Abstract
In this study, the synthesis and biological activities of previously and newly synthesized oleanolic acid derivatives containing seven-membered cyclic amines at the C28 position were described. The obtained compounds were fully characterized via 1H and 13C NMR spectroscopy, and the bioactivity was evaluated by Ellman’s method. Among the tested compounds, 2,3-indolo-oleanolic acid was found to be the most active compound with an IC50 value of 0.78 µM against acetylcholinesterase. These results are significant due to the fact that research on the inhibition of acetylcholinesterase and butyrylcholinesterase enzymes by oleanolic acid, in particular indoloderivatives, is limited.
1. Introduction
Progressive and irreversible neurodegenerative diseases, such as Alzheimer’s disease (AD), characterized by memory loss, behavioral, and many other cognitive impairments, are socially significant diseases that require active attention from scientists. Currently available therapies for AD primarily focus on relieving its symptoms by targeting the cholinergic system [1]. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) are enzymes involved in the breakdown of the neurotransmitter acetylcholine in cholinergic synapses. AChE and BChE remain the most promising and therapeutic targets for symptomatic AD treatment. The “cholinergic hypothesis” [2] underlies the rationale behind using AChE and BChE inhibitors. Inhibiting cholinesterase activity can compensate for the cholinergic deficiency, leading to improvements in learning and memory [3]. In recent years, the development of drugs for AD has been dominated by research into multitargeted treatments that can slow down the progression of the disease. Therefore, new strategies for the search and synthesis of cholinesterase inhibitors remain relevant.
Natural products, in particular triterpenoids, are actively used in organic and medicinal chemistry. At present, the obtained semi-synthetic analogs are considered new classes of antitumor, antiviral, antimicrobial, and other agents [4,5,6,7].
One of the most promising candidates for future clinical trials includes triterpenoids conjugated or annulated with heterocyclic moieties [8]. Thus, indolo-derivatives, along with different biological activities such as anticancer activity [9], exhibited good enzyme inhibition properties. For example, 2,3-indolo-betilinic acid was found to be the most active inhibitor of α-glucosidase, with an IC50 of 1.8 μM, being 221-fold more active than the market drug acarbose [10]. Further modification by amidation with glycine and L-phenylalanine at C28 led to derivatives with IC50 values of 0.04 and 0.05 μM, being 3784- and 4730-fold more active than acarbose [11]. 2,3-Indolo-oleanolic with cyclohexane amide at C28 was found to be a NO production inhibitor with an IC50 2.66 μM [12]. The indolo-glycyrrhetinic acid with a trifluoromethyl substitute in the indole ring exhibited non-competitive inhibition of PTP1B with an IC50 2.5 µM [13].
Compared to other types, the inhibition of enzymes of the esterase family by indole acids has not been studied, although the influence of modification of the C28 position by amines has been noted in the literature. For example, a derivative of betulinic acid with a pyrrolidine substituent exhibited inhibitory properties against BChE with Ki values of 0.39 ± 0.04 µM, while the replacement of the pyrrolidine substituent with piperidinyl led to selective inhibition of AChE (Ki 1.00 ± 0.09 µM) [14]. The amides of lupane, oleanane, and ursane types of triterpene acids with a fragment of 1,3- and 1,4-diazabicyclononane and their methylated salts turned out to be highly specific inhibitors of BChE with a degree of inhibition above 95% [15]. A derivative of glycyrrhetic acid with a triethyldiamine moiety at position C30 exhibited selective butyrylcholinesterase inhibitory activity with a Ki value of 5.43 µM [16].
In line with these data, the main goal of this research was the study of the acetylcholine inhibitory activity of oleanolic acid derivatives, including those with a modified C28 position as well as in combination with the A-indole cycle.
2. Results and Discussion
At first, a library of 3-oxo-oleanolic acid 1 and its homopiperazine amides 2–7 (Figure 1), obtained as described previously [17,18,19], were screened against AChE and BChE inhibitory activity.
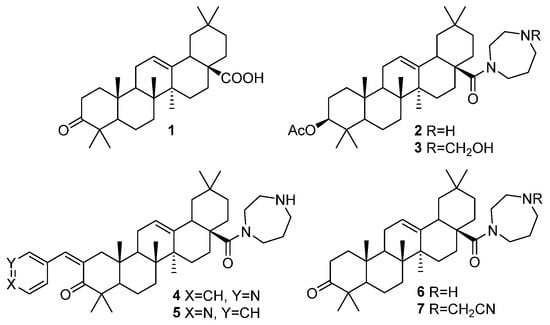
Figure 1.
Previously synthesized oleanolic acid [17] and its homopiperazine amides 2 and 3 [18], 4–7 [19].
Acylation of 2 by chloroacetonitrile in the precence of K2CO3 in DMF afforded N-substituted derivative 8 in an 80% yield. (Scheme 1).
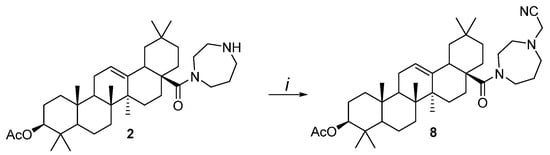
Scheme 1.
Reagents and conditions: i. ClCH2CN, K2CO3, DMF, ∆, 3 h.
The series of C28-modified derivatives were synthesized based on 2,3-indolo-oleanolic acid 9 (Scheme 2). Homopiperazinamide 11 was synthesized according to [19]. Treatment of 11 with chloroacetonitrile led to 12 with a 77% yield. Acylation of 2,3-indolo-erythrodiol, obtained by the reduction of acid 9, with diethyl chlorophosphate in the presence of DMAP in pyridine, gave diethoxyphosphoryl derivative 10 with yields of 85%.
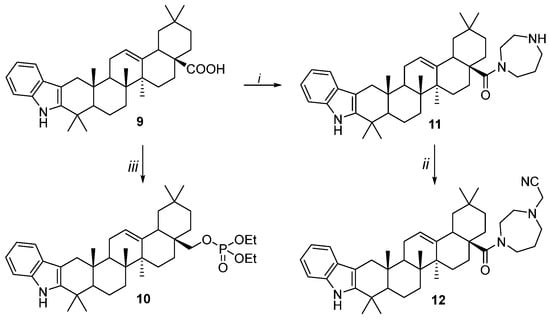
Scheme 2.
Reagents and conditions: i. 1. (COCl)2, Et3N, CH2Cl2, 25 °C, 2 h; 2. Homopiperazine, Et3N, CH2Cl2, ∆, 2 h; ii. ClCH2CN, K2CO3, DMF, ∆, 3 h; iii. (EtO)2POCl, DMAP, Py, 0 °C, 6 h.
The structure of the newly synthesized compounds was confirmed by 1H and 13C NMR spectra. Thus, for cyanomethyl derivatives 8 and 12, the signals of the carbon atom of the CN group were observed at δ 115 ppm. In the 1H and 13C NMR spectra of compound 10, the signals of proton and carbon atoms of ethoxy groups were observed in the range δH 4.10–4.17 ppm, δC 63.62 and 63.66 ppm.
All compounds were tested for their ability to inhibit the activity of recombinant human acetylcholinesterase and human butyrylcholinesterase from blood plasma in vitro according to the Ellman method [20] on a Shimadzu UV-1800 spectrophotometer by measuring the color intensity change at a wavelength of 412 nm for 2 min at 25 °C. Donepezil was used as a reference drug (Table 1).

Table 1.
Acetylcholinesterase and butyrylcholinesterase inhibitory activity of compounds 1–12.
Among hopopiperazinamides, moderate activity was observed for 3-oxo-derivative 6 (IC50 17.7 µM), while the introduction of indolo-group into the oleanolic acid molecule led to an increase in the IC50 to 107 µM (compound 11). N-Cyanomethylation of compounds 6 and 11 improved the activity of the 2,3-indolo derivative 12 (IC50 12.8 µM), while for the 3-oxo-homopiperazine amide 7, the IC50 value increased 1.5 times (IC50 27.7 µM). The introduction of a hydroxymethylene group into the polycyclic ring led to moderate inhibition of butyrylcholinesterase with an IC50 value of 38.8 µM for 3-acetoxy derivative 3, which was not detected for other compounds with a cyanomethyl group. The products of the Claisen–Schmidt reaction with a 3- or 4-pyridine group in the C2 position were inactive (compound 5, 156 µM) or moderately active (compound 4, 27.4 µM).
At the same time, 2,3-indolo-oleanolic acid 9 was found to be the most active among all the tested compounds, with an IC50 value of 0.78 µM, albeit to a lesser extent compared to the effects of donepezil (0.01 µM). In general, according to the value of the average inhibitory concentration, the following pattern can be revealed for the modified derivatives: 2,3-indolo < 3-oxo < 3-acetoxy.
It should be noted that 2,3-indolo-oleanolic acid was also evaluated for its ability to inhibit various enzymes. For example, it was shown that 9 exhibit PTP1B inhibition properties with an IC50 of 0.61 µM [21]. Additionally, 2,3-indolo-oleanolic acid 9 was found to be a moderate α-glucosidase inhibitor with an IC50 of 5.5 μM and 72-fold more active than the market drug acarbose [10]. Considering that studies of indolo-triterpene acid derivatives are limited only to these enzymes, the results obtained in this article are of great interest.
3. Materials and Methods
The spectra were recorded at the Center for the Collective Use ‘Chemistry’ of the Ufa Institute of Chemistry of the UFRC RAS and RCCU “Agidel” of the UFRC RAS. 1H and 13C NMR spectra were recorded on a “Bruker Avance-III” (Bruker, Billerica, MA, USA, 500 and 125.5 MHz, respectively, δ, ppm, Hz) in CDCl3. Tetramethylsilane was used as an internal standard. Mass spectra were obtained on a liquid chromatograph–mass spectrometer LCMS-2010 EV (Shimadzu, Kyoto, Japan). Melting points were detected on a microtable “Rapido PHMK05” (Nagema, Dresden, Germany). Optical rotations were measured on a polarimeter Perkin-Elmer 241 MC (PerkinElmer, Waltham, MA, USA) in a tube length of 1 dm. Thin-layer chromatography analyses were performed on Sorbfil plates (Sorbpolimer, Krasnodar, Russia), using the solvent system chloroform–ethyl acetate at a ratio of 40:1. Substances were detected by spraying the plates with a 10% sulfuric acid solution with subsequent heating at 100–120 °C for 2–3 min. All chemicals were of reagent grade (Sigma-Aldrich, St. Louis, MO, USA). Compounds 1 [17], 2 and 3 [18], 4–7 and 11 [19], 9 [22] were synthesized as described previously. All spectral data of new compounds are provided in the Supplementary Material file.
3.1. General Synthesis of Compounds 8 and 12
The solutions of 2 (0.58 g, 1 mmol) or 11 (0.61 g, 1 mmol) in DMF (20 mL), K2CO3 (0.14 g, 0.98 mmol) and chloroacetonitrile (0.08 mL, 1.3 mmol) were added and the reaction mixture was stirred at 60 °C for 2 h. After cooling to room temperature, the mixture was poured into ice-cold water and filtered. The residue was filtered off, washed with water and dried, then purified by column chromatography on SiO2 using a mixture of petroleum ether—EtOAc (4:1) as the eluent.
3.1.1. N-[3β-Acetoxy-olean-12(13)-en-(homopiperazine-N-cyanomethyl)]-28-amide (8)
Yield 80% (0.50 g), Rf 0.3, mp: 105 °C, [α]D20 + 14 (c 0.01, CHCl3). 1H NMR (δ, ppm, CDCl3, 500 MHz): 0.71, 0.81, 0.82, 0.86, 0.89, 0.90, 1.10 (21H, s, 7CH3), 1.12–2.10 (25H, m, CH, CH2), 2.00 (3H, s, CH3), 2.65–2.80 (2H, m, CH2), 3.00–3.10 (2H, m, CH2), 3.50–3.70 (6H, m, 3CH2), 4.40–4.50 (1H, t, J = 15.4, 8.6 Hz, H-3), 5.22 (1H, s, H-12). 13C NMR (δ, ppm, CDCl3, 125.5 MHz): 15.36, 16.62, 16.88, 18.18, 21.27, 22.48, 23.34, 23.48, 24.05, 25.86, 27.95, 27.99, 29.89, 30.30, 30.35, 32.74, 33.04, 34.03, 36.95, 37.65, 38.04, 39.14, 41.93, 43.68, 46.44, 46.97, 47.29, 47.66, 47.74, 55.34, 80.89 (C-3), 115.20 (CN), 121.33 (C-12), 144.80 (C-13), 170.92 (C(O)CH3), 175.67 (C-28). Anal. Calcd for C39H61N3O3: C, 75.56, H, 9.92, N, 6.78. Found: C, 75.61, H, 9.85, N, 6.64. MS (APCI) m/z 620.50 [M+H]+, calcd for C39H61N3O3: 619.94.
3.1.2. N-[3,2b]-Indolo-olean-12(13)-en-(homopiperazine-N-cyanomethyl)-28-amide (12)
Yield 77% (0.50 g), Rf 0.4, mp: 138.1 °C, [α]D20 + 33 (c 0.01, CHCl3). 1H NMR (δ, ppm, CDCl3, 500 MHz): 0.87, 0.92, 0.95, 0.97, 1.20, 1.21, 1.30 (21H, s, 7CH3), 1.33–2.20 (23H, m, CH, CH2), 2.70–3.75 (10H, m, 5CH2), 4.10–4.15 (1H, m, CH2CN), 5.38 (1H, s, H-12), 7.03–7.44 (4H, m, Harom), 7.80 (1H, s, NH). 13C NMR (δ, ppm, CDCl3, 125.5 MHz): 14.21, 15.58, 16.77, 19.39, 21.05, 22.65, 23.30, 23.47, 24.12, 25.84, 28.13, 30.01, 30.45, 31.00, 32.43, 33.11, 34.00, 34.17, 36.76, 38.18, 39.38, 42.23, 43.91, 46.56, 47.07, 47.49, 47.94, 53.34, 60.40, 106.96 (C-2), 110.32 (Carom), 115.33 (CN), 118.00 (Carom), 118.86 (Carom), 120.91 (Carom), 121.81 (C-12), 128.30 (Carom), 136.17 (Carom), 140.88 (C-3), 144.61 (C-13), 175.67 (C-28). Anal. Calcd for C43H60N4O: C, 79.58, H, 9.32, N, 8.63. Found: C, 79.64, H, 9.45, N, 8.58. MS (APCI) m/z 649.50 [M+H]+, calcd for C43H60N4O: 648.98.
3.2. [3,2b]-Indolo-olean-12(13)-En-28-diethoxyphosphoryl (10)
To a solution of compound 9 (0.53 g, 1 mmol) in pyridine (6 mL), diethyl chlorophosphate (0.28 mL, 1.9 mmol) and 4-dimethylaminopyridine (cat. amount) were added and the reaction mixture was stirred at 20 °C for 12 h. After the reaction was complete, the mixture was poured into H2O/H+, the precipitate was filtered and washed until neutral pH, dried, then purified by column chromatography on SiO2 using a mixture of petroleum ether—EtOAc (9:1) as the eluent.
Yield 85% (0.55 g), Rf 0.6, mp: 110 °C, [α]D20 + 17 (c 0.01, CHCl3). 1H NMR (δ, ppm, CDCl3, 500 MHz): 0.89, 0.91, 0.96, 1.06, 1.23, 1.33, 1.36 (21H, s, 7CH3), 1.40–2.80 (21H, m, CH, CH2), 3.61–3.66 (1H, dd, J = 9.6, 3.6, H-28), 4.02–4.06 (1H, dd, J = 9.6, 3.6, H-28), 4.10–4.17 (10H, m, 2 OCH2CH3), 5.30 (1H, s, H-12), 7.03–7.43 (4H, m, Harom), 7.79 (1H, s, NH). 13C NMR (δ, ppm, CDCl3, 125.5 MHz): 15.75, 16.22, 16.27, 16.49, 19.35, 21.86, 23.30, 23.61, 23.66, 25.47, 25.86, 30.93, 30.99, 31.23, 32.04, 33.17, 33.98, 34.04, 36.64, 36.71, 36.98, 38.00, 39.95, 41.88, 42.28, 46.29, 53.21, 63.62 (OCH2), 63.66 (OCH2), 73.94 (C-28), 106.74 (C-2), 110.43 (Carom), 117.90 (Carom), 118.82 (Carom), 120.90 (Carom), 123.25 (C-12), 128.21 (Carom), 136.21 (Carom), 140.92 (C-2), 143.35 (C-13). Anal. Calcd for C40H60NO4P: C, 73.93, H, 9.31, N, 2.16, P, 4.77. Found: C, 73.87, H, 9.25, N, 2.22, P, 4.67. MS (APCI) m/z 650.50 [M+H]+, calcd for C40H60NO4P: 649.90.
3.3. Biological Assay
In Vitro Cholinesterase Inhibition Assay
The inhibitory activity of compounds against human AChE and human BChE was measured using the Ellman’s method [20]. Stock solutions (0.01 M) of compounds were dissolved in H2O, or ethanol. In the case of compounds soluble in ethanol, the final concentration of ethanol in the cuvette was 0.1% vol. Enzyme-catalyzed substrate hydrolysis was carried out in 0.1 m phosphate buffer (pH 8.0 for AChE and pH 7.0 for BChE) containing 0.25 U recombinant human AChE or 0.25 U human BChE from blood plasma, 0.1 mM 5,5′-dithiobis-(2-nitrobenzoic acid) and 1 mM acetylthiocholine (ATC) or butyrylthiocholine (BTC) as substrates. All assays were performed at 25 °C using a UV-1800 Shimadzu (Shimadzu Co., Kyoto, Japan) spectrophotometer at 412 nm. The tested compounds were pre-incubated with the enzyme for 5 min at 25 °C prior to the addition of the substrate. The rate of substrate hydrolysis was calculated as measured by the optical density change at 412 nm change over 2 min. The sample without a substrate was used as a blank. The sample without an inhibitor was used as a control. Experiments were performed in triplicate. The percentage of AChE/BChE inhibition was determined by comparison of the rates of reaction of test samples relative to the control sample. The percentage of inhibition as a function of the compound concentration was plotted using OriginPro 8.5 software (OriginLab Corporation, Northampton, MA, USA). IC50 (the concentration of the compound producing 50% enzyme activity inhibition) was calculated using the Hill Equation:
where E is the enzyme activity and [I] is the compound concentration.
4. Conclusions
In this work, a series of previously and newly synthesized homopiperazine amides of oleanolic acid were evaluated for their acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity. As a result, 2,3-indolo-oleanolic acid was found to be highly active for AChE, with an IC50 value of 0.78 µM. Among all the tested compounds, homopiprazine amide substituted with a hydroxymethylene group in the amine cycle exhibited a moderate inhibition of butyrylcholinesterase with an IC50 value of 38.8 µM, which was not detected for other compounds.
Supplementary Materials
The following supporting information can be downloaded online. Figures S1–S6: 1H and 13C NMR data of compounds 8, 10, and 12.
Author Contributions
Investigation, A.V.P.; methodology, K.A.P. and I.V.Z.; writing—original draft preparation, A.V.P., K.A.P. and I.V.Z.; writing—review and editing, A.V.P.; project administration, A.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation (project No. 22-73-00266), https://rscf.ru/project/22-73-00266/.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the financial support from the government assignment for FRC Kazan Scientific Center of RAS as part of biochemical studies of cholinesterase inhibitors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moreta, M.P.; Burgos-Alonso, N.; Torrecilla, M.; Marco-Contelles, J.; Bruzos-Cidón, C. Efficacy of Acetylcholinesterase Inhibitors on Cognitive Function in Alzheimer’s Disease. Review of Reviews. Biomedicines 2021, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Maloney, A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 2006, CD005593. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Dai, S.Y.; Deng, F.H.; Peng, L.H.; Li, C.; Pei, Y.H. Recent advances in medicinal chemistry of oleanolic acid derivatives. Phytochemistry 2022, 203, 113397. [Google Scholar] [CrossRef]
- Żwawiak, J.; Pawełczyk, A.; Olender, D.; Zaprutko, L. Structure and Activity of Pentacyclic Triterpenes Codrugs. A Review. Mini Rev. Med. Chem. 2021, 21, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340. [Google Scholar] [CrossRef]
- Oliveira-Costa, J.F.; Meira, C.S.; Neves, M.V.G.D.; Dos Reis, B.P.Z.C.; Soares, M.B.P. Anti-Inflammatory Activities of Betulinic Acid: A Review. Front. Pharmacol. 2022, 13, 883857. [Google Scholar] [CrossRef]
- Nistor, G.; Trandafirescu, C.; Prodea, A.; Milan, A.; Cristea, A.; Ghiulai, R.; Racoviceanu, R.; Mioc, A.; Mioc, M.; Ivan, V.; et al. Semisynthetic Derivatives of Pentacyclic Triterpenes Bearing Heterocyclic Moieties with Therapeutic Potential. Molecules 2022, 27, 6552. [Google Scholar] [CrossRef]
- Gao, C.X.; Tang, C.H.; Wu, T.J.; Hu, Y.; Peng, Y.L.; Liu, M.L.; Liu, Q.W.; Chen, H.F.; Yang, Z.H.; Zheng, X. Anticancer activity of oleanolic acid and its derivatives modified at A-ring and C-28 position. J. Asian Nat. Prod. Res. 2023, 25, 581–594. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Smirnova, I.E.; Kazakova, O.B.; Petrova, A.V.; Ha, N.T.; Viet, D.Q. Synthesis and evaluation of 2,3-indolotriterpenoids as new α-glucosidase inhibitors. Med. Chem. Res. 2017, 26, 2737–2742. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.T.; Tu, A.L.T.; Thanh, T.N.; Thi, C.B.; Babkov, D.A.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.; Patel, N.K.; Gangwal, R.P.; Sangamwar, A.T.; Bhutani, K.K. Oleanolic acid analogs as NO, TNF-α and IL-1β inhibitors: Synthesis, biological evaluation and docking studies. Bioorg. Med. Chem. Lett. 2014, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- De-la-Cruz-Martínez, L.; Duran-Becerra, C.; González-Andrade, M.; Páez-Franco, J.C.; Germán-Acacio, J.M.; Espinosa-Chávez, J.; Torres-Valencia, J.M.; Pérez-Villanueva, J.; Palacios-Espinosa, J.F.; Soria-Arteche, O.; et al. Indole- and Pyrazole-Glycyrrhetinic Acid Derivatives as PTP1B Inhibitors: Synthesis, In Vitro and In Silico Studies. Molecules 2021, 26, 4375. [Google Scholar] [CrossRef]
- Loesche, A.; Kahnt, M.; Serbian, I.; Brandt, W.; Csuk, R. Triterpene-Based Carboxamides Act as Good Inhibitors of Butyrylcholinesterase. Molecules 2019, 24, 948. [Google Scholar] [CrossRef] [PubMed]
- Heise, N.; Friedrich, S.; Temml, V.; Schuster, D.; Siewert, B.; Csuk, R. N-methylated diazabicyclo[3.2.2]nonane substituted triterpenoic acids are excellent, hyperbolic and selective inhibitors for butyrylcholinesterase. Eur. J. Med. Chem. 2022, 227, 113947. [Google Scholar] [CrossRef]
- Schwarz, S.; Lucas, S.D.; Sommerwerk, S.; Csuk, R. Amino derivatives of glycyrrhetinic acid as potential inhibitors of cholinesterases. Bioorg. Med. Chem. 2014, 22, 3370–3378. [Google Scholar] [CrossRef]
- Heller, L.; Schwarz, S.; Perl, V.; Köwitsch, A.; Siewert, B.; Csuk, R. Incorporation of a Michael acceptor enhances the antitumor activity of triterpenoic acids. Eur. J. Med. Chem. 2015, 101, 391–399. [Google Scholar] [CrossRef]
- Kazakova, O.; Rubanik, L.; Smirnova, I.; Poleschuk, N.; Petrova, A.; Kapustsina, Y.; Baikova, I.; Tret’yakova, E.; Khusnutdinova, E. Synthesis and in vitro activity of oleanolic acid derivatives against Chlamydia trachomatis and Staphylococcus aureus. Med. Chem. Res. 2021, 30, 1408–1418. [Google Scholar] [CrossRef]
- Smirnova, I.; Petrova, A.; Giniyatullina, G.; Smirnova, A.; Volobueva, A.; Pavlyukova, J.; Zarubaev, V.; Loc, T.V.; Tran Thi Phoung, T.; Hau, V.T.B.; et al. Synthesis, Anti-Influenza H1N1 and Anti-Dengue Activity of A-Ring Modified Oleanonic Acid Polyamine Derivatives. Molecules 2022, 27, 8499. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Qiu, W.W.; Shen, Q.; Yang, F.; Wang, B.; Zou, H.; Li, J.Y.; Li, J.; Tang, J. Synthesis and biological evaluation of heterocyclic ring-substituted maslinic acid derivatives as novel inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2009, 19, 6618–6622. [Google Scholar] [CrossRef] [PubMed]
- Finlay, H.J.; Honda, T.; Gribble, G.W. Synthesis of novel [3,2-b]indole fused oleanolic acids as potential inhibitors of cell proliferation. Arkivoc 2002, XII, 38–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).