Abstract
The synthesis of previously unknown 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one has been proposed and structurally characterized via a single-crystal X-ray diffraction analysis, 1H, 13C–{1H}, 1H–13C HMQC, and 1H–13C HMBC NMR spectroscopy, and IR spectroscopy.
1. Introduction
The furan ring is a component of many medications with a wide spectrum of action [1,2,3,4]. The development of methods for obtaining new furan derivatives is one of the most dynamic areas of modern organic and medicinal chemistry [5,6]. Of particular interest in this case are condensed biheterocyclic systems containing a furan and another heterocycle [7,8,9,10,11]. Thus, based on tetrasubstituted furans containing ester and acetyl groups in positions 3 and 4, the corresponding furo[3,4-d]piridazines were obtained in a reaction with hydrazine (1,2-binucleophile) [11,12]. In this article, we will focus on the synthesis of a new fused biheterocycle—4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one—and the characterization of its fine structure using NMR (nuclear magnetic resonance), FTIR (Fourier-transform infrared spectroscopy), and an X-ray diffraction analysis. This study will contribute to our understanding of the properties of furo-containing biheterocycles and may have important implications for the development of new biologically active substances.
2. Results and Discussion
We have proposed a method for the synthesis of an original biheterocycle, 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one 2, based on the reaction of acetyl-containing furan-3-carboxylates 1a, b [13] with hydrazine hydrate. It turned out that boiling furancarboxylates 1a, b with an excess of hydrazine hydrate in acetic acid (method A) leads to the preparation of the target 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one 2 with yields up to 32%. Changing the synthesis conditions, namely, the reaction of furan-3-carboxylate 1b with an excess of hydrazine hydrate in ethanol solution in the presence of catalytic amounts of trifluoroacetic acid at room temperature (method B), led to the formation of biheterocycle 2 with a yield of 44% (Scheme 1).
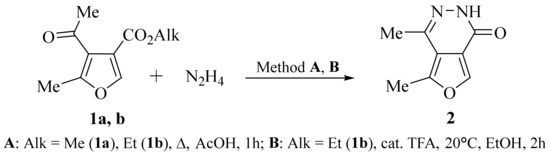
Scheme 1.
Synthesis of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one 2.
The synthesized 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one 2 can exist in the form of lactim or lactam tautomeric (Figure 1). Studying the structure of biheterocycle 2 using IR and 1H, 13C–{1H} NMR spectroscopy, as well as an X-ray structural analysis, unambiguously confirmed that its molecule exists in the lactam form in solution and in the solid phase.
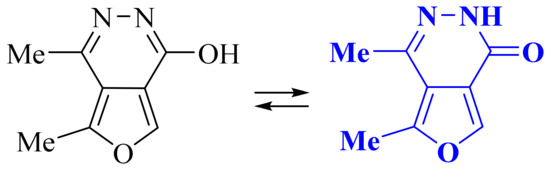
Figure 1.
Tautomeric forms of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one 2.
Indeed, the IR spectrum of biheterocycle 2 contains an intense band at 1668 cm−1 (C=O), which belongs to the valence vibrations of the amide carbonyl group, as well as a broadened band of the amino group of the amide fragment at 3167 cm−1 (NH).
The 13C–{1H} NMR spectrum of biheterocycle 2 contains a carbon atom signal at 157.92 ppm (C=O), characteristic of the carbonyl group of the amide fragment. In addition, the cross peak 11.44 (NH)/140.51 (C4) in the 1H–13C HMBC experiment spectrum also confirms the existence of the synthesized bicycle 2 in solution in the lactam form.
The assignment of signals of protons and carbon atoms in the 1H and 13C NMR spectra was carried out using heteronuclear (1H–13C HMQC, 1H–13C HMBC) experiments. The key cross peaks used for interpretation in the 1H–13C HMBC spectra are as follows: 2.34 (C4CH3)/115.26 (C4a)/140.51 (C4); 2.61 (C5CH3)/115.26 (C4a)/150.92 (C5); 8.43 (C7H)/115.26 (C4a)/117.71 (C7a)/150.92 (C5); and 11.44 (NH)/117.71 (C7a)/140.51 (C4) (Figure 2).
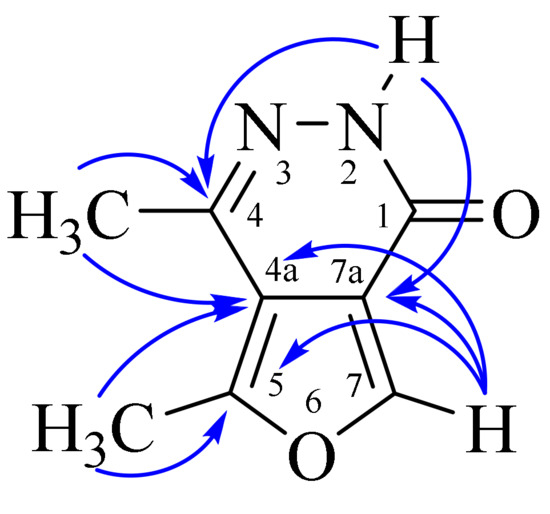
Figure 2.
Key correlations in 1H–13C HMBC spectra.
The lactam molecular and crystal structures of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one (2) have been determined via an X-ray analysis. Light-brown crystals were grown from a saturated ethanol solution by slow solvent evaporation.
The title compound (2) crystallizes in the monoclinic space group P21/c. Figure 3 represents the molecular structures of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one.
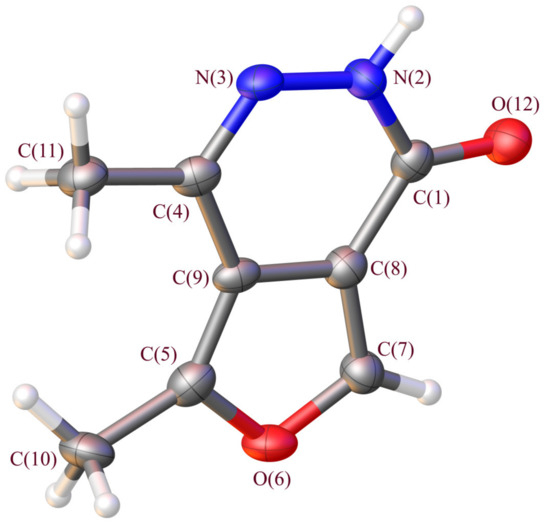
Figure 3.
Geometry of molecule 2 in the crystal. Ellipsoids of anisotropic displacements are shown with 50% probability.
The molecule has a planar conformation, and the deviation of atoms from the least-square plane is lower than 0.039(3) Å. The pyridazinone fragment exists in the lactam form.
The distribution of bond lengths in a molecule indicates the presence of a conjugation in it, which ensures the planar conformation of the entire molecule (see Supplementary Materials Table S2).
The crystal structure of compound 2 is primarily determined by intermolecular hydrogen bonds (N2-H2….O12′ (−x,−1/2 + y,1/2 − z)) and stacking interactions between planar conjugated molecules. The hydrogen bond parameters are as follows: N2-H2 0.87(3), N2….O12′ 2.787(4), H2….O12′ 1.97(3) Å, and angle N2-H2….O12′ 156(3)°. Due to these H-bonds, ribbons are formed along the b axis of the crystal (Figure 4).
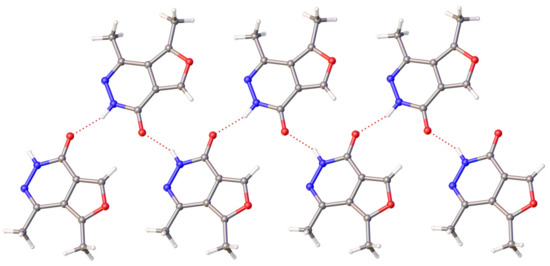
Figure 4.
Hydrogen bonding in the crystal of molecule 2. H-bonds are shown by dotted lines.
Figure 5 shows a fragment of the packaging of crystal 2, which demonstrates interplanar interactions, due to which the formation of double layers is observed. The interplanar distances are 3.19(1) Å, and the angle between the planes of molecules is 0.16(12)°.
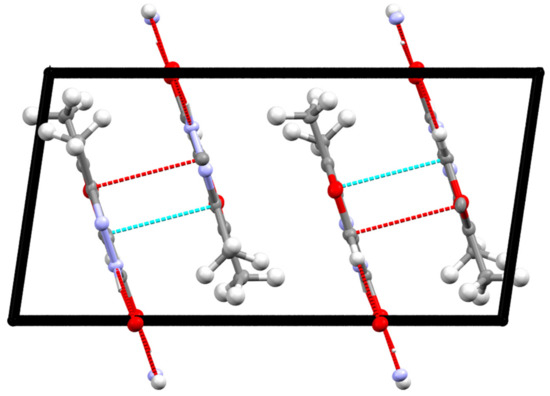
Figure 5.
Stacking interaction in crystal 2. Short contacts are shown by dotted lines. Projection along the b axis.
3. Materials and Methods
Alkyl 4-acetyl-5-methylfuran-3-carboxylates (1a, b) [13] were prepared according to published methods. All reagents were of commercial grade and used without purification.
Method A: To a solution of 0.2 g (1.09 mmol) of methyl 4-acetyl-5-methylfuran-3-carboxylate 1a [13] in 5 mL of glacial acetic acid, 0.275 g (5.5 mmol) of hydrazine hydrate was added. The reaction mixture was refluxed for 1 h. Then the solvent was evaporated and the residue was crystallized from ethanol. Yield 0.017 g (10%), light-brown crystals, mp. 273–275 °C (EtOH).
IR: 1345, 1546, 1617, 1668 (C=O), 3167 (NH).
1H NMR: 2.34 (3H, s, C4CH3), 2.61 (3H, s, C5CH3), 8.43 (1H, s, C7H), 11.44 (1H, s, NH). (Atom labeling shown in Figure 2).
13C{1H} NMR: 13.87 [C5CH3], 19.92 [C4CH3], 115.26 (C4a), 117.71 (C7a), 140.51 (C4), 150.92 (C5), 157.92 (C=O).
Found, %: C 58.53; H 4.91, N 17.06. C8H8N2O2. Calculated, %: C 58.23; H 4.84, N 16.94.
To a solution of 0.2 g (1.02 mmol) of ethyl 4-acetyl-5-methylfuran-3-carboxylate 1b [13] in 5 mL of glacial acetic acid, 0.255 g (5.1 mmol) of hydrazine hydrate was added. The reaction mixture was refluxed for 1 h. Then the solvent was evaporated and the residue was crystallized from ethanol. Yield 0.054 g (32%), light-brown crystals, mp. 273–275 °C (EtOH).
Method B: To a solution of 0.15 g (0.077 mmol) of ethyl 4-acetyl-5-methylfuran-3-carboxylate 1b [13] in 5 mL of ethanol, 0.077 g (1.53 mmol) of hydrazine hydrate and catalytic amount of trifluoroacetic acid were added. The reaction mixture was kept for 2 h at room temperature. Then the solvent was evaporated and the residue was crystallized from ethanol. Yield 0.055 g (44%), light-brown crystals, mp. 273–275 °C (EtOH).
A mixing test with a sample obtained by methods A and B did not result in a depression in the melting point.
Elemental analysis was performed with a Euro Vector EA 3000 analyzer (CHN Dual). Melting points were determined on a PTP-M melting point apparatus.
IR spectra (Figure S1) were registered on Shimadzu IR Prestige-21 spectrometers with samples in KBr pellets. 1H, 13C–{1H} NMR spectra (Figures S2 and S3), 1H–13C HMQC (Figure S4), and 1H–13C HMBC (Figure S5) experiments were performed using a Jeol ECX400A spectrometer (400 MHz for 1H nuclei and 100 MHz for 13C nuclei) in DMSO-d6. The residual signals of the solvent (DMSO-d6: 2.50 ppm for 1H nuclei and 39.6 ppm for 13C nuclei) were used as internal standard.
X-ray diffraction analysis was performed at 108 K on a Bruker D8 QUEST automatic three-circle diffractometer (graphite monochromator, λMoKα = 0.71073 Å, ω- and φ-scan with a step of 0.5°) at the Distributed Spectral-Analytical Center of Shared Facilities for Study of Structure, Composition and Properties of Substances and Materials of FRC Kazan Scientific Center of RAS.
An X-ray diffraction analysis of 2 was performed on a Bruker D8 QUEST automatic three-circle diffractometer with a PHOTON III two-dimensional detector and an I_S DIAMOND microfocus X-ray tube (Mo Kα radiation, λ = 0.71073 Å) at cooling conditions. Data collection and the processing of diffraction data were performed using APEX3 software package. Structure 2 was solved by the direct method using the SHELXT program [14]. It was refined by the full-matrix least-squares method over F2 using the SHELXL program [15]. Crystal 2 was found to be two-component twin and twin rot matrix (−0.694 0 −0.424)/(0 −1 0)/(−1.223 0 0.694), with the estimated BASF line = 0.46. Final model was refined using a combined diffraction data set (HKLF 5), with parameter BASF refined to 0.33285. All calculations were performed in the WinGX software package [16]. The calculations of the geometry of molecules and intermolecular interactions in crystals were carried out using the PLATON program [17]. The drawings of molecule were performed using the MERCURY [18] programs. Non-hydrogen atoms were refined using the anisotropic approximation. The position of the hydrogen atom H(N2) was determined using difference Fourier map. The remaining hydrogen atoms were placed in geometrically calculated positions, and all hydrogen atoms were included in the refinement in the “riding” model.
Crystal 2 monoclinic at 108(2) K a = 7.024(4), b = 7.659(4), c = 13.714(7) Å, β = 98.312(17)o, V = 730.1(6) Å3, Z = 4 ρ calc 1.493 g/cm3, and μ 0.110 mm−1. Reflections collected: 19508, independent reflections: 1464, and observed reflections: I ≥ 2σ(I) 831. Final R-factors were as follows: R factors [I >= 2σ (I)] R1 = 0.0802, wR2 = 0.1895, R factors (all reflections) R1 = 0.1442, wR2 = 0.2182, and GOOF on F2 1.003
Crystallographic data, experimental parameters, and refinement of the condensed biheterocyclic structure were deposited at the Cambridge Crystallographic Data Center; registration numbers and the most important characteristics are provided in Tables S1–S3 (see Supplementary Materials).
4. Conclusions
A method has been proposed for the synthesis under mild conditions of a previously unknown representative of biheterocyclic compounds—4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one—with a free α-position of the furan ring based on the reaction of acetyl-containing furan-3-carboxylates with excess hydrazine hydrate. Its fine structure is characterized via NMR and IR spectroscopy, as well as an X-ray diffraction analysis. The existence of its molecule in lactam form in solution and in the solid phase has been established.
Supplementary Materials
Figure S1: IR spectrum of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one in KBr; Figure S2: 1H NMR spectrum of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one in DMSO-d6; Figure S3: 13C–{1H} NMR spectrum of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one in DMSO-d6; Figure S4: 1H–13C HMQC spectrum of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one in DMSO-d6; Figure S5: 1H–13C HMBC spectrum of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one in DMSO-d6; Figure S6: Geometry of the 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one in the crystal; Figure S7: Hydrogen bonding in the crystal 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one; Figure S8: Stacking interaction in the crystal 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one; Table S1: Crystallographic data, experimental parameters, and refinement of the 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one; Table S2: Bond lengths (d) in the molecule of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one; Table S3: Angles (τ) in the molecule of 4,5-dimethylfuro[3,4-d]pyridazin-1(2H)-one. CCDC 2321540 (accessed on 13 December 2023) contains the supplementary crystallographic data for this paper.
Author Contributions
Conceptualization, S.V.M. and V.V.P., synthesis, K.A.G., methodology, V.V.P.; investigation, R.I.B. and I.A.L.; writing—original draft preparation, S.V.M., V.V.P. and R.I.B.; writing—review and editing, S.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by an internal grant of the Herzen State Pedagogical University of Russia (project № 3VG). The X-ray diffraction study was financially supported within the framework of the state assignment for the ‘Kazan Scientific Center of the Russian Academy of Sciences’ Federal Research Center.
Data Availability Statement
The data presented in this study are available in this article (and Supplementary Material).
Acknowledgments
NMR and IR spectral studies were performed at the Center for Collective Use «Physico-chemical methods for the study of nitro compounds, coordination compounds, biologically active substances, and nanostructured materials» of the Interdisciplinary Resource Center for Collective Use «Modern physico-chemical methods of formation and research of materials for the needs of industry, science, and education» of the Herzen State Pedagogical University of Russia. The structural studies were carried out using the equipment of the A. E. Arbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Patel, P.; Shakya, R.; Asati, V.; Kurmi, B.D.; Verma, S.K.; Gupta, G.D.; Rajak, H. Harish Rajak Furan and benzofuran derivatives as privileged scaffolds as anticancer agents: SAR and docking studies (2010 to till date). J. Mol. Struct. 2024, 1299, 137098. [Google Scholar] [CrossRef]
- Iroegbu, A.O.; Sadiku, E.R.; Ray, S.S.; Hamam, Y. Sustainable Chemicals: A Brief Survey of the Furans. Chem. Afr. 2020, 3, 481. [Google Scholar] [CrossRef]
- Loğoğlu, E.; Yilmaz, M.; Katircioğlu, H.; Yakut, M.; Mercan, S. Synthesis and biological activity studies of furan derivatives. Med. Chem. Res. 2010, 19, 490. [Google Scholar] [CrossRef]
- Lukevits, É.; Demicheva, L. Biological activity of furan derivatives (review). Chem. Heterocycl. Compd. 1993, 29, 243. [Google Scholar] [CrossRef]
- Deepthi, A.; Babu, B.P.; Balachandran, A.L. Synthesis of Furans–Recent Advances. Org. Prep. Proc. Inter. 2019, 51, 409. [Google Scholar] [CrossRef]
- Duc, D.X. Recent Progress in the Synthesis of Furan. Mini-Rev. Org. Chem. 2019, 16, 422. [Google Scholar] [CrossRef]
- Manna, S.K.; Giri, S.; Mondal, S.; Sana, R.N.; Samal, A.K.; Mandal, A. A Detailed Review on C-Fused Furan/3,4-Fused Furan Analog and its Potential Applications. ChemistrySelect 2023, 8, e202203150. [Google Scholar] [CrossRef]
- Gould, K.J.; Hacker, N.P.; McOmie, J.F.W.; Perry, D.H. Benzocyclobutenes. Part 4. Synthesis of benzocyclobutene-1,2-diones by pyrolytic methods. J. Chem. Soc. Perkin Trans. 1980, 1, 1834. [Google Scholar] [CrossRef]
- Oleinik, A.F.; Adamskaya, E.V.; Okinshevich, O.V.; Pershin, G.N. Synthesis and antimicrobial activity of derivatives of 2-aryl-3,4-bis(carboxy)furans. Pharm. Chem. J. 1983, 17, 408. [Google Scholar] [CrossRef]
- Du, Q.; Neudörfl, J.-M.; Schmalz, H.-G. Chiral Phosphine–Phosphite Ligands in Asymmetric Gold Catalysis: Highly Enantioselective Synthesis of Furo[3,4-d]-Tetrahydropyridazine Derivatives through [3 + 3]-Cycloaddition. Chem. Eur. J. 2018, 24, 2379. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wei, Y.; Tong, X. Phosphine-Catalyzed [3 + 2] Annulations of γ-Functionalized Butynoates and 1C, 3O-Bisnucleophiles: Highly Selective Reagent-Controlled Pathways to Polysubstituted Furans. Org. Lett. 2011, 13, 3068. [Google Scholar] [CrossRef]
- Bakavoli, M.; Feizyzadeh, B.; Rahimizadeh, M. Investigation of hydrazine addition to functionalized furans: Synthesis of new functionalized 4,4′-bipyrazole derivatives. Tetrahedron. Lett. 2006, 47, 8965. [Google Scholar] [CrossRef]
- Gomonov, K.A.; Pelipko, V.V.; Litvinov, I.A.; Baichurin, R.I.; Makarenko, S.V. Synthesis of substituted furan-3-carboxylates from alkyl 3-bromo-3-nitroacrylates. Mend. Comm. 2023, 33, 11. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shield, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).