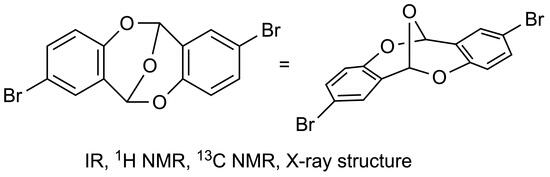
2,8-Dibromo-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine
Abstract
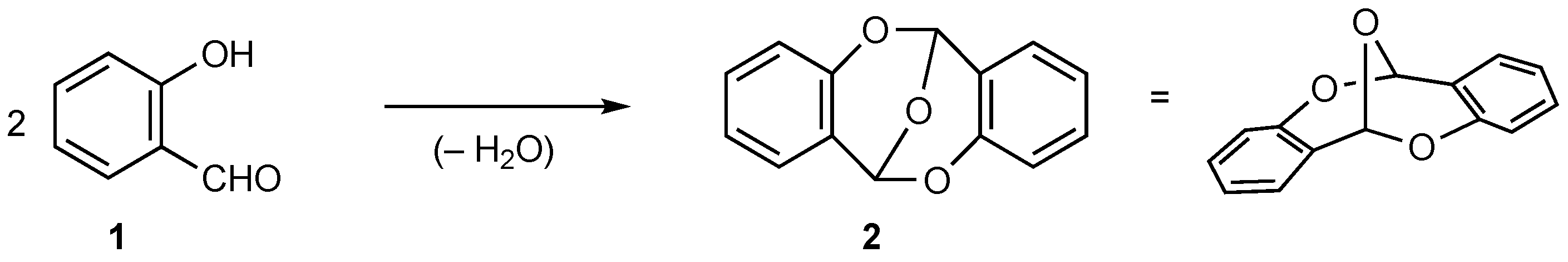
1. Introduction
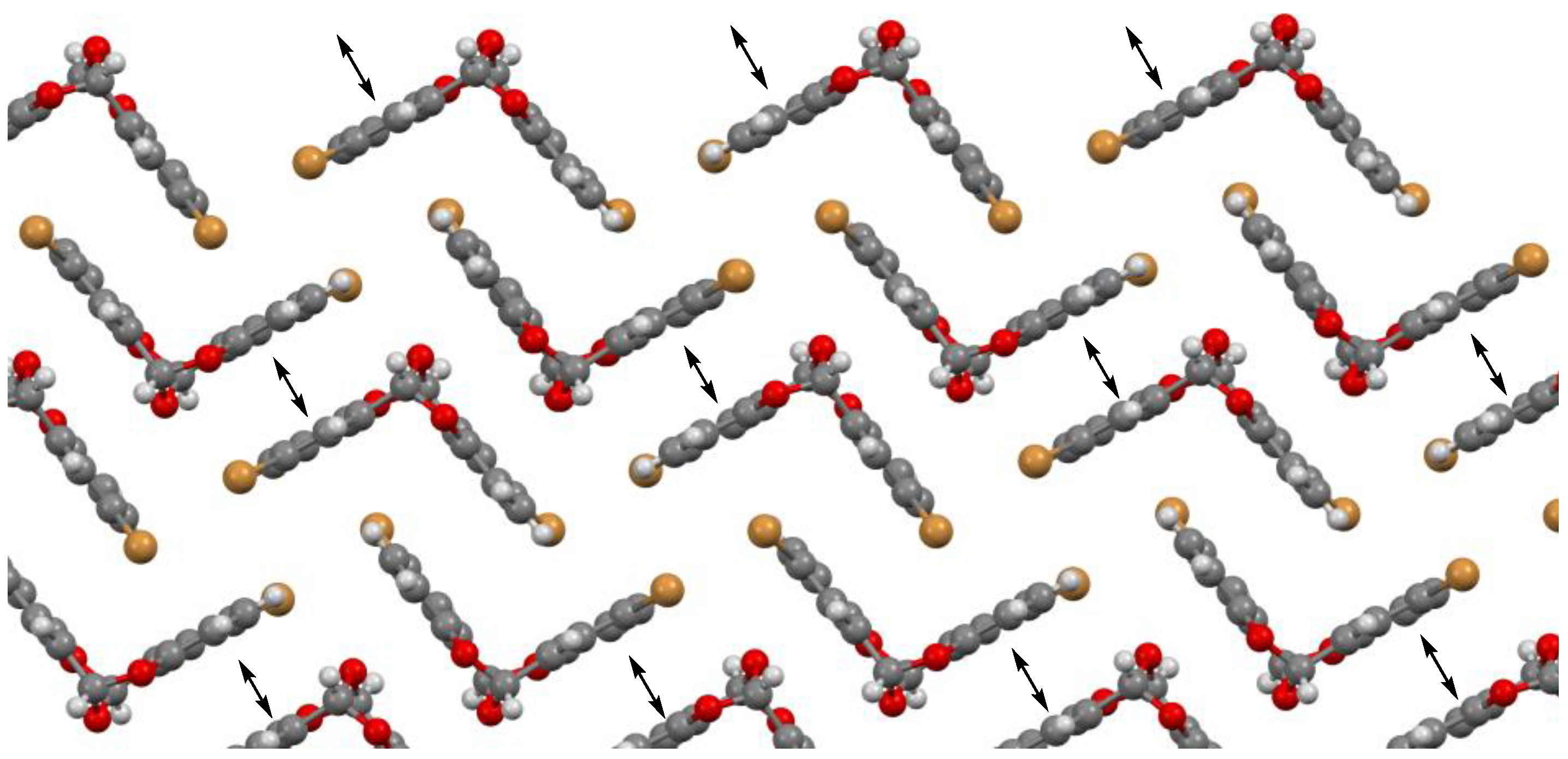
2. Results
3. Experimental
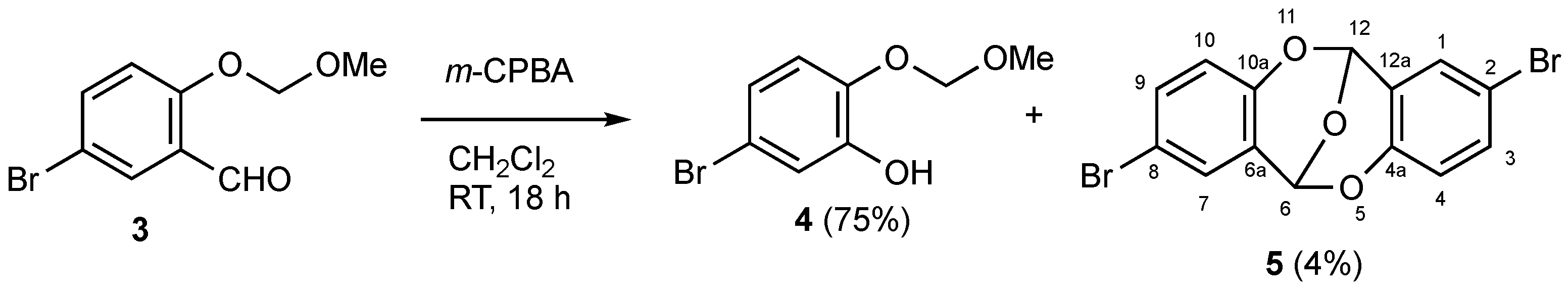
3.1. Reaction Leading to Formation of 5
3.2. X-ray Structure Determination of 5
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ettling. Ueber die Distillationsproducte des salicyligsauren und benzoësauren Kupferoxyds. Liebigs Ann. Chem. 1845, 53, 77–90. [Google Scholar] [CrossRef]
- Cahours, A. Untersuchungen über das Phenol (Phenylhydrat). Liebigs Ann. Chem. 1851, 78, 225–228. [Google Scholar] [CrossRef][Green Version]
- Perkin, W.H. Ueber Benzosalicyl- und Disalicylwasserstoff. Liebigs Ann. Chem. 1868, 145, 295–301. [Google Scholar] [CrossRef]
- Adams, R.; Fogler, M.F.; Kreger, C.W. The structure of disalicyl aldehyde. J. Am. Chem. Soc. 1922, 44, 1126–1133. [Google Scholar] [CrossRef]
- Fiedler, H. Derivate des 2-Hydroxy-3-methoxy-benzaldehyds. Arch. Pharm. 1964, 297, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Endo, Z. Action of phosphorus pentoxide on organic compounds I. Reaction between salicylaldehyde and phosphorus pentoxide. Nippon Kagaku Kaishi 1940, 61, 231–233. [Google Scholar]
- Nevesely, T.; Daniliuc, C.G.; Gilmour, R. Sequential energy transfer catalysis: A cascade synthesis of angularly-fused dihydrocoumarins. Org. Lett. 2019, 21, 9724–9728. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Tanaka, M.; Katsube, T.; Okudo, M.; Iwase, N.; Shigetomi, M.; Kanda, T.; Nakanishi, T. Pyrrolopyridazinone Compound. European Patent 1982986 A1, 22 October 2008. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Bachet, B.; Brassy, C.; Guidi-Morosini, C. Epoxy-8,16 Dihydro-8,16 Dinaphto[2,1-b:2′,1′-f][dioxocinne-1,5]. Acta Crystallogr. Sect. C 1986, 42, 1630–1632. [Google Scholar] [CrossRef]
- Vol’eva, V.B.; Belostotskaya, I.S.; Shishkin, O.V.; Struchkov, Y.T.; Ershov, V.V. Synthesis and structures of anhydrodimers of salicylaldehydes. Russ. Chem. Bull. 1995, 44, 1489–1491. [Google Scholar] [CrossRef]
- Wang, L.-H.; Lin, D.-D. The crystal structure of 4,10-ethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5. Z. Kristallogr. New Cryst. Struct. 2019, 234, 673–674. [Google Scholar] [CrossRef]
- Stomberg, R.; Li, S.; Lindquist, K. Crystal structure of 4,10-dimethoxy-6,12-epoxy-6H,12H-dibenzo[b,f][1,5]dioxocin, C16H14O5. Z. Kristallogr. Cryst. Mater. 1995, 210, 967–968. [Google Scholar] [CrossRef]
- Ragot, J.P.; Prime, M.E.; Archibald, S.J.; Taylor, R.J.K. A novel route to preussomerins via 2-arylacetal anions. Org. Lett. 2000, 2, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, v1.171.42.94a; Rigaku Oxford Diffraction, Rigaku Corporation: Tokyo, Japan, 2023.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
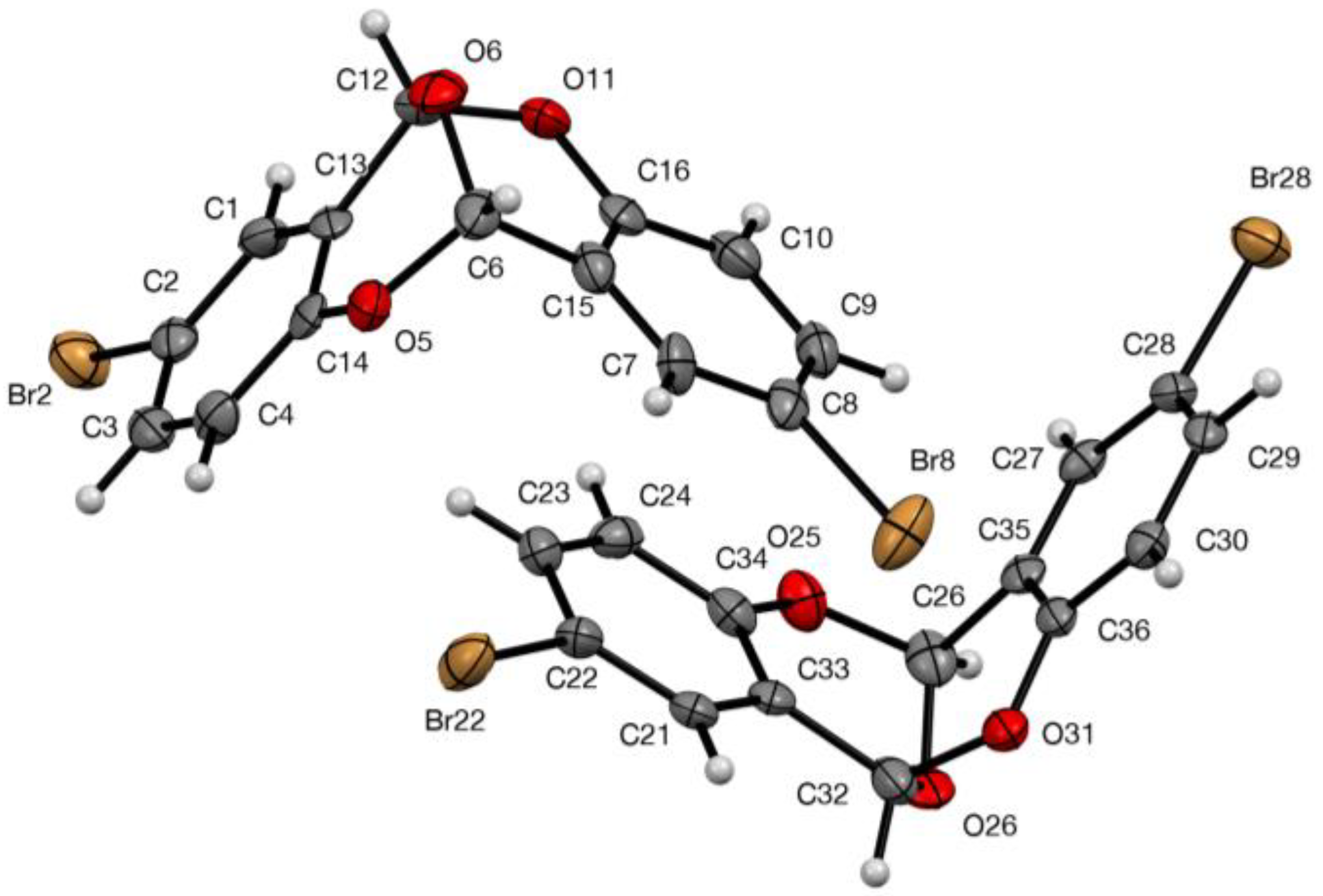


| Compd | Bridging C–O Length (s) | Angle at Bridging O | Angle(s) at Ring Os | Angle between Mean Planes | Ref |
|---|---|---|---|---|---|
| 5 | 1.404 (10), 1.410 (11) | 109.6 (6) | 111.6 (6), 112.4 (7) | 72.9 | This work |
| 5 | 1.403 (11), 1.407 (11) | 109.9 (6) | 112.5 (6), 112.7 (6) | 73.5 | This work |
| 6 FADVOV | 1.418 | 108.6 | 112.3 (2) | 71.7 | [10] |
| 2 ZIZSAC | 1.549 | 106.5 | 117.9 (9) | 65.9 | [11] |
| 7 TOLDAC | 1.415(2), 1.417(2) | 108.0(1) | 111.4(1), 111.9(1) | 73.5 | [12] |
| 8 ZOLBOR | 1.411(3), 1.416(3) | 107.8(2) | 111.5(2), 111.7(2) | 72.75 | [13] |
| 9 UGIPIJ | 1.408(5), 1.414(5) | 109.3(3) | 112.2(2), 112.3(3) | 72.75 | [14] |
| 10 UGIPEF | 1.413(2), 1.417(2) | 111.0(1) | 112.6(1), 113.1(1) | 73.6 | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Cordes, D.B.; Ler, A.J.; McKay, A.P. 2,8-Dibromo-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine. Molbank 2023, 2023, M1729. https://doi.org/10.3390/M1729
Aitken RA, Cordes DB, Ler AJ, McKay AP. 2,8-Dibromo-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine. Molbank. 2023; 2023(3):M1729. https://doi.org/10.3390/M1729
Chicago/Turabian StyleAitken, R. Alan, David B. Cordes, An Jie Ler, and Aidan P. McKay. 2023. "2,8-Dibromo-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine" Molbank 2023, no. 3: M1729. https://doi.org/10.3390/M1729
APA StyleAitken, R. A., Cordes, D. B., Ler, A. J., & McKay, A. P. (2023). 2,8-Dibromo-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine. Molbank, 2023(3), M1729. https://doi.org/10.3390/M1729