Supramolecular Self-Assembly of the Zwitterionic Sn(IV)-Porphyrin Complex
Abstract
:1. Introduction
2. Results and Discussion
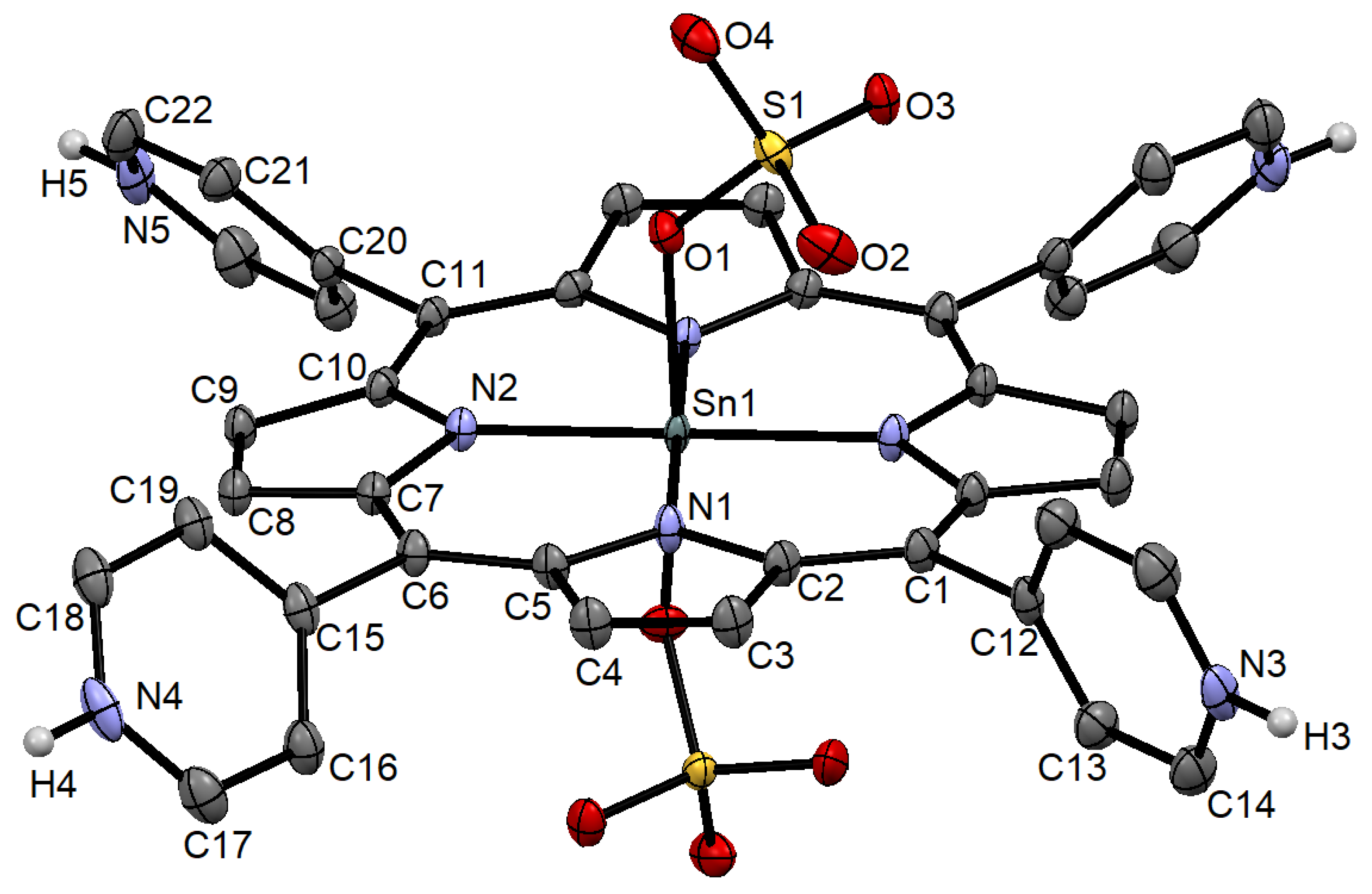
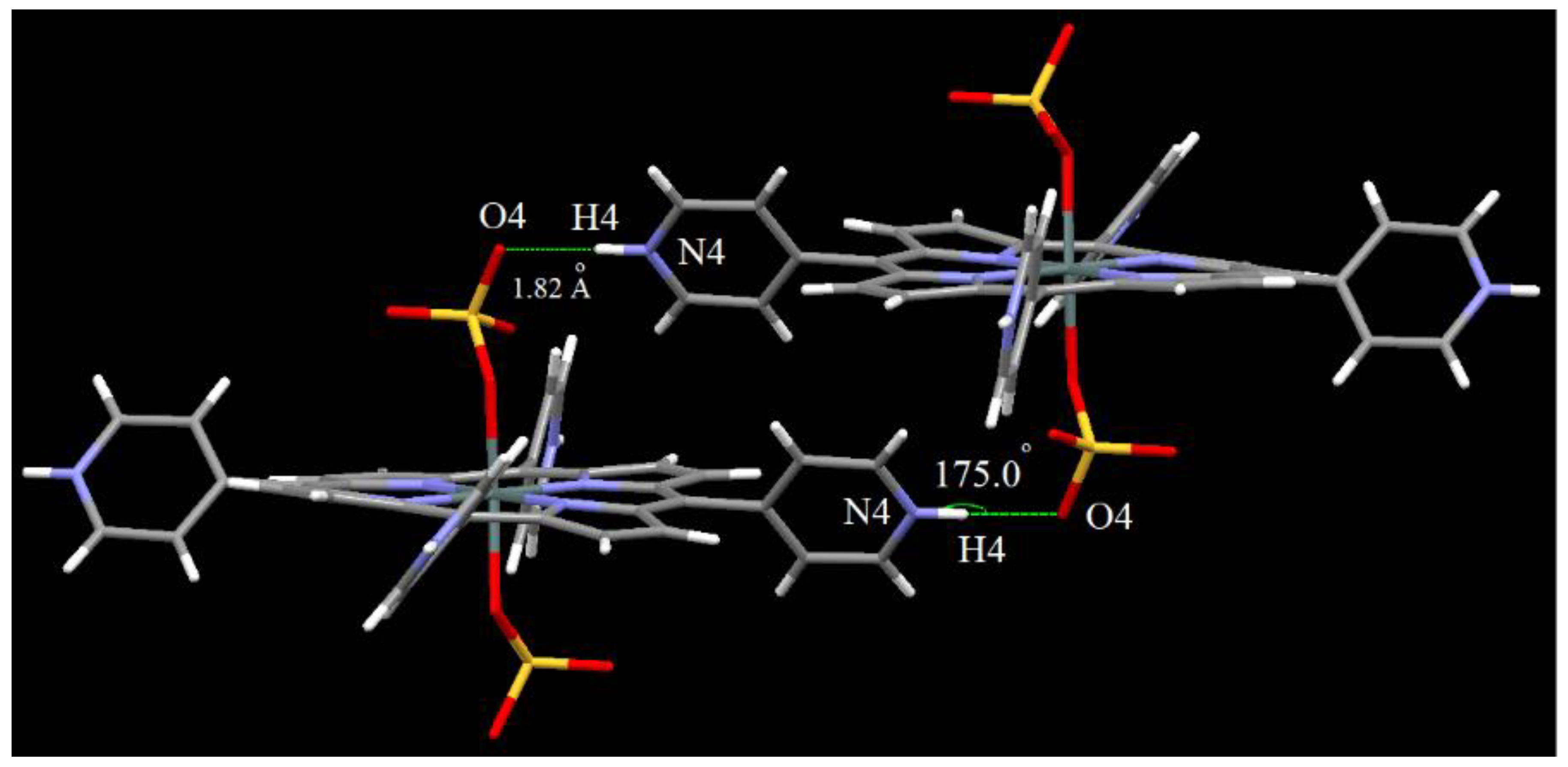
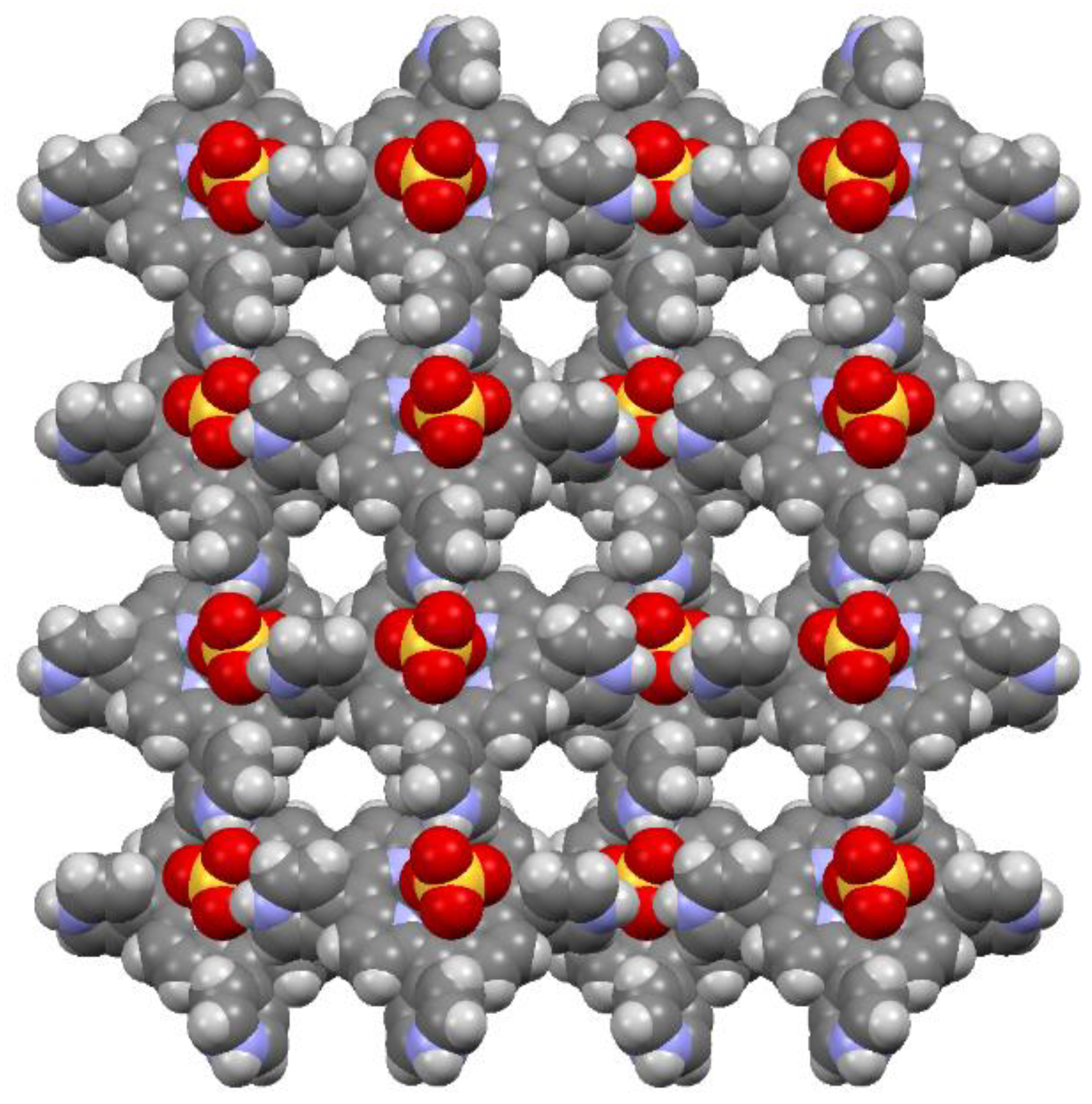
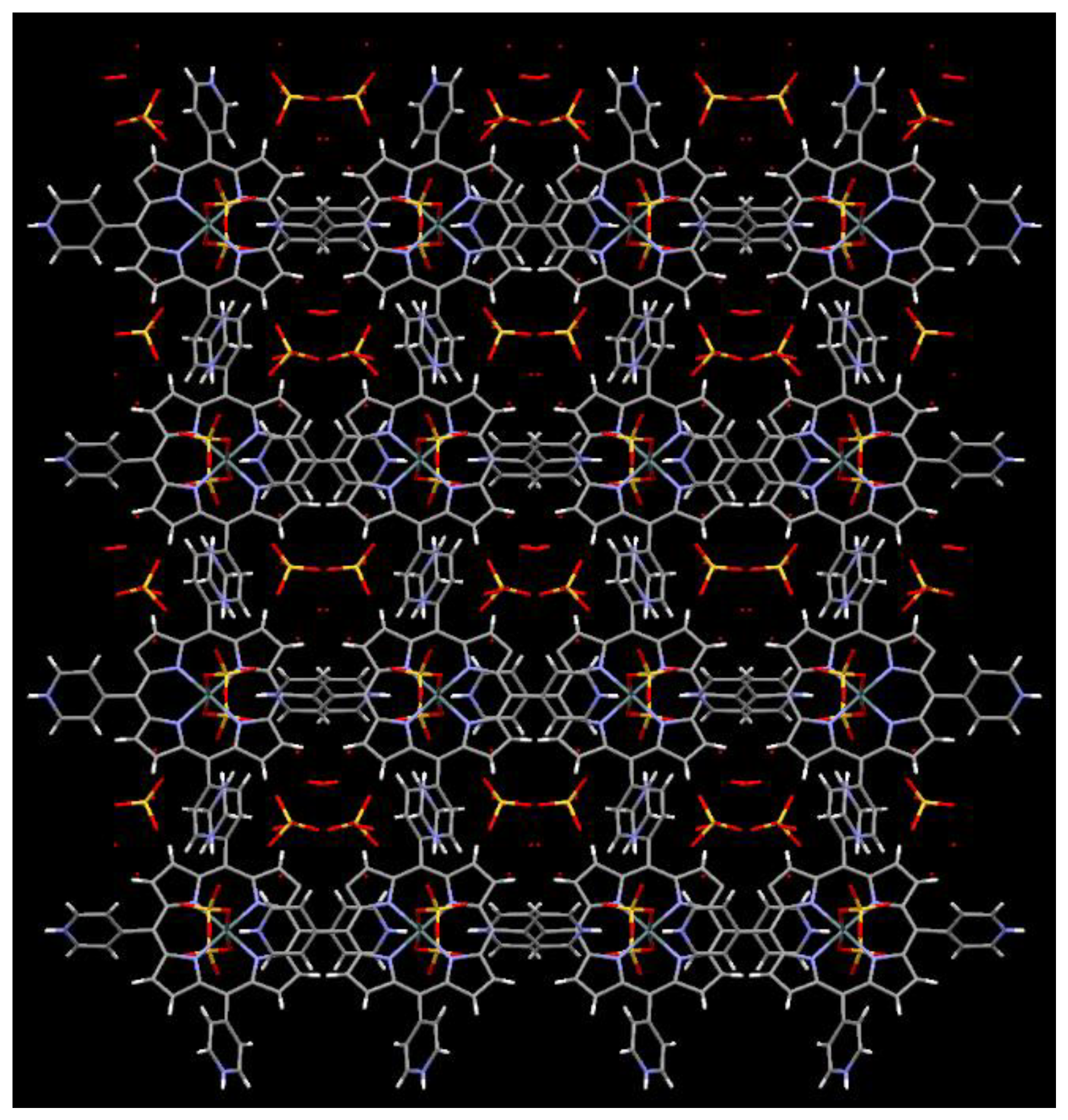
2.1. X-ray Crystal Structure Analysis
2.2. Spectroscopic Characterization
3. Materials and Methods
3.1. Synthesis of [Sn(OSO3)2(TPyHP)](HSO4)2∙8H2O (1)
3.2. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambron, J.-C.; Heitz, V.; Sauvage, J.-P. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 6, pp. 1–42. [Google Scholar]
- Chou, J.-H.; Nalwa, H.S.; Kosal, M.E.; Rakow, N.A.; Suslick, K.S. Applications of Porphyrins and Metalloporphyrins to Materials Chemistry. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 6, pp. 43–131. [Google Scholar]
- Suslick, K.S.; Rakow, N.A.; Kosal, M.E.; Chou, J.-H. The materials chemistry of porphyrins and metalloporphyrins. J. Porphyr. Phthalocyanines 2000, 4, 407–413. [Google Scholar] [CrossRef]
- Milic, T.N.; Chi, N.; Yablon, D.G.; Flynn, G.W.; Batteas, J.D.; Drain, C.M. Controlled hierarchical self-assembly and deposition of nanoscale photonic materials. Angew. Chem. Int. Ed. 2002, 41, 2117–2119. [Google Scholar] [CrossRef]
- Drain, C.M.; Hupp, J.T.; Suslick, K.S.; Wasielewski, M.R.; Chen, X. A perspective on four new porphyrin-based functional materials and devices. J. Porphyr. Phthalocyanines 2002, 6, 243–258. [Google Scholar] [CrossRef]
- Vinodu, M.; Goldberg, I. New assembly modes of porphyrin-based networks. CrystEngComm 2003, 5, 204–207. [Google Scholar] [CrossRef]
- Goldberg, I. Crystal engineering of porphyrin framework solids. Chem. Commun. 2005, 10, 1243–1254. [Google Scholar] [CrossRef]
- George, S.; Goldberg, I. Self-Assembly of Supramolecular Porphyrin Arrays by Hydrogen Bonding: New Structures and Reflections. Cryst. Growth Des. 2006, 6, 755–762. [Google Scholar] [CrossRef]
- Hasobe, T. Photo-and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef]
- Madueno, R.; Raisanen, M.T.; Silien, C.; Buck, M. Functionalizing hydrogen-bonded surface networks with self-assembled monolayers. Nature 2008, 454, 618–621. [Google Scholar] [CrossRef]
- Ohkawa, H.; Takayama, A.; Nakajima, S.; Nishide, H. Cyclic Tetramer of a Metalloporphyrin Based on a Quadruple Hydrogen Bond. Org. Lett. 2006, 8, 2225–2228. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The nature of .Pi.-.Pi. Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Watson, M.D.; Fechtenkotter, A.; Müllen, K. Big Is Beautiful-“Aromaticity” Revisited from the Viewpoint of Macromolecular and Supramolecular Benzene Chemistry. Chem. Rev. 2001, 101, 1267–1300. [Google Scholar] [CrossRef]
- Zang, L.; Che, Y.; Moore, J.S. One-Dimensional Self-Assembly of Planar π-Conjugated Molecules: Adaptable Building Blocks for Organic Nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef]
- Northrop, B.H.; Zheng, Y.-R.; Chi, K.-W.; Stang, P.J. Self-Organization in Coordination-Driven Self-Assembly. Acc. Chem. Res. 2009, 42, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Farha, O.K.; Shultz, A.M.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Active-Site-Accessible, Porphyrinic Metal-Organic Framework Materials. J. Am. Chem. Soc. 2011, 133, 5652–5655. [Google Scholar] [CrossRef]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-Harvesting Metal-Organic Frameworks (MOFs): Efficient Strut-to-Strut Energy Transfer in Bodipy and Porphyrin-Based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.F.J.; Antonietti, M. Ionic self-assembly: Facile synthesis of supramolecular materials. Adv. Mater. 2003, 15, 673–683. [Google Scholar] [CrossRef]
- Fallon, G.D.; Langford, S.J.; Lee, M.A.-P.; Lygris, E. Self-assembling Mixed Porphyrin Trimers—The Use of Diaxial Sn(IV) Porphyrin Phenolates as an Organising Precept. Inorg. Chem. Commun. 2002, 5, 715–718. [Google Scholar] [CrossRef]
- Jo, H.J.; Jung, S.H.; Kim, H.-J. Synthesis and Hydrogen-Bonded Supramolecular Assembly of trans-Dihydroxotin(IV) Tetrapyridylporphyrin Complexes. Bull. Korean Chem. Soc. 2004, 25, 1869–1873. [Google Scholar]
- Dvivedi, A.; Pareek, Y.; Ravikanth, M. SnIV Porphyrin Scaffolds for Axially Bonded Multiporphyrin Arrays: Synthesis and Structure Elucidation by NMR Studies. Chem. Eur. J. 2014, 20, 4481–4490. [Google Scholar] [CrossRef]
- Jo, H.J.; Kim, S.H.; Kim, H.-J. Supramolecular Assembly of Tin(IV) Porphyrin Cations Stabilized by Ionic Hydrogen-Bonding Interactions. Bull. Korean Chem. Soc. 2015, 36, 2348–2351. [Google Scholar] [CrossRef]
- Amati, A.; Cavigli, P.; Demitri, N.; Natali, M.; Indelli, M.T.; Iengo, E. Sn(IV) Multiporphyrin Arrays as Tunable Photoactive Systems. Inorg. Chem. 2019, 58, 4399–4411. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Lee, C.-J.; Kim, H.-J. Hexacoordinated Sn(IV) porphyrin-based square-grid frameworks exhibiting selective uptake of CO2 over N2. Bull. Korean Chem. Soc. 2022, 43, 103–109. [Google Scholar] [CrossRef]
- Kim, H.-J. Assembly of Sn(IV)-Porphyrin Cation Exhibiting Supramolecular Interactions of Anion···Anion and Anion···π Systems. Molbank 2022, 2022, M1454. [Google Scholar] [CrossRef]
- Hawley, J.C.; Bampos, N.; Abraham, R.J.; Sanders, J.K.M. Carboxylate and carboxylic acid recognition by tin (IV) porphyrins. Chem. Commun. 1998, 6, 661–662. [Google Scholar] [CrossRef]
- Chen, Q.-C.; Xiao, Z.-Y.; Fite, S.; Mizrahi, A.; Fridman, N.; Zhan, X.; Keisar, O.; Cohen, Y.; Gross, Z. Tuning Chemical and Physical Properties of Phosphorus Corroles for Advanced Applications. Chem. Eur. J. 2019, 25, 11383–11388. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, H.-J. Bis(methanesulfonato-κ O)(5,10,15,20-tetraphenylporphyrinato-κ4N,N’,N”,N’”)tin(IV) chloroform trisolvate. Acta Cryst. 2012, E68, m626. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Bruker. SHELXTL (Ver. 6.10): Program for Solution and Refinement of Crystal Structures; Bruker AXS Inc.: Madison, WI, USA, 2000. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, H.-J. Supramolecular Self-Assembly of the Zwitterionic Sn(IV)-Porphyrin Complex. Molbank 2023, 2023, M1723. https://doi.org/10.3390/M1723
Shee NK, Kim H-J. Supramolecular Self-Assembly of the Zwitterionic Sn(IV)-Porphyrin Complex. Molbank. 2023; 2023(3):M1723. https://doi.org/10.3390/M1723
Chicago/Turabian StyleShee, Nirmal Kumar, and Hee-Joon Kim. 2023. "Supramolecular Self-Assembly of the Zwitterionic Sn(IV)-Porphyrin Complex" Molbank 2023, no. 3: M1723. https://doi.org/10.3390/M1723
APA StyleShee, N. K., & Kim, H.-J. (2023). Supramolecular Self-Assembly of the Zwitterionic Sn(IV)-Porphyrin Complex. Molbank, 2023(3), M1723. https://doi.org/10.3390/M1723





