2-(1-Methoxycarbonyl-2-phenyleth-1-yl)-1-benzylpyridin-1-ium Bromide
Abstract
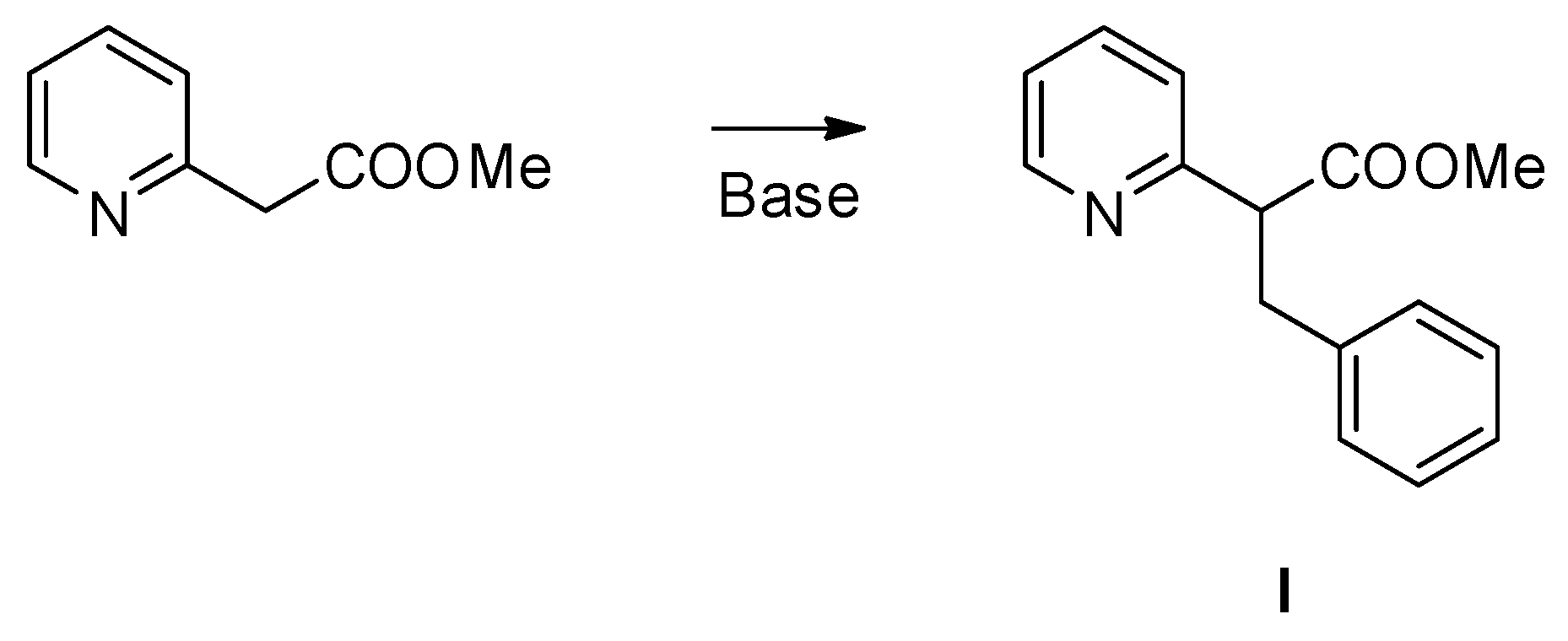
:1. Introduction
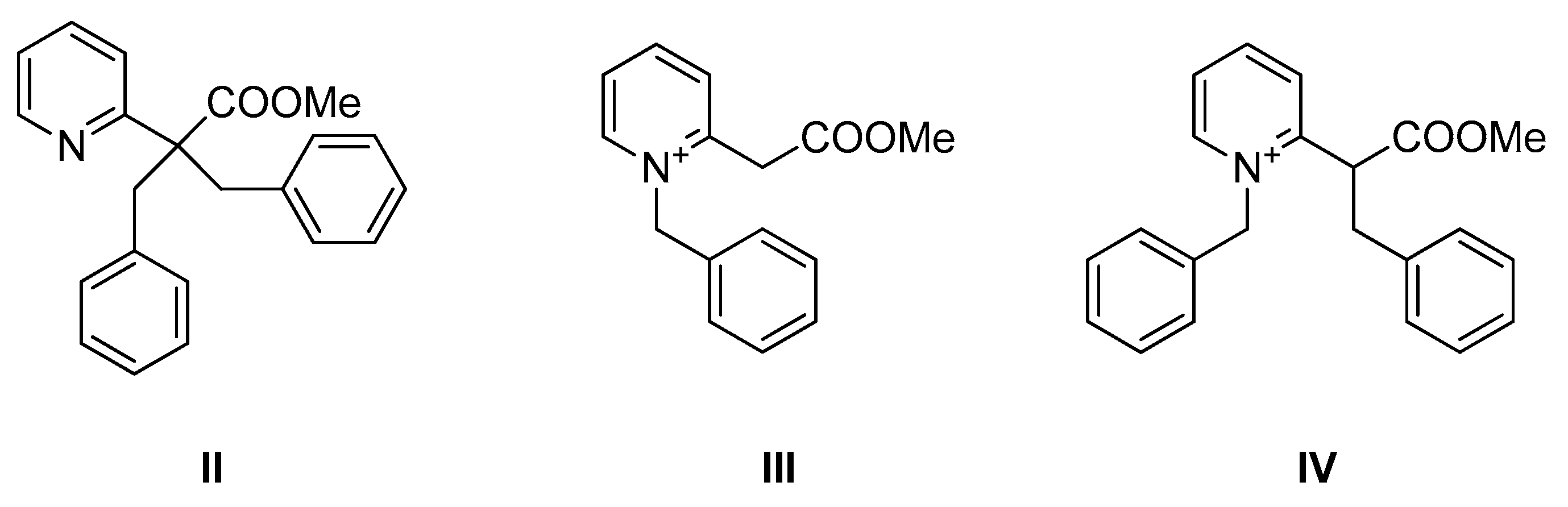
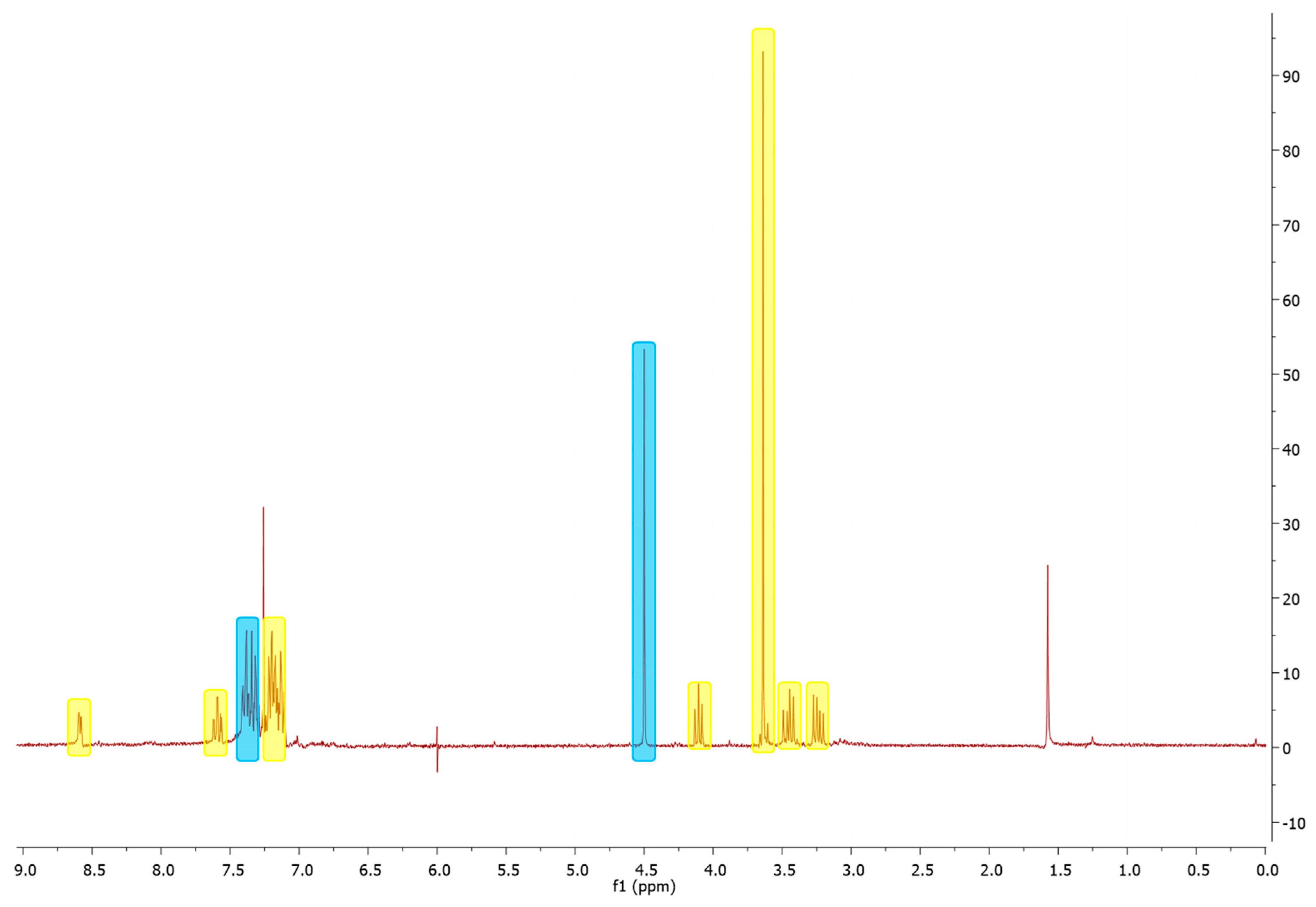
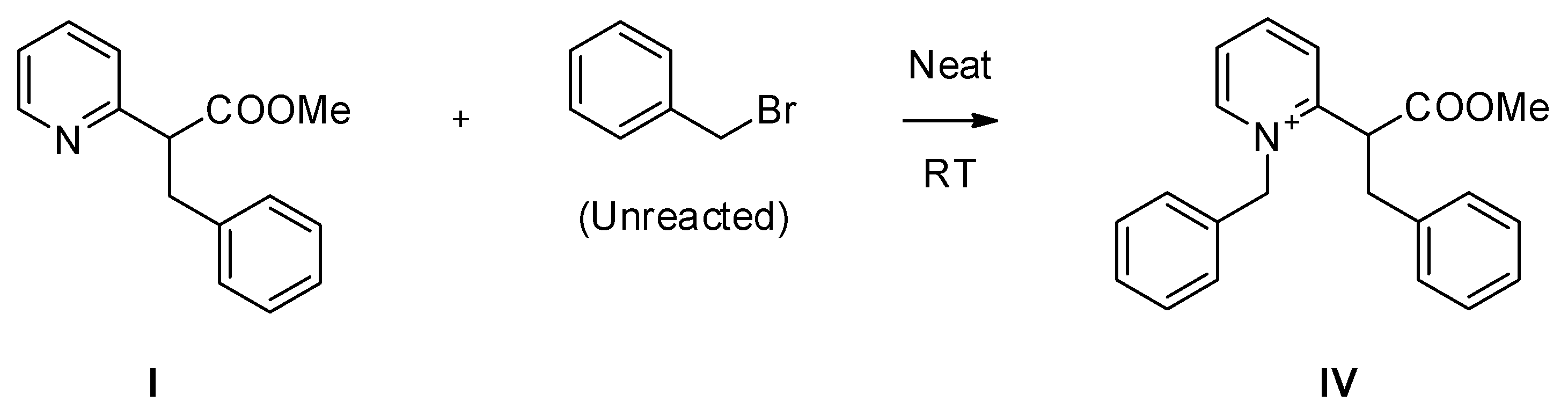
2. Results
3. Material and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, S.; Zhang, X.; Zeng, S.; Xu, Y.; Nie, W.; Zhou, Y.; Xu, T.; Chen, P. Quaternary Ammonium Salts: Insights into Synthesis and New Directions in Antibacterial Applications. Bioconjug. Chem. 2023, 34, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, K.; Ke, Y.; Tang, Y.; Liu, L.; Huang, T.; Li, C.; Tang, Z.; Chen, T. Transition-metal-free and base promoted C-C bond formation via C-N bond cleavage of organoammonium salts. Org. Biomol. Chem. 2021, 19, 8237–8240. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xue, W.; Yu, B.; Pang, H.; Yu, L.; Wang, Q.; Zhu, D. Development of a Trimethylamine-Catalyzed Novel Synthesis of Azoxystrobin. Org. Process Res. Dev. 2023, 27, 1276–1282. [Google Scholar] [CrossRef]
- Szymaniak, D.; Maćkowiak, A.; Ciarka, K.; Praczyk, T.; Marcinkowska, K.; Pernak, J. Synthesis and Characterization of Double-Salt Herbicidal Ionic Liquids Comprising both 4-Chloro-2-methylphenoxyacetate and trans-Cinnamate Anions. ChemPlusChem 2020, 85, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Yao, S.; Dong, B.; Li, X.; Song, H. Synthesis, characterization and physical properties of novel cholinium-based organic magnetic ionic liquids. J. Mol. Liq. 2017, 240, 152–161. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, S.; Cheng, W.; Ren, J. Hydroxyl-functionalized ionic liquid: A novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate. Tetrahedron Lett. 2008, 49, 3588–3591. [Google Scholar] [CrossRef]
- Manikandan, C.; Ganesan, K. Solid-Supported Synthesis of Flexible Dimeric Pyridinium Salts and Their Catalytic Activities. Synlett 2016, 27, 1527–1530. [Google Scholar] [CrossRef]
- Qu, B.; Mangunuru, H.P.R.; Wei, X.; Fandrick, K.R.; Desrosiers, J.-N.; Sieber, J.D.; Kurouski, D.; Haddad, N.; Samankumara, L.P.; Lee, H.; et al. Synthesis of Enantioenriched 2-Alkyl Piperidine Derivatives through Asymmetric Reduction of Pyridinium Salts. Org. Lett. 2016, 18, 4920–4923. [Google Scholar] [CrossRef] [PubMed]
- Faustini, G.; Longhena, F.; Bruno, A.; Bono, F.; Grigoletto, J.; La Via, L.; Barbon, A.; Casiraghi, A.; Straniero, V.; Valoti, E.; et al. Alpha-synuclein/synapsin III pathological interplay boosts the motor response to methylphenidate. Neurobiol. Dis. 2020, 138, 104789. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, A.; Longhena, F.; Faustini, G.; Ribaudo, G.; Suigo, L.; Camacho-Hernandez, G.A.; Bono, F.; Brembati, V.; Newman, A.H.; Gianoncelli, A.; et al. Methylphenidate Analogues as a New Class of Potential Disease-Modifying Agents for Parkinson’s Disease: Evidence from Cell Models and Alpha-Synuclein Transgenic Mice. Pharmaceutics 2022, 14, 1595. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, A.; Casiraghi, A.; Longhena, F.; Straniero, V.; Valoti, E. Structural Analogues of Methylphenidate as Parkinson’s Disease-Modifying Agents. IT202000019303A1, 5 August 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suigo, L.; Straniero, V.; Valoti, E. 2-(1-Methoxycarbonyl-2-phenyleth-1-yl)-1-benzylpyridin-1-ium Bromide. Molbank 2023, 2023, M1738. https://doi.org/10.3390/M1738
Suigo L, Straniero V, Valoti E. 2-(1-Methoxycarbonyl-2-phenyleth-1-yl)-1-benzylpyridin-1-ium Bromide. Molbank. 2023; 2023(4):M1738. https://doi.org/10.3390/M1738
Chicago/Turabian StyleSuigo, Lorenzo, Valentina Straniero, and Ermanno Valoti. 2023. "2-(1-Methoxycarbonyl-2-phenyleth-1-yl)-1-benzylpyridin-1-ium Bromide" Molbank 2023, no. 4: M1738. https://doi.org/10.3390/M1738
APA StyleSuigo, L., Straniero, V., & Valoti, E. (2023). 2-(1-Methoxycarbonyl-2-phenyleth-1-yl)-1-benzylpyridin-1-ium Bromide. Molbank, 2023(4), M1738. https://doi.org/10.3390/M1738







