Abstract
A new heteroleptic Cu(I) complex, [Cu(L)(PPh3)2][BF4] (L = 6-(furan-2-yl)-2,2′-bipyridine; PPh3 = triphenylphosphine), was successfully synthesized and characterized. Its molecular structure was determined using X-ray crystallography, and NMR as well as HR-ESI-MS data confirm the compound’s integrity in solution. The complex exhibits emission solely in the solid state (λem = 576 nm) and demonstrates a photoluminescence quantum yield of 2.5%.
1. Introduction
Luminescent copper(I) heteroleptic Cu(N^N)(P^P)-type complexes (N^N = chelating diimines, P^P = phosphines) hold promise as phosphorescent active materials for organic, light-emitting devices (OLEDs), light-emitting electrochemical cells (LECs), and other applications [1,2,3,4], providing attractive alternatives to the well-known heavy metal counterparts.
To achieve optimized photophysical properties, a careful selection of both N^N ligands (which should be sterically demanding, rigid, and highly conjugated) and P^P ligands (preferably bidentate, with a large bite angle) is necessary [5,6,7,8,9]. These characteristics will also influence the relative thermodynamic stability of the various species formed through ligand exchange (i.e., homoleptic vs. heteroleptic), thereby determining if the preferred heteroleptic complex will predominate in solution [10].
Most of the reported complexes incorporate an aromatic diimine core (e.g., 2,2′-bipyridine, 1,10-phenanthroline, etc.) modified at various positions, along with a diphosphine ligand. Only 10% of these contain triphenylphosphine [7]. In the latter case, the main drawbacks include the absence of emission properties in solution at room temperature and lower thermodynamic stability due to ligand exchange. Nevertheless, there are a few examples where these compounds, in the solid state (either neat or doped in a polymer matrix), exhibit intriguing photophysical properties [7].
We recently published [11] a study on the synthesis, characterization, and photophysical properties of a Cu(I) heteroleptic complex utilizing a 2-2′-bipyridine derivative substituted at position 6 with a thiophenyl group (i.e., 6-(thiophen-2-yl)-2,2′-bipyridine) and triphenylphosphine. The compound was stable in solution, although non-emissive, and exhibited orange photoluminescence (λem = 600 nm) in the solid state. Given this background, we deemed it worthwhile to synthesize and investigate a similar complex containing the N^N type ligand 6-(furan-2-yl)-2,2′-bipyridine. By doing so, we could also elucidate the impact of changing the heteroatom (from S to O) on the compound’s photophysical properties.
2. Results and Discussion
2.1. Characterization with NMR Spectroscopy
The 1H-NMR spectrum (Figure 1) of complex 1 ([CuL(PPh3)2]BF4) was recorded in CDCl3. A single set of sharp signals indicate the integrity of the compound in the solution. The 1H chemical shifts for both the ligands and the complex are reported in Table 1. Among these, the one assigned to H6′ experienced the greatest impact from complexation, featuring a 1.10 ppm upfield shift. Notably, a similar trend was also observed for the furan protons, suggesting the presence of intramolecular stacking, potentially involving the furan moiety and the phenyl rings of the phosphine ligands.
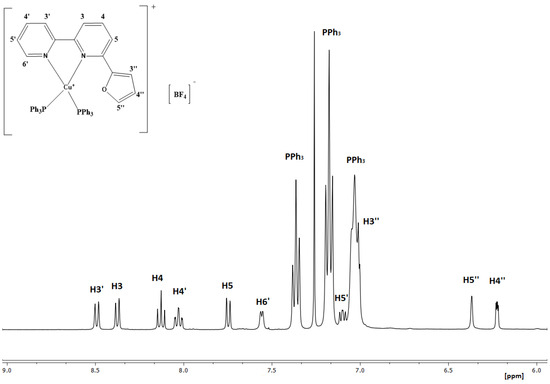
Figure 1.
1H NMR spectrum of 1 in CDCl3 (500 MHz, 298 K).

Table 1.
1H NMR data (δ, ppm) for L and complex 1.
2.2. Absorption Spectrum
Figure 2 displays the diffuse reflectance spectrum (DRS) of [CuL(PPh3)2]BF4 (1). The broad spectral features spanning from 250 to 480 nm are characteristic of Cu(N^N)(P^P)-type complexes. In particular, electronic transitions corresponding to ligand-centered (LC) π→π* and n→π* transitions are observed within the 230–350 nm range, while higher energy bands (>350 nm) can be attributed to MLCT transitions [12,13,14].
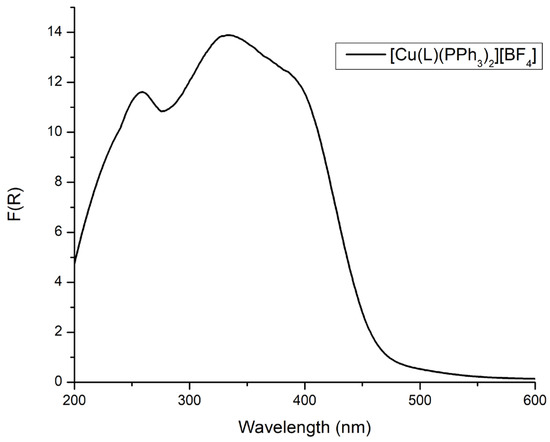
Figure 2.
The Kubelka–Munk spectrum of the solid [CuL(PPh3)2][BF4].
2.3. IR Spectroscopy
In the ATR-IR spectrum of 1, (Figure 3) the presence of both ligands and the counter ion is evident. The most distinctive bands were identified at 1594, 1053, and 516 cm−1, corresponding to C=N, B-F, and C-P stretching vibrations, respectively [12].
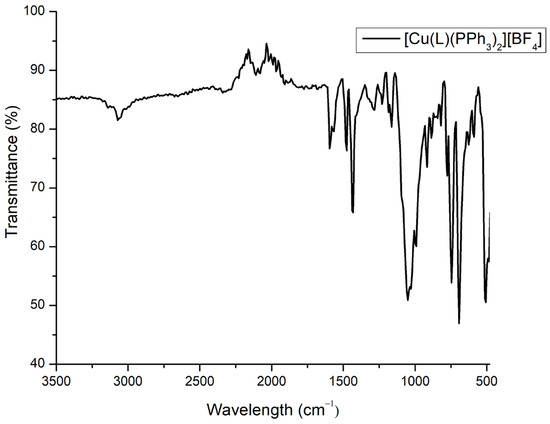
Figure 3.
The ATR-IR spectrum of the complex 1.
2.4. Emission Spectrum-Quantum Yield Calculation
Complex 1 is non-emissive in solution. Nevertheless, upon excitation of the solid at 400 nm at room temperature, a broad, non-structured emission band, typically found in compounds of this nature [9,15,16], centered at λem = 576 nm emerges (Figure 4). The calculated absolute photoluminescence quantum yield ΦPL is relatively low, at approximately 2.5%. The large Stoke’s shift (56,818 cm−1) implies a considerable energy loss in the MLCT-excited state, likely resulting from structural relaxation and consequently promoting radiationless deactivation [12,13,14]. The photophysical characteristics of the parent compound and [CuL′(PPh3)2]BF4 (where L′ = 6-(thiophen-2-yl)-2,2′-bipyridine) [11] previously reported by our group are nearly identical in terms of the quantum yield. However, there is a slight difference in the position of the emission maximum, with a 24 nm blue shift.
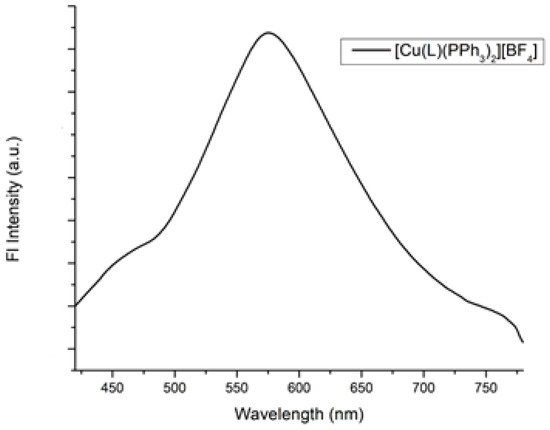
Figure 4.
The emission spectrum of the solid 1.
2.5. Description of the Structure
Table 2 presents selected bond distances (Å) and angles (°) for the coordination sphere of Cu(I) in the cation, while Figure 5 depicts the structure. The Cu(1) center of the mononuclear complex 1 sits in a distorted tetrahedron constructed by the two nitrogen atoms [N(1), N(2)] from the diimine ligand L (6-(furan-2-yl)-2,2′-bipyridine) and two phosphorus atoms [P(1), P(2)] that belong to two coordinated PPh3 molecules. The Cu-P and Cu-N bond distances agree well with literature values, falling within the expected range for comparable complexes (Cu-N: 2.06–2.14 Å, Cu-P: 2.24–2.28 Å) [9,11,15,16,17,18,19]. Nevertheless, it is worth noting that the Cu(1)-N(2) bond is noticeably elongated (2.21 Å) due to significant steric hindrance from the two coordinated PPh3 molecules and the distortion of the bipyridine ligand away from planarity. The two rings of the heterocyclic ligand are non-coplanar, forming a dihedral angle of 18.4° [N(1)-C(5)-C(6)-N(2)], while the pyridyl-N(2)-furan rings are nearly coplanar, with a torsion angle of 5.3° [N(2)-C(10)-C(11)-O(1)].

Table 2.
Selected structural characteristics of compound 1.
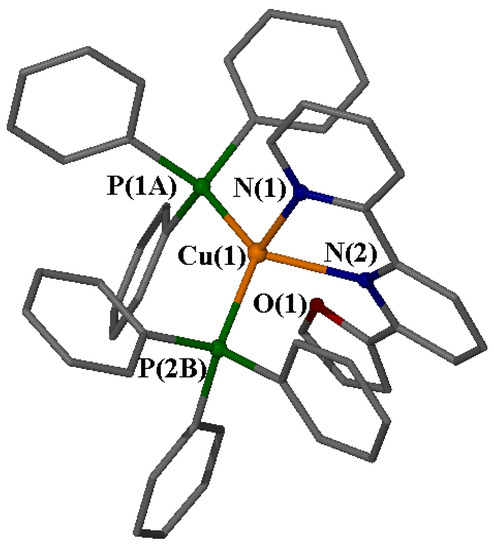
Figure 5.
A “ball-and-stick” presentation of the cation in compound 1 with a partial labelling scheme. Hydrogen atoms have been omitted for clarity.
Several noteworthy non-covalent intramolecular interactions exist. The calculated Cu(1)···O(1) distance of 3.15 Å indicates a weak interaction between the two atoms, which significantly differs from the corresponding value in the homoleptic complex (4.73 Å) [20]. The dihedral angle defined by [O(1)-C(14)-P(1A)-C(1A)] is 87.2°, suggesting that these two rings are almost vertical. Consequently, the H atom of the furan ring is located at a distance of 2.71 Å from the phenyl ring centroid, potentially indicating a T-shaped stacking interaction. Strong intramolecular π ··· π interactions (approximately 3.8 Å) exist between the N(2) ring and a phenyl ring of P(2B). The crystal holds BF4− through strong intermolecular interactions, including non-conventional C-H···F hydrogen bonds, which likely contribute to anion disordering. These interactions are illustrated in Figure 6. Figure 7 presents a packing diagram along the α axis.
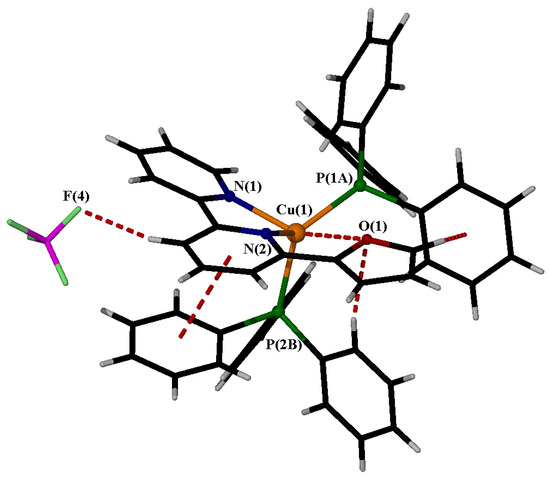
Figure 6.
Intramolecular and intermolecular interactions in the cation of 1 (red dotted lines).
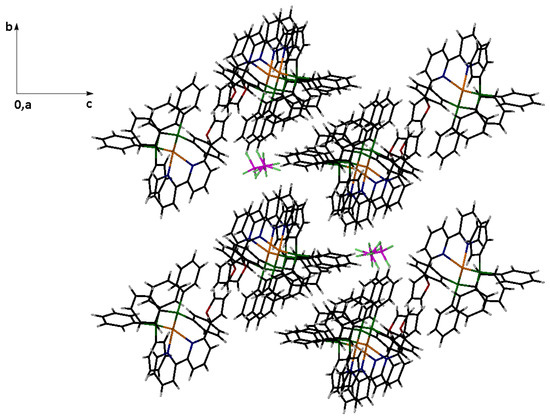
Figure 7.
A packing diagram of 1 down to the α axis of the unit cell. All lattice constituents are shown.
3. Materials and Methods
3.1. Materials
All chemicals (Cu(CH3CN)4]BF4, PPh3, NH4OAc, 2-acetylfuran, 2-acetylpyridine, Me2NH.HCl, I2 and paraformaldehyde) and solvents used were purchased from commercial sources and required no further purification. The ligand L, 6-(furan-2-yl)-2,2-bipyridine was synthesized following a well-documented procedure [20].
3.2. Methods
An elemental analysis for carbon (C), hydrogen (H), and nitrogen (N) was conducted with a PerkinElmer 2400 Series II analyzer. A Thermo Scientific LTQ Orbitrap XL™ system was used to acquire the high-resolution electrospray ionization mass spectrum (HR-ESI-MS) of the compound (Figure S1). Proton nuclear magnetic resonance (1H NMR), including 1H-1H-COSY (Figure S2) and 1H-1H NOESY NMR spectra (Figure S3), was recorded on a Bruker Avance spectrometer operating at 400.13 MHz (1H) and processed using Topspin 4.07 software (Bruker Biospin GmbH, Ettlingen, Germany). The UV-Vis spectrum (Figure S4) was recorded using a dual-beam Shimadzu UVPC 2401 spectrometer. The IR spectrum was collected using an Agilent Cary 630 ATR-IR spectrometer. The diffuse reflectance–absorbance spectrum (DRS–absorbance) was measured on an Agilent Cary 60 UV–Vis spectrophotometer utilizing a xenon source lamp and an external reflectance probe (Barrelino™, Harrick Scientific Products, Inc., New York, NY, USA). An emission study was performed on a Jasco FP-8300 fluorimeter with a xenon lamp source and an integrated sphere for solid samples. The equation Q = S2/S0 − S1 was used to calculate the photoluminescence absolute quantum yield of the complex in the solid state, where S2 represents the integrated emission intensity of the sample, while S0 and S1 denote the integrated excitation intensities of the standard and the sample, respectively.
3.3. Crystal Structure Determination
The diffraction data of a suitable yellow polyhedral-shaped crystal of compound 1 (0.60 × 0.30 × 0.20 mm3) were collected using a Bruker D8 Quest Eco diffractometer (Ettlingen, Germany) outfitted with a Photon II detector (Ettlingen, Germany) and a TRIUMPH (curved graphite) monochromator that employed Mo Ka radiation (λ = 0.71073 Å) [21]. A wide-frame algorithm was applied to integrate the collected frames (comprising φ and ω scans) utilizing Bruker SAINT software. The multi-scan method (SADABS) [22] was applied to correct the data for absorption effects. The P-1 space group was assigned, and the structure was solved employing the Bruker SHELXT Software Package. Subsequent refinement was achieved through full-matrix least-squares techniques on F2 (SHELXL 2018/3) [23], facilitated by the ShelXle interface [24]. Anisotropic treatment was methodically applied to non-H atoms, while organic H atoms were positioned in calculated ideal locations and subsequently refined as riding on their corresponding carbon atoms. Geometric calculations were executed utilizing PLATON [25], and molecular graphics were generated utilizing X-Seed [26]. A few residual electron densities, which were ascribed to solvent molecules, proved difficult to model and were consequently addressed by implementing the SQUEEZE routine within PLATON. The counter ion (BF4−) was treated as disordered in two positions (partial occupancies of 52% and 48%) utilizing the DSR program [27].
Crystal data for C50H40CuBF4N2OP2 (M = 897.13 g/mol) are summarized as follows: triclinic, space group P-1 (no. 2), a = 10.8869(3) Å, b = 14.6179(4) Å, c = 15.1433(5) Å, α = 95.3500(2)°, β = 94.526(2)°, γ = 90.832(2)°, V = 2391.40(12) Å3, Z = 2, T = 296(2) K, µ(MoKα) = 0.576 mm−1, Dcalc = 1.246 g/cm3, 80,512 reflections measured (2.694° ≤ θ ≤ 24.998°), 8393 unique (Rint = 0.0473) which were used in all calculations. The final R1 was 0.0350 (I > 2σ(I)) and wR2 was 0.0873 (all data). (Table S1)
Full details on the structure can be found in the CIF file deposited with the CCDC. CCDC2281603 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/(accessed on 14 July 2023).
3.4. Synthesis
Synthesis of the Complex [Cu(L)(PPh3)2][BF4] (1)
A mixture of [Cu(CH3CN)4]BF4 (0.05 mmol) and 26.3 mg of PPh3 (0.1 mmol) in 5 mL of Ar-degassed dichloromethane were stirred for an hour, followed by the addition of 11.2 mg of L (0.05 mmol). The initially colorless solution turned yellow-orange immediately, and was stirred for an additional 1 h. Subsequently, the solvent was removed under reduced pressure until it reached a volume of approximately 1 mL, followed by the addition of diethyl ether. This led to the precipitation of a yellow-colored solid, which was promptly collected and subjected to vacuum-drying. Yield: 89.4% (calculated using the mass (m = 40.12 mg) of the precipitate). Suitable crystals of [CuL(PPh3)2]BF4 were obtained within a week by layering an acetone solution of the complex with heptane. It should also be mentioned that we have successfully synthesized the compound on a scale of up to 0.1 mmol. As the complex’s synthetic procedure is relatively simple, we believe that it is possible to further increase the scale. C50H40N2BOF4P2Cu: calc.% C, 66.94; H, 4.49; N, 3.12. Found C, 66.89; H, 4.44; N, 3.08. 1H NMR (400 MHz, CDCl3) (ppm) L: 8.51 (d, J = 8.2 Hz, 1H); 8.40 (d, J = 7.8 Hz, 1H); 8.15 (t, J = 8 Hz, 1H); 8.05 (td, J = 8 Hz, 1.5 Hz, 1H); 7.77 (d, J = 7.8 Hz, 1H); 7.58 (d, J = 4.6 Hz, 1H); 7.12 (m, 1H); 7.05 (m, 1H); 6.39 (d, J = 7.9 Hz, 1H); 6.25 (dd, J = 3.4 Hz, 1.7 Hz, 1H). PPh3: 7.39, 7.20, 7.05 (30H). ATR-IR (cm−1) 3062w (=C-H aromatic), 1594m (C=N bipyridyl), 1567m (C=C aromatic), 1053s (B-F BF4) and 516m (=C-P, PPh3). HR ESI MS: cal. m/z = 547.0974, found m/z = 547.0995 for C32H25CuN2PO [Cu(L)(PPh3)]+. UV-Vis (chloroform): λmax (nm), ε (M−1 cm−1) 242 (41223); 252 (40795); 266 (39630); 333 (13200) (Figure S4).
4. Conclusions
A novel yellow emitter (λem = 576 nm) formulated as [CuL(PPh3)2]BF4 (L = 6-(furan-2-yl)-2,2′-bipyridine) was successfully prepared and structurally characterized (NMR, X-ray diffraction). The compound is stable in both the solid state and in solution, exhibiting a photoluminescent quantum yield of 2.5% in the solid state. Comparison of the photophysical properties of the studied compound and [CuL′(PPh3)2]BF4 (with L′ denoting 6-(thiophen-2-yl)-2,2′-bipyridine) [11] revealed that both display nearly identical quantum yields. However, a minor discrepancy arises in the emission peak position, resulting in a 24 nm blueshift. We are currently engaged in the synthesis and characterization of similar compounds to fully elucidate the impact of the five-membered ring heteroatom change on their photophysical behavior.
Supplementary Materials
The following supporting information can be downloaded online: Figure S1: HR-ESI-MS spectrum of the complex in acetone (top) and theoretical spectrum for the fragment C32H25CuN2PO [Cu(L)(PPh3)]+; Figure S2: 1H-1H-COSY NMR spectrum of the complex; Figure S3: 1H-1H-NOESY NMR spectrum of the complex; Figure S4: UV-Vis spectrum of the complex in chloroform; Table S1: Crystal data and structure refinement for C50 H40BCuF4N2OP2 at 296(2) K; File S1: crystal structure of the compound (*.mol); File S2: crystal structure of the compound (*.cif) file; File S3 (checkcif, pdf file).
Author Contributions
Conceptualization: G.M.; supervision: G.M.; formal analysis: P.K., D.G. and J.C.P.; investigation: P.K. and D.G.; X-ray crystallography: J.C.P. and D.G.; writing—original draft: D.G. and J.C.P.; writing—review and editing: G.M. and J.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CCDC 2281603 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/ (accessed on 14 July 2023). All other data in this study can be found in the Supplementary Materials.
Acknowledgments
The authors would like to thank The Network of Research Supporting Laboratories at the University of Ioannina for providing access to MS, NMR and X-ray diffraction facilities.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Zhang, Q.; Ding, J.; Cheng, Y.; Wang, L.; Xie, Z.; Jing, X.; Wang, F. Novel Heteroleptic CuI Complexes with Tunable Emission Color for Efficient Phosphorescent Light-Emitting Diodes. Adv. Funct. Mater. 2007, 17, 2983–2990. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Cheng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly Efficient Electroluminescence from Green-Light-Emitting Electrochemical Cells Based on CuI Complexes. Adv. Funct. Mater. 2006, 16, 1203–1208. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Holler, M.; Moudam, O.; Nierengarten, J.-F.; Zhou, Z.; Wegh, R.T.; Welter, R. Highly Luminescent CuI Complexes for Light-Emitting Electrochemical Cells. Adv. Mater. 2006, 18, 1313–1316. [Google Scholar] [CrossRef]
- Min, J.; Zhang, Q.; Sun, W.; Cheng, Y.; Wang, L. Neutral Copper(I) Phosphorescent Complexes from Their Ionic Counterparts with 2-(2′-Quinolyl)Benzimidazole and Phosphine Mixed Ligands. Dalton Trans. 2011, 40, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Li, G.F.; Zhang, X.Y.; Li, R.F.; Liu, X.F. Synthesis and Properties of Two New Cu(I) Complexes Based on 5,6-Substituted Imidazole-2,9-Dimethyl-1,10-Phenanthroline and Triphenylphosphine. Russ. J. Gen. Chem. 2016, 86, 387–390. [Google Scholar] [CrossRef]
- Leoni, E.; Mohanraj, J.; Holler, M.; Mohankumar, M.; Nierengarten, I.; Monti, F.; Sournia-Saquet, A.; Delavaux-Nicot, B.; Nierengarten, J.-F.; Armaroli, N. Heteroleptic Copper(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands: Rationalization of the Photophysical and Electrochemical Properties. Inorg. Chem. 2018, 57, 15537–15549. [Google Scholar] [CrossRef]
- Beaudelot, J.; Oger, S.; Peruško, S.; Phan, T.-A.; Teunens, T.; Moucheron, C.; Evano, G. Photoactive Copper Complexes: Properties and Applications. Chem. Rev. 2022, 122, 16365–16609. [Google Scholar] [CrossRef]
- Steen, R.O.; Nurkkala, L.J.; Angus-Dunne, S.J.; Schmitt, C.X.; Constable, E.C.; Riley, M.J.; Bernhardt, P.V.; Dunne, S.J. The Role of Isomeric Effects on the Luminescence Lifetimes and Electrochemistry of Oligothienyl-Bridged Dinuclear Tris(2,2′-bipyridine)Ruthenium(II) Complexes. Eur. J. Inorg. Chem. 2008, 2008, 1784–1794. [Google Scholar] [CrossRef]
- Safin, D.A.; Mitoraj, M.P.; Robeyns, K.; Filinchuk, Y.; Velde, C.M.L.V. Luminescent Mononuclear Mixed Ligand Complexes of Copper(I) with 5-Phenyl-2,2′-Bipyridine and Triphenylphosphine. Dalton Trans. 2015, 44, 16824–16832. [Google Scholar] [CrossRef]
- Kaeser, A.; Mohankumar, M.; Mohanraj, J.; Monti, F.; Holler, M.; Cid, J.-J.; Moudam, O.; Nierengarten, I.; Karmazin-Brelot, L.; Duhayon, C.; et al. Heteroleptic Copper(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands. Inorg. Chem. 2013, 52, 12140–12151. [Google Scholar] [CrossRef]
- Kouvatsis, P.; Glykos, D.; Plakatouras, J.C.; Malandrinos, G. [6-(Thiophen-2-Yl)-2,2′-Bipyridine]Bis(Triphenylphosphine) Copper(I) Tetrafluoroborate. Molbank 2023, 2023, M1605. [Google Scholar] [CrossRef]
- Shi, L.; Li, B.; Lu, S.; Zhu, D.; Li, W. Synthesis, Characterization and Oxygen-Sensing Properties of a Novel Luminescent Cu(I) Complex. Appl. Organomet. Chem. 2009, 23, 379–384. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Liu, W.; Qin, W. Study on the Synthesis, Characterization, Photophysical Performance and Oxygen-Sensing Behavior of a Luminescent Cu(I) Complex with Large Conjugation Plane. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 104, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zuo, Q. A Series of Blue-Green-Yellow-Red Emitting Cu(I) Complexes: Molecular Structure and Photophysical Performance. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117280. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Cao, X.-F.; Gu, W.; Su, B.-T.; Zhang, F.; Wen, H.-R.; Hong, R. Luminescent Mononuclear Copper(I) Heteroleptic Complexes with 6-Cyano-2,2′-Bipyridine. Inorg. Chem. Commun. 2012, 15, 65–68. [Google Scholar] [CrossRef]
- Chen, J.-L.; Fu, X.-F.; Wang, J.-Y.; Guo, Z.-H.; Xiao, Y.-L.; He, L.-H.; Wen, H.-R. A Series of New Emissive Mononuclear Copper(I) Bipyridyl Complexes Bearing the Methoxycarbonyl Groups. Inorg. Chem. Commun. 2015, 53, 88–91. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Huang, J.-S.; Zhong, M.-H. [6-(4-Bromophenyl)-2,2′-Bipyridine-κ 2 N,N′]Bis(Triphenylphosphane-κ P)Copper(I) Tetrafluoridoborate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, m1187. [Google Scholar] [CrossRef]
- Polson, M.I.J.; Hanan, G.S.; Taylor, N.J. [4′-(2-Bromo-5-Pyridyl)-2,2′:6′,2″-Terpyridine-κ 3 N,N′,N″]Bis(Triphenylphosphine-κ P)Copper(I) Tetrafluoridoborate Dichloromethane Hemisolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, m205. [Google Scholar] [CrossRef]
- Paderina, A.; Ramazanov, R.; Valiev, R.; Müller, C.; Grachova, E. So Close, Yet so Different: How One Donor Atom Changes Significantly the Photophysical Properties of Mononuclear Cu(I) Complexes. Inorg. Chem. 2022, 61, 11629–11638. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E.; Kopecky, P.; Neuburger, M.; Zampese, J.A. The Intramolecular Aryl Embrace: From Light Emission to Light Absorption. Dalton Trans. 2011, 40, 12584. [Google Scholar] [CrossRef]
- Bruker. APEX 3. In SAINT, SHELXT; Bruker AXS Inc.: Fitchburg, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.J. X-Seed—A Software Tool for Supramolecular Crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
- Kratzert, D.; Krossing, I. Recent Improvements in DSR. J. Appl. Crystallogr. 2018, 51, 928–934. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).