibitory activity against human soluble epoxide hydrolase (sEH) of multiple series of adamantyl-containing 1,3-disubstituted ureas and thioureas, and the same findings have been reported by our colleagues [1,2,3]. sEH is involved in the metabolism of epoxy fatty acids to corresponding vicinal diols through a catalytic addition of water [4,5]. The resulting dihydroxyepoxyeicosatrienoic acids promote various pathological states, such as pain and inflammation [6]. Thus, inhibition of sEH could be beneficial in the treatment of cardiovascular, neuronal, and renal diseases [7,8]. 1,3-Disubstituted ureas containing lipophilic moieties such as adamantyl, bornyl, or 4-(trifluoromethoxy)phenyl (Figure 1) are among the most potent sEH inhibitors active in nanomolar concentrations [9,10,11].
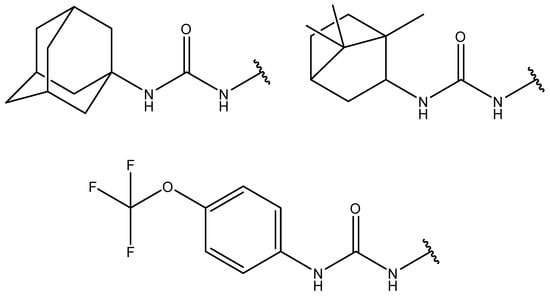
Figure 1.
Most common fragments of soluble epoxide hydrolase inhibitors.
This makes the synthesis and evaluation of new adamantyl-containing 1,3-disubstituted thioureas as soluble epoxide hydrolase inhibitors relevant. The present study focuses on the preparation and identification of 1-(3-chlorophenyl)-3-(6-((1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)amino)hexyl)thiourea.
2. Results and Discussion
We chose to use imine derived from camphor as a fragment for our new class of soluble epoxide hydrolase inhibitors for a number of reasons. First of all, substitution of adamantane with natural monoterpene fragments [2] or their simultaneous introduction into the same molecule [12] can provide great benefits in the field of green chemistry. Secondly, compounds containing imines derived from camphor possess various biological activities, including strong antiviral activity [13]. Finally, the imine group, unlike the urea and thiourea groups, is slightly basic and is capable of forming salts with acids. These salts could be water-soluble, or could at least possess significantly higher water solubility compared to free imine.
Based on our previous experience, we proposed a method for the preparation and isolation of 1-(3-chlorophenyl)-3-(6-((1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)amino)hexyl)thiourea (3) (Scheme 1).
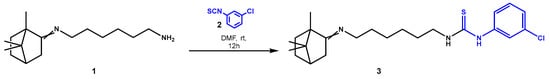
Scheme 1.
Synthesis of compound 3 from 6-((1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)amino)hexan-1-amine (1) and 3-chlorophenylisothiocyanate (2).
Starting compound 1 has very poor solubility in hexane and diethyl ether, so we used DMF as a solvent for this reaction. We usually use anhydrous DMF for the reactions including isocyanates due to its water sensitivity. However, for isothiocyanates, this is not necessary. We also did not use Et3N in this reaction. Commonly, we use Et3N to increase the basicity of the medium and thus to speed up the reaction. However, to remove Et3N from the reaction mass, we rinse it with 1 N HCl and then with water. For this reaction, we could not use HCl due to the possibility of salt formation by the product. For the same reason, we did not use excess amine in this reaction. Excess amine is used to speed up the reaction and to make sure that no isocyanate or isothiocyanate remains. Excess amine is also removed by treating the reaction mass with 1 N HCl. Since we could not do this, we used the starting material in the equimolar ratio and increased the reaction time from 8 to 12 h.
After the reaction mass was stirred for 12 h, the formation of pale-yellow precipitate of crude compound 3 was observed. After the solvent was filtered off, the precipitate was washed with distilled water and dried in vacuo. Crystallization from ethanol produced pure compound 3 as a white solid.
3. Materials and Methods
1H and 13C NMR spectra were recorded with a Bruker DPX 300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) (at frequencies of 300 and 75 MHz) in DMSO-d6 solution with TMS as the standard. The J values are given in Hz. The IR spectrum was recorded on a FT-801 FT-IR spectrometer (LLC Simex, Novosibirsk, Russia). The MS spectrum was recorded on an Agilent MS 5977b (Agilent Technologies, Inc., Santa Clara, CA, USA) using electron impact ionization (EI). The melting point was measured using a Büchi M-565 (Büchi Labortechnik AG, Flawil, Switzerland) and was calculated as the mean of 3 separate experiments. The elemental analysis was performed on a Perkin-Elmer Series II 2400 Elemental Analyzer (Perkin Elmer Inc., Waltham, MA, USA). The TLC analysis was carried out on Merck silica gel chromatography plates with fluorescent indicator F254 (1.05554); sorbent: Silica 60, with a layer thickness of 200 um; a pore size of 60 Å, and a particle size of 10-12 um; binder: organic polymer (Merck KGaA, Darmstadt, Germany). The solvents and reagents were purchased from commercial sources. The NMR signals of the bornylidene fragment were assigned according to data found in the literature [13].
Synthesis of 1-(3-chlorophenyl)-3-(6-((1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)amino)hexyl)thiourea (3) was performed according to the following procedure. Labelling of atoms in the compound 3 is given in Figure 2. For detailed spectral data see Supplementary Materials.
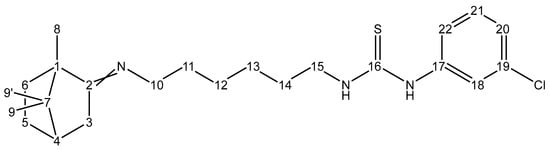
Figure 2.
Carbon atoms labeling for compound 3.
Into a flat-bottom flask equipped with a magnetic stirrer, we added 300 mg (1.20 mmol) of compound 1, 5 mL of hexane and 200 mg (1.18 mmol) of compound 2. The resulting mixture was left to stir for 12 h at rt. After that, pale-yellow precipitate of crude compound 3 was filtered off, washed with 30 mL of distilled water, and dried in vacuo. The resulting crude compound 3 was purified by crystallization from ethanol. Yield 298 mg, 0.70 mmol, 60% yield, white solid, m.p. = 112.7 °C. FT-IR (ATR, cm−1): 549 (C-Cl), 738 (C=S), 1478 (C-N), 1545 (NH), 1673 (C=N), 2942 (CH). Mass spectrum, m/z (Irel.%): 421 (2% [M + 1]+), 250 (20% [M-Cl-Ph-NCS]+), 169 (100%, [Cl-Ph-NCS]+). 1H NMR (DMSO-d6), δ, ppm: 0.68 (s, 3H, H-9), 0.84 (s, 3H, H-9), 0.87 (s, 3H, H-8), 1.13–1.22 (m, 2H, (H-5endo, H-6endo)), 1.26–1.35 (m, 4H, H-12, H-13), 1.48–1.55 (m, 4H, H-11, H-14), 1.55–1.61 (m, 1H, H-6exo), 1.75–1.84 (m, 2H, (H-3endo, H-5exo)), 1.88 (t, 1H, J = 4.5 Hz, H-4), 2.27 (br.d, 1H, J = 17.2 Hz, H-3exo), 3.02–3.20 (m, 2H, H-10), 3.38–3.49 (m, 2H, H-15), 7.05–7.13 (m, 1H, H-20), 7.25–7.34 (m, 2H, H-21, H-22), 7.70 (s, 1H, H-18), 7.86 (br.s, 1H, NH-C-15), 9.48 (br.s, 1H, NH-C-17). 13C{1H} NMR (DMSO-d6), δ, ppm: 12.2 (C-8), 19.4 (C-9), 19.9 (C-9), 27.0 (C-12), 27.3 (C-13), 27.6 (C-5), 29.0 (C-11), 30.8 (C-14), 32.6 (C-6), 35.4 (C-3), 43.9 (C-4), 44.4 (C-15), 47.0 (C-7), 51.8 (C-10), 53.7 (C-1), 121,3 (C-22), 122.4 (C-18), 123.9 (C-20), 130.7 (C-21), 133.2 (C-19), 141.8 (C-17), 180.4 (C-16), 180.8 (C-2). Calcd. for C23H34ClN3S: C 65.77; H 8.16; N 10.00; S 7.63. Found: C 65.80; H 8.15; N 10.05; S 7.60. M = 420.06.
4. Conclusions
In this work, we presented a method for the preparation of 1-(3-chlorophenyl)-3-(6-((1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)amino)hexyl)thiourea from 6-((1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)amino)hexan-1-amine and 3-chlorophenylisothiocyanate in DMF with 60% yield. The compound, which was synthesized for the first time, was identified via 1H and 13C{1H} NMR, MS, FT-IR, and elemental analyses.
Supplementary Materials
1H NMR, 13C NMR and FT-IR spectra. Figure S1. 1H NMR spectrum of compound 3. Figure S2. 13C NMR spectrum of compound 3. Figure S3. FT-IR spectrum of compound 3.
Author Contributions
Conceptualization, V.B.; investigation, D.Z.; writing, review, and editing, D.Z. and V.B.; project administration, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was performed with financial support from the Russian Science Foundation (project No. 19-73-10002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, Y.; Sun, J.; Tong, H.; Wang, J.; Cao, R.; Xu, H.; Chen, L.; Morisseau, C.; Zhang, M.; Shi, Y.; et al. Design and Synthesis of Dual-Targeting Inhibitors of sEH and HDAC6 for the Treatment of Neuropathic Pain and Lipopolysaccharide-Induced Mortality. J. Med. Chem. 2024, 67, 2095–2117. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, V.; Morisseau, C.; Pitushkin, D.; Fayzullin, R.R.; Karlov, D.; Vernigora, A.; Kuznetsov, Y.; Abbas, S.M.H.; Butov, G.M.; Hammock, B.D. Ureas derived from camphor and fenchone reveal enantiomeric preference of human soluble epoxide hydrolase. Rusults Chem. 2022, 4, 100653. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, V.V.; Danilov, D.V.; D’yachenko, V.S.; Rasskazova, E.V.; Butov, G.M. Synthesis and Properties of 1,3-Disubstituted Ureas and Their Isosteric Analogs Containing Polycyclic Fragments: I. Synthesis of 1-(Adamantan-1-yl)-3-(fluoro, chlorophenyl)ureas. Russ. J. Org. Chem. 2020, 56, 735–740. [Google Scholar] [CrossRef]
- Luo, A.; Wu, Z.; Li, S.; McReynolds, C.B.; Wang, D.; Liu, H.; Huang, C.; He, T.; Zhang, X.; Wang, Y.; et al. The soluble epoxide hydrolase inhibitor TPPU improves comorbidity of chronic pain and depression via the AHR and TSPO signaling. J. Transl. Med. 2023, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.-H.; Morisseau, C.; Zhang, M.; Dong, H.-J.; Zhu, Q.-M.; Huo, X.-K.; Sun, C.-P.; Hammock, B.D.; Ma, X.-C. Genetic deletion or pharmacological inhibition of soluble epoxide hydrolase attenuated particulate matter 2.5 exposure mediated lung injury. J. Hazard. Mater. 2023, 458, 131890. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Sig. Transduct. Target Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Mehta, R.; Goswami, S.K.; Hammock, B.D.; Morisseau, C.; Hwang, S.H.; Mallappa, O.; Azeemuddin, M.M.; Rafiq, M.; Manjula, S.N. Neuroprotective effect of herbal extracts inhibiting soluble epoxide hydrolase (sEH) and cyclooxygenase (COX) against chemotherapy-induced cognitive impairment in mice. Biochem. Biophys. Res. Commun. 2023, 667, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.T.; Yang, J.; Morisseau, C.; He, Q.; Hammock, B.; Youn, J.H. Effects of Individual Circulating FFAs on Plasma and Hepatic FFA Epoxides, Diols, and Epoxide-Diol Ratios as Indices of Soluble Epoxide Hydrolase Activity. Int. J. Mol. Sci. 2023, 24, 10760. [Google Scholar] [CrossRef] [PubMed]
- Codony, S.; Entrena, J.M.; Calvó-Tusell, C.; Jora, B.; González-Cano, R.; Osuna, S.; Corpas, R.; Morisseau, C.; Pérez, B.; Barniol-Xicota, M.; et al. Synthesis, In Vitro Profiling, and In Vivo Evaluation of Benzohomoadamantane-Based Ureas for Visceral Pain: A New Indication for Soluble Epoxide Hydrolase Inhibitors. J. Med. Chem. 2022, 65, 13660–13680. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, V.; Morisseau, C.; Karlov, D.; Pitushkin, D.; Vernigora, A.; Rasskazova, E.; Butov, G.M.; Hammock, B.D. Bioisosteric substitution of adamantane with bicyclic lipophilic groups improves water solubility of human soluble epoxide hydrolase inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127430. [Google Scholar] [CrossRef] [PubMed]
- Hammock, B.D.; McReynolds, C.B.; Wagner, K.; Buckpitt, A.; Cortes-Puch, I.; Croston, G.; Lee, K.S.S.; Yang, J.; Schmidt, W.K.; Hwang, S.H. Movement to the Clinic of Soluble Epoxide Hydrolase Inhibitor EC5026 as an Analgesic for Neuropathic Pain and for Use as a Nonaddictive Opioid Alternative. J. Med. Chem. 2021, 64, 1856–1872. [Google Scholar] [CrossRef] [PubMed]
- Munkuev, A.A.; Zakharenko, A.L.; Kornienko, T.E.; Dyrkheeva, N.S.; Ilina, E.S.; Suslov, E.V.; Issa, F.; Achara, C.; Reynisson, J.; Volcho, K.P.; et al. Synthesis of adamantane-monoterpene conjugates with 1,3,4-thiadiazol-2(3H)-imine linker and evaluation of their inhibitory activity against TDP1. Med. Chem. Res. 2024, 33, 324–335. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.I.; Shernyukov, A.V.; Gatilov, Y.V.; Razumova, Y.V.; Zarubaev, V.V.; Tretiak, T.S.; Pokrovsky, A.G.; Kiselev, O.I.; Salakhutdinov, N.F. Discovery of a new class of antiviral compounds: Camphor imine derivatives. Eur. J. Med. Chem. 2015, 105, 263–273. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).