Abstract
The one-pot template reaction between 2-(diphenylphosphino)benzaldehyde, benzylamine and copper(I) iodide yields the dinuclear copper complex (P∩N)2Cu2I2, as revealed by single-crystal X-ray diffraction.
1. Introduction
P∩N ligands are used in many areas of coordination chemistry due to their advantageous and tunable ligand properties. Many architectures are feasible. Besides the length of the bridge between the phosphorus and nitrogen atoms, the properties of the N-donor atom can vary: for example, the nitrogen atom can be part of a N heterocycle or it can be found as part of an amine, imine, or amide group. A huge number of variations in P∩N ligands have been realized, and their use covers practically all areas of coordination chemistry from basic synthetic and structural research to more applied fields like catalysis and optoelectronics [1,2,3,4,5,6,7,8]. Many P∩N ligands, e.g., Ph2P(s-py) (with s-py = substituted 2-pyridyl-type moiety), are commercially available and, hence, broadly used.
An easy variation in P∩N ligand properties can be realized by imine formation, according to Scheme 1. Commercially available 2-(diphenylphosphino)benzaldehyde is a particularly attractive platform as it can give access to a wide variety of P∩N ligands. Imine formation is a very efficient process, which does not require demanding reaction procedures. In principle, all types of primary amines—aromatic or aliphatic—react in high yields under mild conditions [9,10,11,12,13,14,15,16,17,18,19,20,21]. In this context, of particular interest might be the use of oligo-amines, which lead to the formation of polydentate P∩N ligands [22,23], or the use of chiral amines [24].
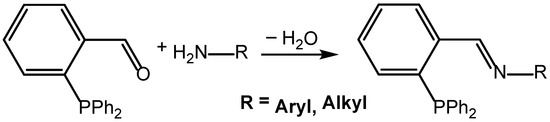
Scheme 1.
Versatile access to P∩N ligands starting from 2-(diphenylphosphino)benzaldehyde and a primary amine.
Our interest in P∩N copper(I) complexes comes from the fact that many of them feature interesting luminescence properties. For example, Ph2P(s-py)-type ligands form complexes of the type (P∩N)3Cu2X2 (X = Cl, Br, I) with a 3:2 stoichiometry with one bridging bidentate P∩N ligand and two monodentate P∩N ligands bound via the P atoms [25]. The halides build the bridge between the two copper atoms. It should be noted that other stoichiometries are also known for Ph2P(s-py)-type ligands [2,25,26,27]. These complexes often show extremely high emission quantum yield with comparable low emission lifetimes due to thermally activated delayed fluorescence, which makes them interesting candidates for OLED applications. It has been shown that OLEDs using those emitters are, indeed, promising alternatives to expensive emitter molecules based on iridium [1,28,29,30,31,32].
To investigate whether ligands bearing an imine group, as shown in Scheme 1, also form photophysically interesting complexes, the synthesis of a copper(I) iodide complex was undertaken with R = benzyl. The ligand with R = Bn is not new but has already been used for the complexation of Re(I) [33], Fe [34], Co [35], Pd [36,37,38,39,40], Rh [41], Ru [42], Au [9,43], Pt [9,44], Ir [45], and Cu [46].
2. Results and Discussion
The original idea was to synthesize a complex with a 3:2 stoichiometry, like those of the type (P∩N)3Cu2X2 (vide supra). Therefore, the stoichiometry, as described in Section 3, follows this 3:2 relation.
The title compound was synthesized in a very simple one-pot reaction (Scheme 2). Such a template synthesis facilitates the procedure even more as it saves the isolation/purification step of the P∩N ligand. First, 2-(diphenylphosphino)benzaldehyde and benzylamine were stirred for 3 h under ambient conditions in dichloromethane [33]; then, solid copper(I) iodide was added. Immediately, a dark-orange solution was formed. After stirring overnight, the formed orange-red powder was isolated by decantation and dried in vacuo. Not untypical for copper halide complexes bearing phosphane ligands, the resulting compound was only very slightly soluble in standard, non-coordinating solvents. In donating solvents like hot pyridine and acetonitrile, the complex is sparingly soluble; however, it is doubtful that the complex also remains in its dimeric form in solution. Because of this low solubility in non-coordinating solvents, no NMR spectra were recorded.
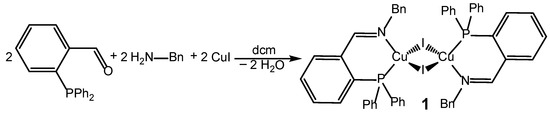
Scheme 2.
Synthesis of title compound 1.
The complex could be recrystallized from hot acetonitrile, yielding well-shaped red crystals. The single-crystal X-ray diffraction analysis revealed that instead of the intended 3:2 stoichiometry, a dinuclear complex with a 1:1 composition was formed, as shown in Figure 1. The copper atom is surrounded by a nitrogen atom, a phosphorus atom, and two iodine atoms in a distorted tetrahedral environment with the iodine atoms in a bridging binding mode. The central {Cu2I2} core is somewhat asymmetric with two similar but yet different Cu–I bond lengths of 2.579(1) Å (Cu1–I1) and 2.698(1) Å (Cu1–I1i). The nitrogen atoms are in a cis-configuration, rendering the whole complex inversion symmetric. The overall structure is very similar to a copper(I) iodide complex, bearing a closely related P∩N ligand [47]. The Cu1–N1 bond length (2.127(3) Å) is in the range typical for sp2-hybridized nitrogen atoms [2,25]. The Cu1–P1 bond length (2.227(1) Å) is in the expected range. The bite angle of the P∩N ligand is 89.79(7)°, and the Cu1–I1–Cu1i angle is 70.10(2)°. Cuprophilic interactions do not play a considerable role in the formation of the dimeric structure, as the Cu…Cu separation is 3.032 Å and, thus, well beyond the sum of the van der Waals radii of 2.8 Å [48]. All lengths and angles are, thus, as expected for a complex with a {Cu2I2} structure [49,50,51].
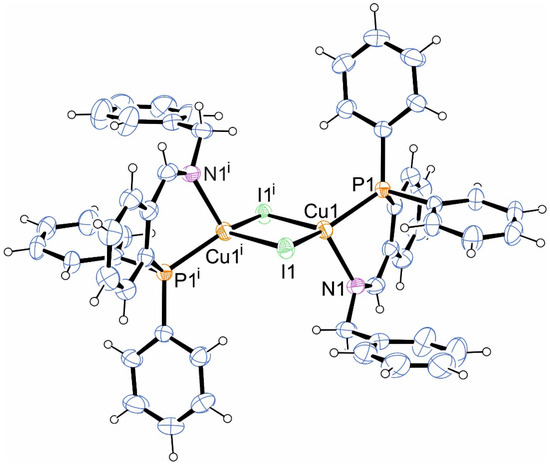
Figure 1.
Molecular structure of title compound (displacement parameters are drawn at 50% probability level). Selected bond length (Å) and angles (°): Cu1–I1 2.579(1), Cu1–I1i 2.698(1), Cu1–N1 2.127(3), Cu1–P1 2.227(1), N1–Cu1–P1 89.79(7), I1–Cu1–I1i 109.90(2), Cu1–I1–Cu1i 70.10(2). Symmetry code: i = −x + 1, −y, −z + 1.
In the ESI mass spectrum, the fragment [L2Cu]+ (L = ligand) can be detected as the most intense signal. Other fragments could not be assigned with high confidence (cf. Figure S2). The IR spectrum features signals at 1628 and 1432 cm−1, which is characteristic of the C=N stretch vibration and the P–phenyl group, respectively [12,52,53,54,55,56]. Contrary to many other related dinuclear complexes, 1 does not feature any observable photoluminescence at room temperature upon excitation with a UV light. This can be explained by an absence of low-lying π* orbitals, including the imine nitrogen atom, as the luminescence of such dinuclear copper(I) complexes is often based on a d→π* metal-to-ligand-transfer-excited state [56].
3. Experimental Methodology
3.1. General
All solvents and starting materials were commercially available and used without further purification. Elemental analyses were carried out by the Center for Chemical Analysis of the Faculty of Natural Sciences of the University Regensburg. The IR spectrum was recorded on a Bruker Alpha II spectrometer equipped with a Platinum ATR module. Single-crystal structure analysis was carried out on an STOE-IPDS diffractometer (STOE & Cie GmbH, Darmstadt, Germany) with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). The structure was solved by direct methods (SIR-97 [57]) and refined with olex2.refine [58] in Olex2 [59]. The H atoms were calculated geometrically, and a riding model was applied in the refinement process.
3.2. Synthesis of Diiodido-bis{N-[2-(diphenylphosphino)benzylidene]benzylamine-κ2N,P}dicopper(I), 1
2-(Diphenylphosphino)benzaldehyde (0.10 g, 0.34 mmol) and benzylamine (37 mg, 0.34 mmol) were dissolved in 30 mL of dichloromethane and stirred for 3 h. Copper(I) iodide (44 mg, 0.23 mmol) was added in one portion. The color of the solution turned immediately dark orange. The reaction mixture was stirred overnight. The orange-red powder formed was isolated by decanting and was dried in a vacuum. As soon as the complex precipitated from the reaction mixture, it was not sufficiently soluble in common weakly or non-coordinating solvents to perform NMR spectroscopy. Nicely shaped, red single crystals suitable for single-crystal X-ray diffraction were obtained by cooling a hot-filtrated solution of the title compound in acetonitrile.
Yield: 0.11 g, 84% based on CuI. Elemental analysis calc. for C52H44Cu2I2N2P2 (1139.79 g·mol−1): C 54.79%; H 3.89%; N 2.46%; found: C 55.67%; H 3.96%; N 2.50%. MS(EI) (dcm/MeOH + 10 mM NH4Ac): m/z 821.3 [L2Cu]+. IR (ATR, cm−1): 3034, 3000, 2883, 2843, 1628, 1477, 1432, 1092, 1024, 766, 744, 691, 513, 480, 430. The IR spectrum can be found in the Supplementary Materials.
Crystal data for C52H44Cu2I2N2P2 (M = 1139.73 g/mol): monoclinic, space group P21/n (no. 14), a = 15.916(3) Å, b = 9.226(1) Å, c = 15.939(3) Å, β = 95.94(2)°, V = 2328.0(7) Å3, Z = 2, T = 297 K, μ(CuKα) = 2.346 mm−1, Dcalc = 1.626 g/cm3, 24633 reflections measured (2.8° ≤ 2Θ ≤ 25.91°), 4505 unique (Rint = 0.042, Rsigma = 0.023), which were used in all calculations. The final R1 was 0.0321 (I > 2σ(I)) and wR2 was 0.0860 (all data). Further crystallographic details can be found in Table S1. CCDC 2334274 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
Supplementary Materials
Figure S1: Elemental analysis of the title compound; Figure S2: MS(EI) spectrum of the title compound; Figure S3: IR-spectrum (ATR) of the title compound; Table S1: Crystal data and data collection and structure refinement details for 1.
Author Contributions
J.S. measured the IR spectrum; M.Z. collected the X-ray data and solved the structure; U.M. conceived the study, prepared the compound, analyzed the data, and wrote this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CCDC 2334274 contains supplementary crystallographic data for this paper. This information can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. The results of the elemental analysis and the mass spectrum can be found in the supporting information.
Acknowledgments
The author thanks Michael Bodensteiner for supporting with updating the cif file.
Conflicts of Interest
The authors declare that they do not have any competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cheng, G.; Zhou, D.; Monkowius, U.; Yersin, H. Fabrication of a Solution-Processed White Light Emitting Diode Containing a Single Dimeric Copper(I) Emitter Featuring Combined TADF and Phosphorescence. Micromachines 2021, 12, 1500. [Google Scholar] [CrossRef] [PubMed]
- Hofbeck, T.; Niehaus, T.A.; Fleck, M.; Monkowius, U.; Yersin, H. P∩N Bridged Cu(I) Dimers Featuring Both TADF and Phosphorescence. From Overview towards Detailed Case Study of the Excited Singlet and Triplet States. Molecules 2021, 26, 3415. [Google Scholar] [CrossRef]
- Aucott, S.M.; Slawin, A.M.Z.; Woollins, J.D. The co-ordination chemistry of 2-(diphenylphosphinoamino)pyridine. J. Chem. Soc. Dalton Trans. 2000, 2559–2575. [Google Scholar] [CrossRef]
- Carroll, M.P.; Guiry, P.J. P,N ligands in asymmetric catalysis. Chem. Soc. Rev. 2014, 43, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Munzeiwa, W.A.; Omondi, B.; Nyamori, V.O. Architecture and synthesis of P,N-heterocyclic phosphine ligands. Beilstein J. Org. Chem. 2020, 16, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B. The Backbone of Success of P,N-Hybrid Ligands: Some Recent Developments. Molecules 2022, 27, 6293. [Google Scholar] [CrossRef]
- Margalef, J.; Biosca, M.; de la Cruz Sánchez, P.; Faiges, J.; Pàmies, O.; Diéguez, M. Evolution in heterodonor P-N, P-S and P-O chiral ligands for preparing efficient catalysts for asymmetric catalysis. From design to applications. Coord. Chem. Rev. 2021, 446, 214120. [Google Scholar] [CrossRef]
- Yersin, H.; Czerwieniec, R.; Monkowius, U.; Ramazanov, R.; Valiev, R.; Shafikov, M.Z.; Kwok, W.-M.; Ma, C. Intersystem crossing, phosphorescence, and spin-orbit coupling. Two contrasting Cu(I)-TADF dimers investigated by milli- to micro-second phosphorescence, femto-second fluorescence, and theoretical calculations. Coord. Chem. Rev. 2023, 478, 214975. [Google Scholar] [CrossRef]
- Chiririwa, H.; Moss, J.R.; Hendricks, D.; Smith, G.S.; Meijboom, R. Synthesis, characterisation and in vitro evaluation of platinum(II) and gold(I) iminophosphine complexes for anticancer activity. Polyhedron 2013, 49, 29–35. [Google Scholar] [CrossRef]
- Kindervater, M.B.; Binder, J.F.; Baird, S.R.; Vogels, C.M.; Geier, S.J.; Macdonald, C.L.B.; Westcott, S.A. The phosphinoboration of 2-diphenylphosphino benzaldehyde and related aldimines. J. Organomet. Chem. 2019, 880, 378–385. [Google Scholar] [CrossRef]
- Shirakawa, E.; Nakao, Y.; Murota, Y.; Hiyama, T. Palladium-iminophosphine-catalyzed homocoupling of alkynylstannanes and other organostannanes using allyl acetate or air as an oxidant. J. Organomet. Chem. 2003, 670, 132–136. [Google Scholar] [CrossRef]
- Sánchez, G.; García, J.; Serrano, J.L.; García, L.; Pérez, J.; López, G. Homoleptic palladium complexes with phosphine-amide or iminophosphine ligands. Inorg. Chim. Acta 2010, 363, 1084–1091. [Google Scholar] [CrossRef]
- Xue, Z.; Linh, N.T.B.; Noh, S.K.; Lyoo, W.S. Phosphorus-Containing Ligands for Iron(III)-Catalyzed Atom Transfer Radical Polymerization. Angew. Chem. Int. Ed. 2008, 47, 6426–6429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xing, J.; Dong, Y.; Xie, S.; Ren, S.; Qi, X.; Sun, H.; Li, X.; Fuhr, O.; Fenske, D. Phosphine-assisted C–H bond activation in Schiff bases and formation of novel organo cobalt complexes bearing Schiff base ligands. New J. Chem. 2018, 42, 4646–4652. [Google Scholar] [CrossRef]
- Zheng, Q.; Zheng, D.; Han, B.; Liu, S.; Li, Z. Chromium complexes supported by the bidentate PN ligands: Synthesis, characterization and application for ethylene polymerization. Dalton Trans. 2018, 47, 13459–13465. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.; Camadanli, S.; Klein, H.-F. Spontaneous Bicyclometalation of a Single Cobalt(I) Complex Stabilized by a δ-C–H Agostic Interaction. Eur. J. Inorg. Chem. 2018, 608–611. [Google Scholar] [CrossRef]
- Nakao, Y.; Hirata, Y.; Ishihara, S.; Oda, S.; Yukawa, T.; Shirakawa, E.; Hiyama, T. Stannylative Cycloaddition of Enynes Catalyzed by Palladium-Iminophosphine. J. Am. Chem. Soc. 2004, 126, 15650–15651. [Google Scholar] [CrossRef] [PubMed]
- Inami, T.; Sako, S.; Kurahashi, T.; Matsubara, S. Methylenecyclopropanes in [4 + 1] Cycloaddition with Enones. Org. Lett. 2011, 13, 3837–3839. [Google Scholar] [CrossRef]
- Best, J.; Wilson, J.M.; Adams, H.; Gonsalvi, L.; Peruzzini, M.; Haynes, A. Reactivity of Rhodium(I) Iminophosphine Carbonyl Complexes with Methyl Iodide. Organometallics 2007, 26, 1960–1965. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Wu, R.; Li, Q.; Wang, Q.-R.; Xu, Y.-C.; Jiang, L. ‘Evans Auxiliary’ Based P–N Ligands for Pd-Catalyzed Asymmetric Allylic Alkylation Reactions. Synlett 2012, 23, 1805–1808. [Google Scholar] [CrossRef]
- St-Coeur, P.-D.; Kinley, S.; Vogels, C.M.; Decken, A.; Morin, P., Jr.; Westcott, S.A. Synthesis, characterization, and anticancer properties of iminophosphineplatinum(II) complexes containing boronate esters. Can. J. Chem. 2017, 95, 207–213. [Google Scholar] [CrossRef]
- Yang, H.; Du, J.; Wang, C.-L.; Zhan, S.-Z. Synthesis, structures, characterizations and catalytic behaviors for hydrogen evolution of copper(II) and copper(I) complexes supported by diiminodiphosphines. Inorg. Chem. Commun. 2021, 130, 108719. [Google Scholar] [CrossRef]
- Gao, P.-S.; Li, N.; Zhang, J.-L.; Zhu, Z.-L.; Gao, Z.-W.; Sun, H.-M.; Zhang, W.-Q.; Xu, L.-W. Highly Efficient Palladium-Catalyzed Allylic Alkylation of Cyanoacetamides with Controllable and Chemoselective Mono- and Double Substitutions. ChemCatChem 2016, 8, 3466–3474. [Google Scholar] [CrossRef]
- Ortega-Gaxiola, J.I.; Valdés, H.; Rufino-Felipe, E.; Toscano, R.A.; Morales-Morales, D. Synthesis of Pd(II) complexes with P-N-OH ligands derived from 2-(diphenylphosphine)-benzaldehyde and various aminoalcohols and their catalytic evaluation on Suzuki-Miyaura couplings in aqueous media. Inorg. Chim. Acta 2020, 504, 119460. [Google Scholar] [CrossRef]
- Zink, D.M.; Bächle, M.; Baumann, T.; Nieger, M.; Kühn, M.; Wang, C.; Klopper, W.; Monkowius, U.; Hofbeck, T.; Yersin, H.; et al. Synthesis, Structure, and Characterization of Dinuclear Copper(I) Halide Complexes with P^N Ligands Featuring Exciting Photoluminescence Properties. Inorg. Chem. 2013, 52, 2292–2305. [Google Scholar] [CrossRef] [PubMed]
- Olmos, M.E.; Schier, A.; Schmidbaur, H. 2-(Diphenylphosphino)-pyridine as an Ambidentate Ligand in Homo- and Hetero-binuclear Complexes of Copper, Silver, and Gold. Z. Naturforsch. 1997, 52, 203–208. [Google Scholar] [CrossRef]
- Neshat, A.; Aghakhanpour, R.B.; Mastrorilli, P.; Todisco, S.; Molani, F.; Wojtczak, A. Dinuclear and tetranuclear copper(I) iodide complexes with P and P^N donor ligands: Structural and photoluminescence studies. Polyhedron 2018, 154, 217–228. [Google Scholar] [CrossRef]
- Ravaro, L.P.; Zanoni, K.P.S.; de Camargo, A.S.S. Luminescent Copper(I) complexes as promising materials for the next generation of energy-saving OLED devices. Energy Reports 2020, 6, 37–45. [Google Scholar] [CrossRef]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Zink, D.M.; Bergmann, L.; Ambrosek, D.; Wallesch, M.; Volz, D.; Mydlak, M. Singlet harvesting copper-based emitters: A modular approach towards next-generation OLED technology. Transl. Mater. Res. 2014, 1, 015003. [Google Scholar] [CrossRef]
- Zink, D.M.; Volz, D.; Baumann, T.; Mydlak, M.; Flügge, H.; Friedrichs, J.; Nieger, M.; Bräse, S. Heteroleptic, Dinuclear Copper(I) Complexes for Application in Organic Light-Emitting Diodes. Chem. Mater. 2013, 25, 4471–4486. [Google Scholar] [CrossRef]
- Volz, D.; Chen, Y.; Wallesch, M.; Liu, R.; Fléchon, C.; Zink, D.M.; Friedrichs, J.; Flügge, H.; Steininger, R.; Göttlicher, J.; et al. Bridging the Efficiency Gap: Fully Bridged Dinuclear Cu(I)-Complexes for Singlet Harvesting in High-Efficiency OLEDs. Adv. Mater. 2015, 27, 2538–2543. [Google Scholar] [CrossRef]
- Monkowius, U.; Zabel, M. Tricarbonylchlorido{N-[2-(diphenyl-phosphino)benzylidene]benzylamine-κ2N,P}rhenium(I) dichloromethane solvate. Acta Cryst. 2008, 64, m313. [Google Scholar]
- Xing, J.; Sun, H.; Zheng, T.; Qi, X.; Li, X.; Fuhr, O.; Fenske, D. Syntheses and properties of 2-azaallyl Iron(I) complexes via Csp3-H bond activation. J. Organomet. Chem. 2018, 868, 61–65. [Google Scholar] [CrossRef]
- Xing, J.; Sun, H.; Xue, B.; Li, X.; Fuhr, O.; Fenske, D. Formation of 2-Azaallyl Cobalt(I) Complexes by Csp3−H BondActivation. Organometallics 2017, 36, 975–980. [Google Scholar] [CrossRef]
- Mogorosi, M.M.; Mahamo, T.; Moss, J.R.; Mapolie, S.F.; Slootweg, J.C.; Lammertsma, K.; Smith, G.S. Neutral palladium(II) complexes with P,N Schiff-base ligands: Synthesis, characterization and catalytic oligomerisation of ethylene. J. Organomet. Chem. 2011, 696, 3585–3592. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Pretorius, M. Synthesis and evaluation of phosphine–N ligands in transition metal-catalysed C–C bond forming reactions. J. Mol. Cataly. A 2008, 284, 77–84. [Google Scholar] [CrossRef]
- Chiririwa, H.; Meijboom, R. (SP-4-2)-Chlorido{N-[2-(diphenylphosphanyl)benzylidene]benzylamine-κ2P,N}(methyl)palladium(II). Acta Cryst. E 2011, 67, m1498. [Google Scholar] [CrossRef] [PubMed]
- Chiririwa, H.; Moss, J.R.; Hendricks, D.; Meijboom, R.; Muller, A. Synthesis, characterisation and in vitro evaluation of palladium(II) iminophosphine complexes for anticancer activity. Transition Met. Chem. 2013, 38, 165–172. [Google Scholar]
- Nobre, S.M.; Monteiro, A.L. Pd complexes of iminophosphine ligands: A homogeneous molecular catalyst for Suzuki-Miyaura cross-coupling reactions under mild conditions. J. Mol. Cataly. A 2009, 313, 65–73. [Google Scholar] [CrossRef]
- Wajda-Hermanowicz, K.; Kochel, A.; Wróbel, R. Coordination studies of nitrogen-containing aryl phosphine ligands PˆN and PˆNˆN with rhodium. J. Organomet. Chem. 2018, 860, 30–48. [Google Scholar] [CrossRef]
- Essoun, E.; Wang, R.; Aquino, M.A.S. Disassembly of diruthenium(II,III) tetraacetate with P–N donor ligands. Inorg. Chim. Acta 2017, 454, 97–106. [Google Scholar] [CrossRef]
- Traut-Johnstone, T.; Kanyanda, S.; Kriel, F.H.; Viljoen, T.; Kotze, P.D.R.; van Zyl, W.E.; Coates, J.; Rees, D.J.G.; Meyer, M.; Hewer, R.; et al. Heteroditopic P,N ligands in gold(I) complexes: Synthesis, structure and cytotoxicity. J. Inorg. Biochem. 2015, 145, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Mahamo, T.; Moss, J.R.; Mapolie, S.F.; Smith, G.S.; Slootweg, J.C.; Lammertsma, K. Platinacycloalkane complexes containing [P,N] bidentate ligands: Synthesis and decomposition studies. Dalton Trans. 2014, 43, 5546–5557. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Tian, Z.; Ge, X.; Gong, Y.; Zheng, H.; Shi, S.; Liu, Z. Lysosome-Targeted Phosphine-Imine Half-Sandwich Iridium(III) Anticancer Complexes: Synthesis, Characterization, and Biological Activity. Organometallics 2019, 38, 1761–1769. [Google Scholar] [CrossRef]
- See Supporting Information of: Kern, T.; Monkowius, U.; Zabel, M.; Knör, G. Mononuclear Copper(I) Complexes Containing Redox-Active 1,2-Bis(aryl-imino)acenaphthene Acceptor Ligands: Synthesis, Crystal Structures and Tuneable Electronic Properties. Eur. J. Inorg. Chem. 2010, 4148–4156. [Google Scholar]
- Beck, J.F.; Schmidt, J.A.R. Isolation and characterization of main group and late transition metal complexes using orthometallated imine ligands. Dalton Trans. 2012, 41, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Egly, J.; Bissessar, D.; Achard, T.; Heinrich, B.; Steffanut, P.; Mauro, M.; Bellemin-Laponnaz, S. Copper(I) complexes with remotely functionalized phosphine ligands: Synthesis, structural variety, photophysics and effect onto the optical properties. Inorg. Chim. Acta 2021, 514, 119971. [Google Scholar] [CrossRef]
- Hirtenlehner, C.; Monkowius, U. Syntheses, crystal structures and blue luminescence of Cu2X2(Ph3P)2[(−)-nicotine]2 (X=Br, I). Inorg. Chem. Commun. 2012, 15, 109–112. [Google Scholar] [CrossRef]
- Kern, T.; Monkowius, U.; Zabel, M.; Knör, G. Synthesis, crystal structure and charge transfer spectra of dinuclear copper(I) complexes bearing 1,2-bis(arylimino)acenaphthene acceptor ligands. Inorg. Chim. Acta 2011, 374, 632–636. [Google Scholar] [CrossRef]
- Jensen, K.A.; Nielsen, P.H. Infrared Spectra of Some Organic Compounds of Group V B Elements. Acta Chem. Scand. 1963, 17, 1875–1885. [Google Scholar] [CrossRef]
- Shi, L.; Li, B.; Lu, S.; Zhu, D.; Li, W. Synthesis, characterization and oxygen-sensing properties of a novel luminescent Cu(I) complex. Appl. Organometal. Chem. 2009, 23, 379–384. [Google Scholar] [CrossRef]
- Kouvatsis, P.; Glykos, D.; Plakatouras, J.C.; Malandrinos, G. [6-(Thiophen-2-yl)-2,2’-bipyridine]bis(triphenylphosphine) Copper(I) Tetrafluoroborate. Molbank 2023, 2023, M1605. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spektroskopische Methoden in der Organischen Chemie, 7th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2005; pp. 33–73. [Google Scholar]
- Beaudelot, J.; Oger, S.; Peruško, S.; Phan, T.-A.; Teunens, T.; Moucheron, C.; Evano, G. Photoactive Copper Complexes: Properties and Applications. Chem. Rev. 2022, 122, 16365–16609. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—olex2 dissected. Acta Cryst. A 2015, 71, 59–75. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).