2,3,5,6-Tetrafluoro-[N-(3-aminopropyl)-ε-caprolactam]-4-pyridine
Abstract
1. Introduction
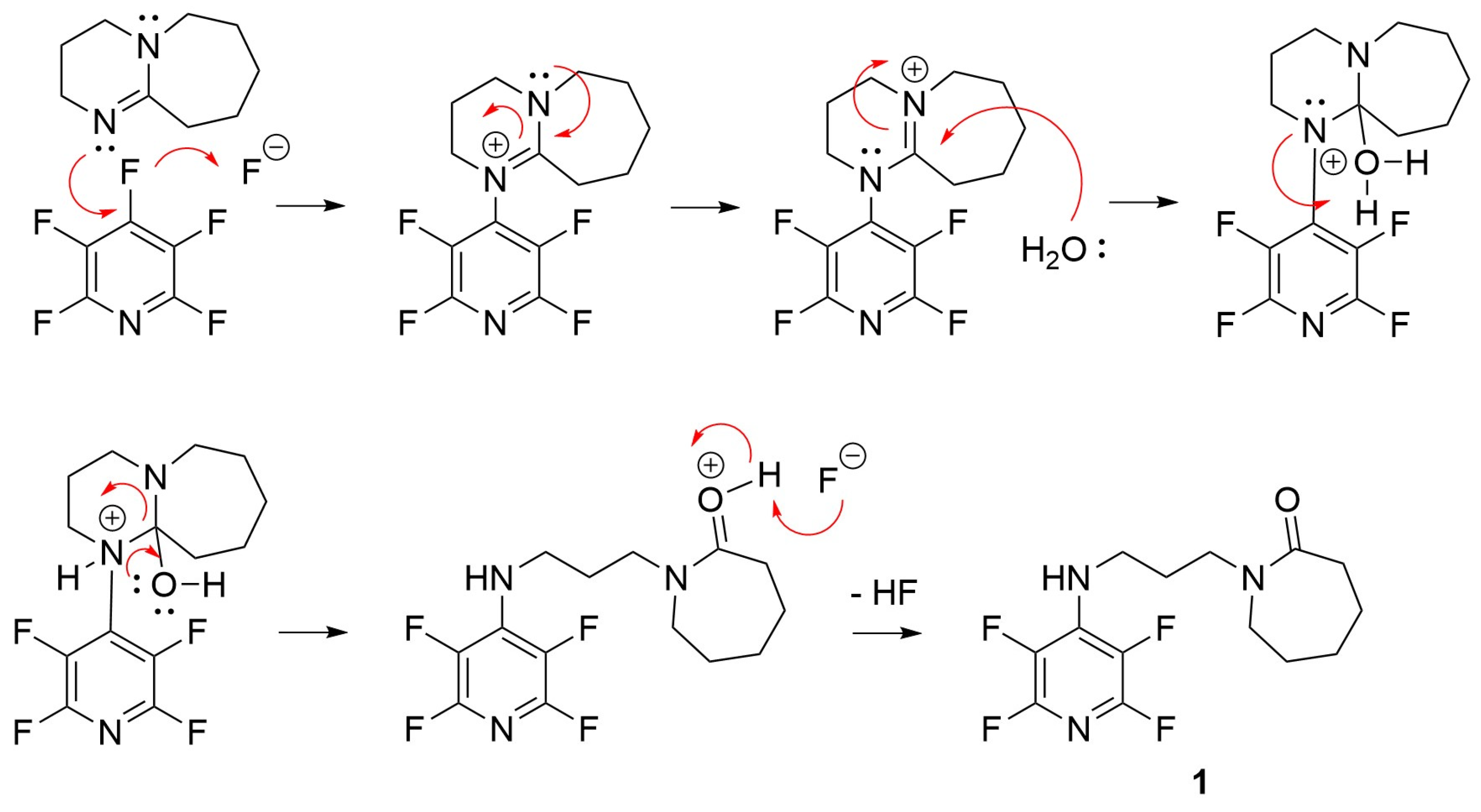
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 2,3,5,6-Tetrafluoro-N-[(propyl)caprolactam]-4-pyridinamine (1)
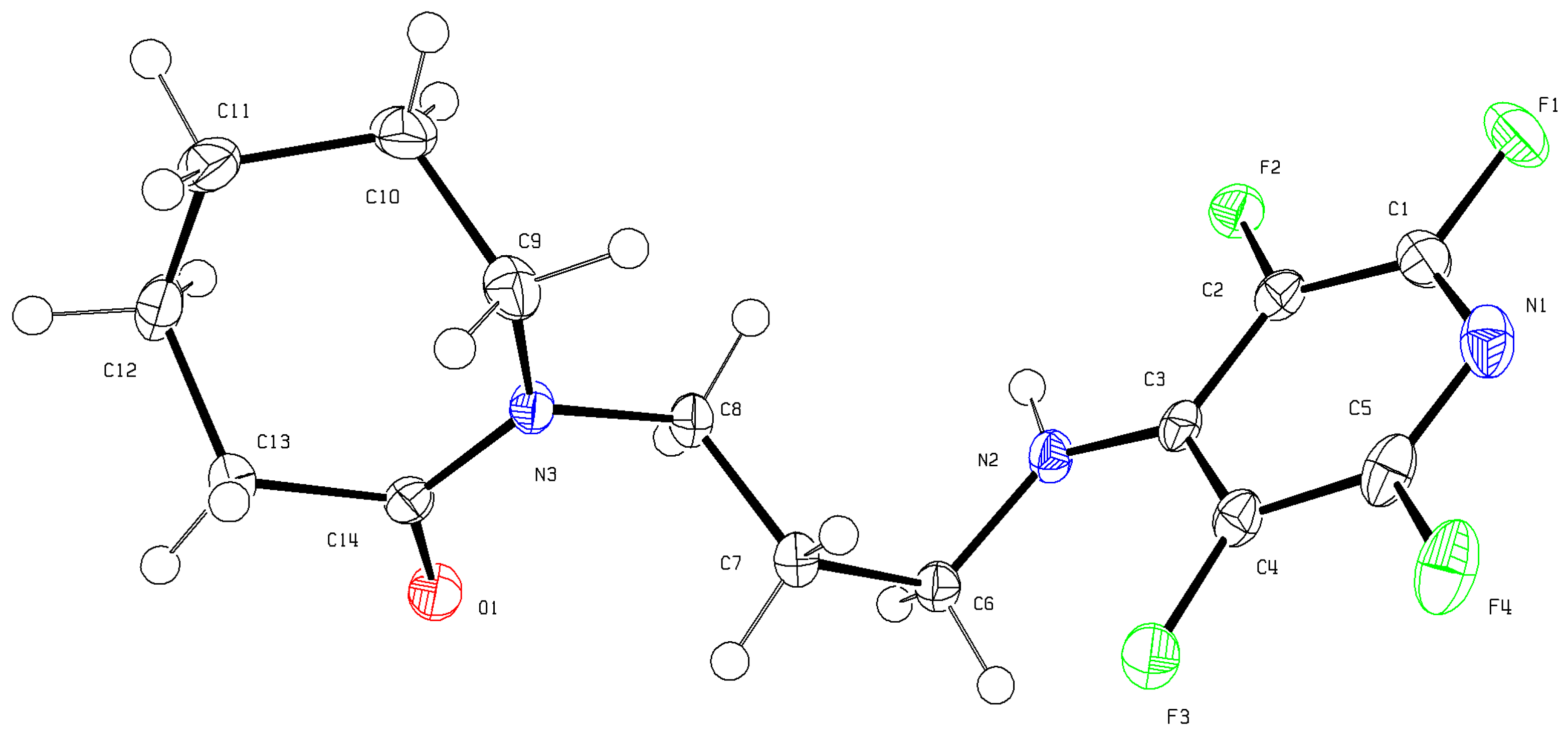
3.2. Single-Crystal XRD Determination
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gais, H.-J.; Vollhardt, J.; Krüger, C. Ein monomeres Kaliumsalz von Bis(trimethylsilyl)methyl(phenyl)sulfon und die Bestimmung der Kopplungskonstanten 1J(13C, 29Si) Si-stabilisierter “Carbanionen”. Angew. Chem. 1988, 100, 1108–1110. [Google Scholar] [CrossRef]
- Patai, S.; Rappoport, Z. The Chemistry of Amidines and Imidiates; John Wiley and Sons: Chichester, UK, 1991; p. 782. [Google Scholar]
- Hyde, A.M.; Calabria, R.; Arvary, R.; Wang, X.; Klapars, A. Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogenous Bases. Org. Process Res. Dev. 2019, 23, 1860–1871. [Google Scholar] [CrossRef]
- Vangala, M.; Shinde, G.P. p-Nitrophenyl carbonate promoted ring-opening reactions of DBU and DBN affording lactam carbamate. Beilstein J. Org. Chem. 2016, 12, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Gierczyk, B.; Schroeder, G.; Brzezinski, B. Reaction of Some Strong N-Bases with Chloropentafluorobenzene in the Presence of Water Molecules. J. Org. Chem. 2003, 68, 3139–3144. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, T.; Houck, M.; Corley, C.A.; Iacono, S.T. Theoretical Explanation of Reaction Site Selectivity in the Addition of a Phenoxy Group to Perfluoropyridine. J. Phys. Chem. A 2019, 123, 9450–9455. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.N.; Hooker, J.M.; Ritter, T. Concerted Nucleophilic Aromatic Substitution with 19F− and 18F−. Nature 2016, 534, 369. [Google Scholar] [CrossRef]
- Sandford, G. Perfluoroheteroaromatic Chemistry: Multifunctional Systems from Perfluorinated Heterocycles by Nucleophilic Aromatic Substitution Processes. In Halogenated Heterocycles: Synthesis, Application and Environment; Iskra, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–31. ISBN 978-3-642-43950-6. [Google Scholar]
- Puffr, R.; Sebenda, J. Polymerization of N-substituted lactams. Makromol. Chem. Macromol. Symp. 1986, 3, 249–264. [Google Scholar] [CrossRef]
- Gautam, R.; Geniza, I.; Iacono, S.T.; Friesen, C.M.; Jennings, A.R. Perfluoropyridine: Discovery, Chemistry, and Applications in Polymers and Material Science. Molecules 2022, 27, 1616. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.M.; Newton, J.; Hardera, J.; Iacono, S.T. Hydrofluoroethers (HFEs): A History of Synthesis. In Perfluoroalkyl Substances: Synthesis, Applications, Challenges and Regulations; Améduri, B., Ed.; RSC Publishing: Croydon, UK, 2022; p. 166. [Google Scholar]
- Friesen, C.M.; Kelly, A.R.; Iacono, S.T. Shaken Not Stirred: Perfluoropyridine Polyalkylether Prepolymers. Macromolecules 2022, 55, 10970–10979. [Google Scholar] [CrossRef]
- Christe, K.O.; Wilson, W.W. Reaction of the fluoride anion with acetonitrile. J. Fluor. Chem. 1990, 47, 117–120. [Google Scholar] [CrossRef]
- Hui, Y.; Webster, R.D. Absorption of Water into Organic Solvents Used for Electrochemistry under Conventional Operating Conditions. Anal. Chem. 2011, 83, 976–981. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friesen, C.M.; Weeks, N.J.; Iacono, S.T. 2,3,5,6-Tetrafluoro-[N-(3-aminopropyl)-ε-caprolactam]-4-pyridine. Molbank 2024, 2024, M1777. https://doi.org/10.3390/M1777
Friesen CM, Weeks NJ, Iacono ST. 2,3,5,6-Tetrafluoro-[N-(3-aminopropyl)-ε-caprolactam]-4-pyridine. Molbank. 2024; 2024(1):M1777. https://doi.org/10.3390/M1777
Chicago/Turabian StyleFriesen, Chadron M., Nathan J. Weeks, and Scott T. Iacono. 2024. "2,3,5,6-Tetrafluoro-[N-(3-aminopropyl)-ε-caprolactam]-4-pyridine" Molbank 2024, no. 1: M1777. https://doi.org/10.3390/M1777
APA StyleFriesen, C. M., Weeks, N. J., & Iacono, S. T. (2024). 2,3,5,6-Tetrafluoro-[N-(3-aminopropyl)-ε-caprolactam]-4-pyridine. Molbank, 2024(1), M1777. https://doi.org/10.3390/M1777





