This Special Issue, “Molecules from Side Reactions II”, belongs to the section Organic Synthesis of the journal Molbank and was launched in 2021, after the first edition, “Molecules from Side Reactions”. “Molecules from Side Reactions I&II” have collected brief papers dealing with the synthesis and characterization of molecules obtained from unexpected and/or unpredictable synthetic routes.
Research concerning side products deserves to be published for at least these two main reasons: (1) side products can be useful starting points and/or intermediates for new syntheses and (2) the rationalization of the reaction conditions through which they were formed might allow for a better understanding of reaction mechanisms.
It is pleasing to see that both Special Issues have been considered useful forums of discussion for research addressing side products by organic chemists of different nationalities.
Within two years, “Molecules from Side Reactions II” collected 17 papers, with Italy being the country that contributed the most research (Figure 1). The geographical attribution was derived from the affiliations of corresponding authors.
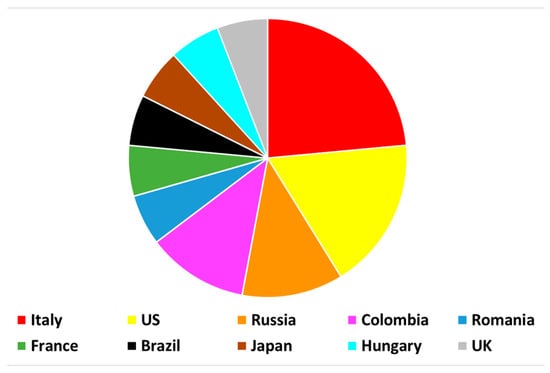
Figure 1.
Geographical distribution of manuscripts belonging to the Special Issue “Molecules from Side Reactions II”.
This Special Issue’s topics cover the following aspects of organic chemistry:
- Synthesis of heterocycles;
- Synthesis of carbohydrates;
- Synthesis of modified nucleosides;
- Mechanisms of reactions;
- Green Chemistry;
- Chemistry of natural substances.
A summary of the research results published in this Special Issue follows.
Politano et al. found that the treatment of ethyl 2-oxocyclohexanecarboxylate with 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate produced, unexpectedly, ethyl salicylate instead of ethyl 2-oxo-3-cyclohexene-1-carboxylate (contribution 1). The discovered methodology has the following advantages over conventional oxidative dehydrogenation reactions [1]: (1) the conversion is metal-free, and (2) the oxoammonium salt oxidant is environmentally friendly [2].
Tetrazoles are non-natural heterocyclic compounds endowed with interesting biological properties [3]. To obtain these compounds, Sepsey Für et al. studied the reaction of pyridazinethiones with hydrazine (contribution 2). Together with the expected E and Z stereoisomers of the desired hydrazones, they surprisingly recovered small amounts of the Schiff bases obtained after the reaction of the hydrazones with acetone present in traces in the glassware.
With the aim to decorate the bioactive isoindolo[2,1-a]quinoline scaffold [4], exploiting a Claisen–Schmidt-type condensation reaction [5], Rodríguez et al. obtained a new N-{2-[(3-oxo-1,3-dihydro-2-benzofuran-1-yl)acetyl]phenyl}acetamide derivative as a side-product (contribution 3).
Multivalent carbohydrates can recognize proteins on cell surfaces and induce biological effects [6,7]. Multivalency has been mimicked artificially by synthesizing polymers containing carbohydrates, functionalized with linkers at the end of which a polymerizable acrylamide moiety can be introduced [8]. When Miyagawa et al. studied the acryloylation reaction of 6-aminohexyl α-d-mannoside (contribution 4), they isolated both the desired N-hexyl α-d-acetylmannosyl acrylamide and the unknown N,N-bis(hexyl α-d-acetylmannosyl) acrylamide monomer, which contains two hexyl mannose units and one acrylamide group.
Rachid et al. noted the unexpected formation of an oxazole ring during the synthesis of a copper(I) cyanide network polymer. The research was conducted using single-crystal X-ray diffraction analysis and disclosed a complex architecture with a network of interatomic interactions (contribution 5).
Nascimento et al. described an interesting conversion of lupulone [9] into an annulated pyrazole through a hetero-cyclization reaction performed with phenylhydrazine. The structure of the obtained polycyclic heterocycle was ascertained through NMR spectroscopy (contribution 6).
Marzano et al. presented the structural analysis of a side product recovered during the attempts to alkylate the inosine N1 position regioselectively [10]. The N1-alkylated inosines were revealed to be fundamental building blocks for the preparation of cyclic inosine diphosphate ribose (cIDPR) analogs [11,12,13] to be exploited as potential intracellular Ca2+ ions mobilizers [14,15]. The side product was identified as an O6-alkylated inosine regioisomer through NMR analysis (contribution 7).
Amrane et al. treated 1-(4-chlorophenoxy)-4-methylphthalazine with the PCl5/POCl3 system to perform the CH3→CCl3 conversion. Unfortunately, a side dichloro methylphosphonic dichloride derivative was obtained as a single product (contribution 8).
A two-step conversion of natural betulin [16,17] to 19β,28-epoxy-18α-olean-3β-ol-2-furoate was realized by Lugemwa through a rearrangement in the E-ring followed by esterification on the A-ring of the obtained allobetulin (contribution 9).
Straniero et al. contributed to the Special Issue with three papers. In the first paper, the authors unexpectedly isolated (3-methylene-2,3-dihydronaphtho[2,3-b][1,4]dioxin-2-yl)methanol instead of the corresponding epoxide upon the exposition of 2,3-dihydronaphtho[2,3-b][1,4]dioxine-2-carbaldehyde to the Johnson–Corey–Chaykovsky reaction conditions [18] (contribution 10).
In the search for novel antimicrobial agents acting through the inhibition of the protein FtsZ [19], Straniero et al. also reported the synthesis and characterization of threo and erythro isomers of 6-fluoro-3-(2,3,6,7,8,9-hexahydronaphtho[2,3-b][1,4]dioxin-2-yl)-2,3-dihydrobenzo[b][1,4]dioxine-5-carboxamide, obtained as side products when the two isomers of the 2-(oxiran-2-yl)-2,3,6,7,8,9-hexahydronaphtho[2,3-b][1,4]dioxines were reacted with 2,6-difluorophenate (contribution 11).
Straniero et al. also found an unexpected conversion at room temperature of a pyridine derivative into the corresponding N-benzylated pyridinium salt and attributed the transformation to the presence of the unreacted benzyl bromide in the crude mixture (contribution 12).
The reaction of 3-methyl-2-thioxoimidazolidin-4-one with 1,3-dehydroadamantane afforded an unexpected side product, namely (Z)-2′-((adamantan-1-yl)thio)-1,1′-dimethyl-2′,3′-dihydro-[2,4′-biimidazolylidene]-4,5,5′(1H,1′H,3H)-trione, which was characterized by Burmistrov et al. via single-crystal X-ray diffraction analysis (contribution 13).
While searching for applications in small molecule activation reactions, a new binuclear manganese complex with two different N,O-ligands was synthesized and characterized by single-crystal X-ray diffraction analysis by Khrizanforova et al. (contribution 14). The authors found an interesting ligand environment in the crystal structure in the proximity of the two manganese centers.
Cely-Veloza et al. reported on a successful good-yielding microwave synthesis of a small series of diethyl 2-((arylamino)methylene)malonates, which were originally produced as side products of a three-component reaction (contribution 15). Interestingly, two compounds showed better IC50 than the positive control against the phytopathogens belonging to the family of Fusarium oxysporum.
In the paper published by Burcă et al., the authors explored the reactivity of triazoles, which are a class of five-membered heterocycles with remarkable biological properties [20]. In detail, they found that a new triazol-3-one unexpectedly formed following the reduction reaction of a heterocyclic thioketone with sodium borohydride in the presence of small amounts of water (contribution 16).
Aitken et al. characterized for the first time, through NMR and single-crystal X-ray diffraction experiments, a new dibromodisalicylaldehyde obtained as a side-product when studying the Baeyer–Villiger oxidation [21,22] of 5-bromo-2-methoxymethoxybenzaldehyde with m-chloroperoxybenzoic acid (contribution 17).
All the papers submitted to this Special Issue have been rigorously pre-checked by Guest Editors (GEs) before being sent for peer-review. The GEs especially thank the reviewers for verifying the correctness of the proposed side structures through a careful analysis of the experimental data reported in the manuscripts. It is pleasing to see that the acceptance rate for “Molecules from Side Reactions II” was 94%, which highlights the very high quality of research submitted to this Special Issue. Lastly, the GEs also wish to thank the Editor in Chief for accepting the Special Issue proposal and the Editorial Office staff for providing all the necessary assistance to carry out this project.
List of Contributions
- Politano, F.; Brydon, W.P.; Nandi, J.; Leadbeater, N.E. Unexpected Metal-Free Dehydrogenation of a β-Ketoester to a Phenol Using a Recyclable Oxoammonium Salt. Molbank 2021, 2021, M1180. https://doi.org/10.3390/M1180.
- Sepsey Für, C.; Keglevich, G.; Bölcskei, H. Unexpected Formation of 4-aryl-1-(Propane-2-ylidenehydrazono)-2,3-diazaspiro[5.5]undec-3-ene by the Reaction of Pyridazinethiones Derivatives with Hydrazine. Molbank 2021, 2021, M1243. https://doi.org/10.3390/M1243.
- Rodríguez, R.; León, O.; Quiroga, F.; Cifuentes, J. N-{2-[(3-Oxo-1,3-dihydro-2-benzofuran-1-yl)acetyl]phenyl}acetamide. Molbank 2021, 2021, M1244. https://doi.org/10.3390/M1244.
- Miyagawa, A.; Ohno, S.; Yamamura, H. N,N-Bis(hexyl α-d-acetylmannosyl) Acrylamide. Molbank 2021, 2021, M1255. https://doi.org/10.3390/M1255.
- Rachid, L.N.; Corfield, P.W.R. Poly[3-methyl-1,3-oxazolidin-2-iminium[µ3-cyanido-tri-µ2-cyanido-κ9C:N-tricuprate(I)]]. Molbank 2021, 2021, M1259. https://doi.org/10.3390/M1259.
- Nascimento, J.E.R.d.; Hartwig, D.; Jacob, R.G.; Silva, M.S. 3-Isobutyl-5,5,7-tris(3-methylbut-2-en-1-yl)-1-phenyl-1,7-dihydro-4H-indazole-4,6(5H)-dione. Molbank 2022, 2022, M1330. https://doi.org/10.3390/M1330.
- Marzano, M.; Terracciano, M.; Piccialli, V.; Mahal, A.; Nilo, R.; D’Errico, S. O6-[(2″,3″-O-Isopropylidene-5″-O-tbutyldimethylsilyl)pentyl]-5′-O-tbutyldiphenylsilyl-2′,3′-O-isopropylideneinosine. Molbank 2022, 2022, M1345. https://doi.org/10.3390/M1345.
- Amrane, D.; Khoumeri, O.; Vanelle, P.; Primas, N. Dichloro{4-(4-chlorophenoxy)phthalazin-1-yl} methylphosphonic dichloride. Molbank 2022, 2022, M1439. https://doi.org/10.3390/M1439.
- Lugemwa, F.N. 19β,28-Epoxy-18α-olean-3β-ol-2-furoate from Allobetulin (19β,28-Epoxy-18α-olean-3β-ol). Molbank 2022, 2022, M1499. https://doi.org/10.3390/M1499.
- Suigo, L.; Lodigiani, G.; Straniero, V.; Valoti, E. (3-Methylene-2,3-dihydronaphtho[2,3-b][1,4]dioxin-2-yl)methanol. Molbank 2022, 2022, M1521. https://doi.org/10.3390/M1521.
- Straniero, V.; Suigo, L.; Lodigiani, G.; Valoti, E. Obtainment of Threo and Erythro Isomers of the 6-Fluoro-3-(2,3,6,7,8,9-hexahydronaphtho[2,3-b][1,4]dioxin-2-yl)-2,3-dihydrobenzo[b][1,4]dioxine-5-carboxamide. Molbank 2023, 2023, M1559. https://doi.org/10.3390/M1559.
- Suigo, L.; Straniero, V.; Valoti, E. 2-(1-Methoxycarbonyl-2-phenyleth-1-yl)-1-benzylpyridin-1-ium Bromide. Molbank 2023, 2023, M1738. https://doi.org/10.3390/M1738.
- Burmistrov, V.; Mokhov, V.; Fayzullin, R.R.; Butov, G.M. (Z)-2′-((Adamantan-1-yl)thio)-1,1′-dimethyl-2′,3′-dihydro-[2,4′-biimidazolylidene]-4,5,5′(1H,1′H,3H)-trione. Molbank 2023, 2023, M1585. https://doi.org/10.3390/M1585.
- Khrizanforova, V.V.; Fayzullin, R.R.; Budnikova, Y.H. Manganese(II) Bromide Coordination toward the Target Product and By-Product of the Condensation Reaction between 2-Picolylamine and Acenaphthenequinone. Molbank 2023, 2023, M1606. https://doi.org/10.3390/M1606.
- Cely-Veloza, W.-F.; Quiroga, D.; Coy-Barrera, E. Diethyl 2-((aryl(alkyl)amino)methylene)malonates: Unreported Mycelial Growth Inhibitors against Fusarium oxysporum. Molbank 2023, 2023, M1630. https://doi.org/10.3390/M1630.
- Burcă, I.; Badea, V.; Deleanu, C.; Bercean, V.; Péter, F. 4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazol-3-one. Molbank 2023, 2023, M1705. https://doi.org/10.3390/M1705.
- Aitken, R.A.; Cordes, D.; Ler, A.J.; McKay, A.P. 2,8-Dibromo-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine. Molbank 2023, 2023, M1729. https://doi.org/10.3390/M1729.
Author Contributions
Writing—original draft preparation, S.D. and A.G.; writing—review and editing, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to Luisa Cuorvo for her technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Y.; Yan, B.; Cheng, Y. State-of-the-Art Review of Oxidative Dehydrogenation of Ethane to Ethylene over MoVNbTeOx Catalysts. Catalysts 2023, 13, 204. [Google Scholar] [CrossRef]
- Miller, S.A.; Bobbitt, J.M.; Leadbeater, N.E. Oxidation of terminal diols using an oxoammonium salt: A systematic study. Org. Biomol. Chem. 2017, 15, 2817–2822. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Meng, Y.; Wu, Y.; Song, C.; Chang, J. Synthesis of isoindolo[1,2-a]isoquinoline and isoindolo[2,1-a]quinoline derivatives via trifluoroacetic acid-mediated cascade reactions. Tetrahedron 2021, 93, 132280. [Google Scholar] [CrossRef]
- Yadav, G.D.; Wagh, D.P. Claisen-Schmidt Condensation using Green Catalytic Processes: A Critical Review. ChemistrySelect 2020, 5, 9059–9085. [Google Scholar] [CrossRef]
- Quintana, J.I.; Atxabal, U.; Unione, L.; Ardá, A.; Jiménez-Barbero, J. Exploring multivalent carbohydrate–protein interactions by NMR. Chem. Soc. Rev. 2023, 52, 1591–1613. [Google Scholar] [CrossRef]
- Laigre, E.; Goyard, D.; Tiertant, C.; Dejeu, J.; Renaudet, O. The study of multivalent carbohydrate–protein interactions by bio-layer interferometry. Org. Biomol. Chem. 2018, 16, 8899–8903. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ibrahim, A.A.; Chong, K.F.; Soldatov, A.V.; Ali, G.A.M. Glycopolymer-Based Materials: Synthesis, Properties, and Biosensing Applications. Top. Curr. Chem. 2022, 380, 45. [Google Scholar] [CrossRef]
- Carbone, K.; Gervasi, F. An Updated Review of the Genus Humulus: A Valuable Source of Bioactive Compounds for Health and Disease Prevention. Plants 2022, 11, 3434. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.M.; Broom, A.D.; Buckheit, R.W. Antiviral Amphipathic Oligo- and Polyribonucleotides: Analogue Development and Biological Studies. J. Med. Chem. 2003, 46, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Solid-Phase Synthesis of a New Diphosphate 5-Aminoimidazole-4-carboxamide Riboside (AICAR) Derivative and Studies toward Cyclic AICAR Diphosphate Ribose. Molecules 2011, 16, 8110–8118. [Google Scholar] [CrossRef]
- Mahal, A.; D’Errico, S.; Borbone, N.; Pinto, B.; Secondo, A.; Costantino, V.; Tedeschi, V.; Oliviero, G.; Piccialli, V.; Piccialli, G. Synthesis of cyclic N 1 -pentylinosine phosphate, a new structurally reduced cADPR analogue with calcium-mobilizing activity on PC12 cells. Beilstein J. Org. Chem. 2015, 11, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Greco, F.; Patrizia Falanga, A.; Tedeschi, V.; Piccialli, I.; Marzano, M.; Terracciano, M.; Secondo, A.; Roviello, G.N.; Oliviero, G.; et al. Probing the Ca2+ mobilizing properties on primary cortical neurons of a new stable cADPR mimic. Bioorg. Chem. 2021, 117, 105401. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Collins, T.J.; Peppiatt, C.M.; Prothero, L.S.; MacKenzie, L.; De Smet, P.; Travers, M.; Tovey, S.C.; Seo, J.T.; Berridge, M.J.; et al. Calcium signalling—An overview. Semin. Cell Dev. Biol. 2001, 12, 3–10. [Google Scholar] [CrossRef]
- Matikainen, N.; Pekkarinen, T.; Ryhänen, E.M.; Schalin-Jäntti, C. Physiology of Calcium Homeostasis. Endocrinol. Metab. Clin. North Am. 2021, 50, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive Review on Betulin as a Potent Anticancer Agent. BioMed Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef]
- Mondal, M.; Connolly, S.; Chen, S.; Mitra, S.; Kerrigan, N.J. Recent Developments in Stereoselective Reactions of Sulfonium Ylides. Organics 2022, 3, 320–363. [Google Scholar] [CrossRef]
- Han, H.; Wang, Z.; Li, T.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Wang, J. Recent progress of bacterial FtsZ inhibitors with a focus on peptides. FEBS J. 2021, 288, 1091–1106. [Google Scholar] [CrossRef]
- Gupta, O.; Pradhan, T.; Chawla, G. An updated review on diverse range of biological activities of 1,2,4-triazole derivatives: Insight into structure activity relationship. J. Mol. Struct. 2023, 1274, 134487. [Google Scholar] [CrossRef]
- Renz, M.; Meunier, B. 100 Years of Baeyer–Villiger Oxidations. Eur. J. Org. Chem. 1999, 1999, 737–750. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, Z.; Peng, J.; Qin, Q.; Jiao, N. Alternative method to Baeyer–Villiger oxidation of cyclobutenones using I 2/DMSO catalytic systems. Green Chem. 2023, 25, 7079–7083. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).