Cocrystal of Codeine and Cyclopentobarbital
Abstract
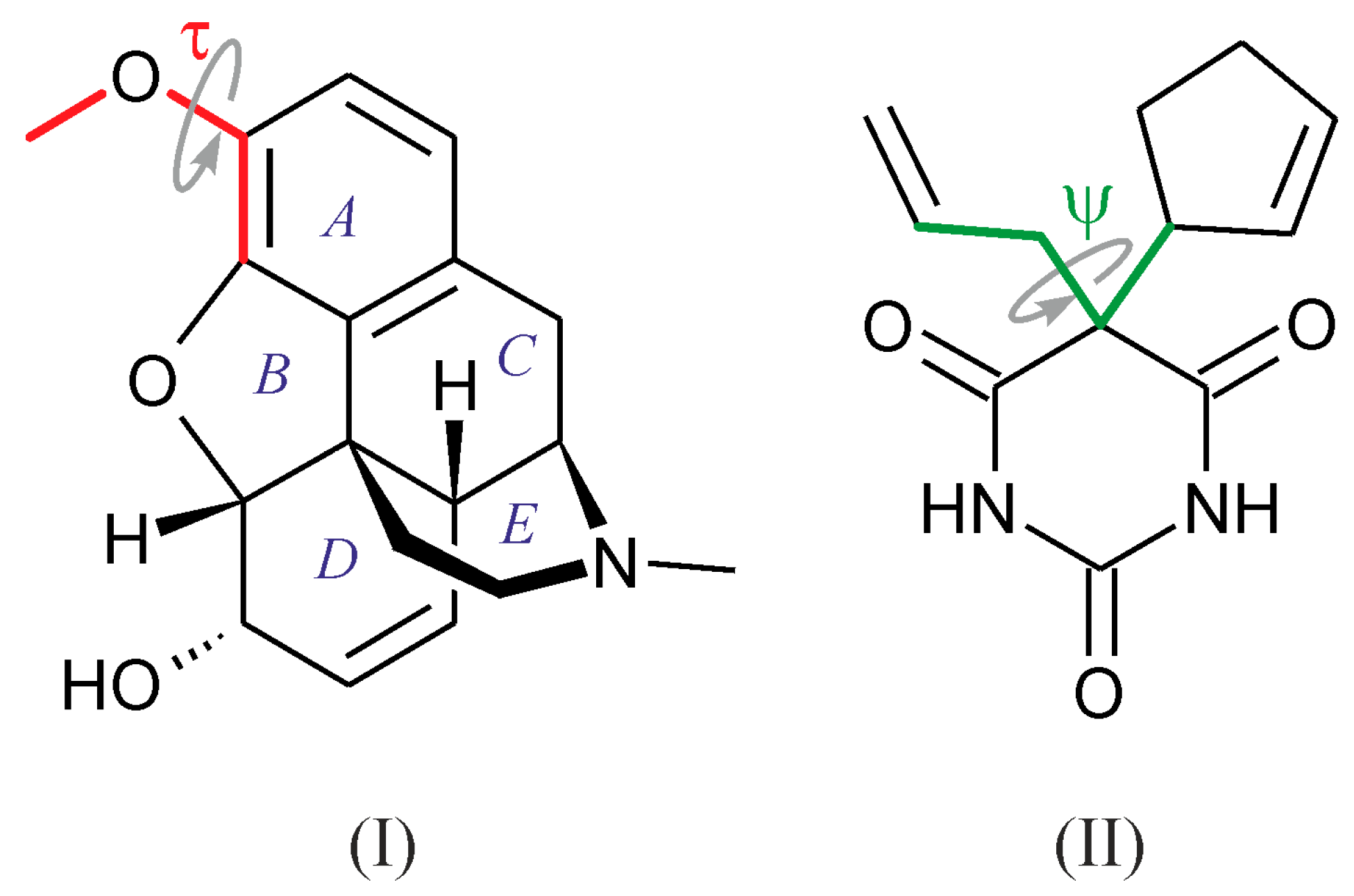
:1. Introduction
2. Results
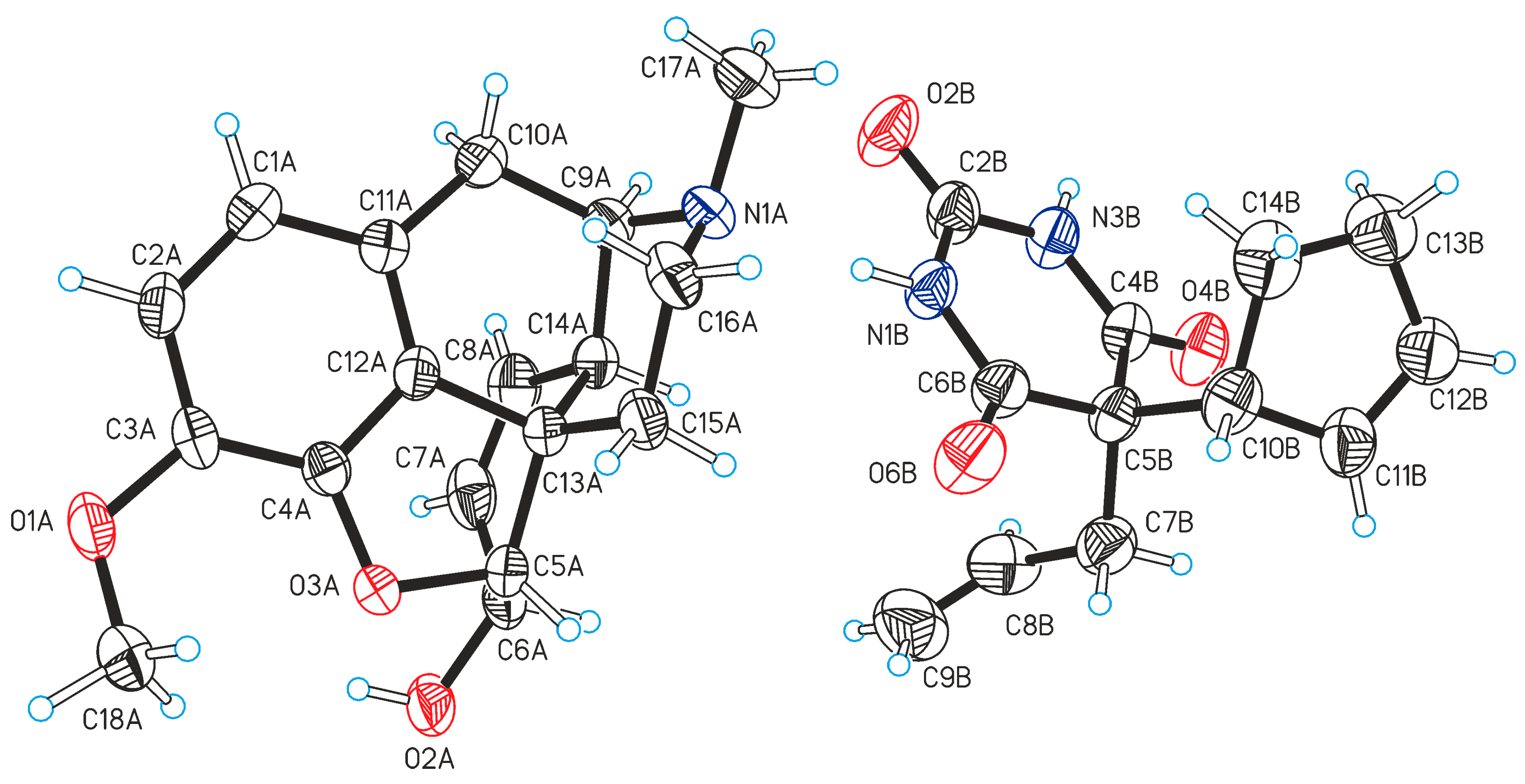
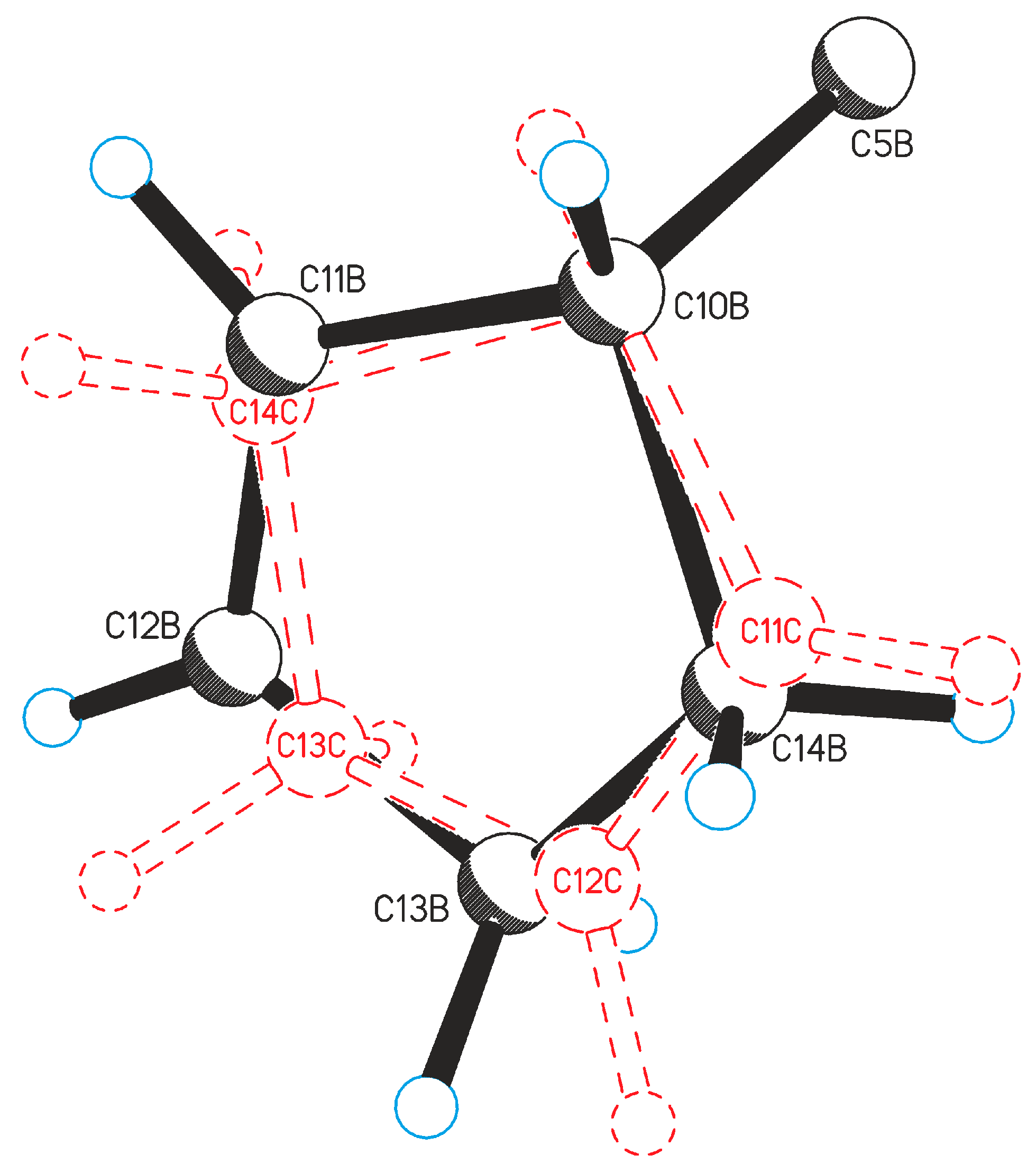
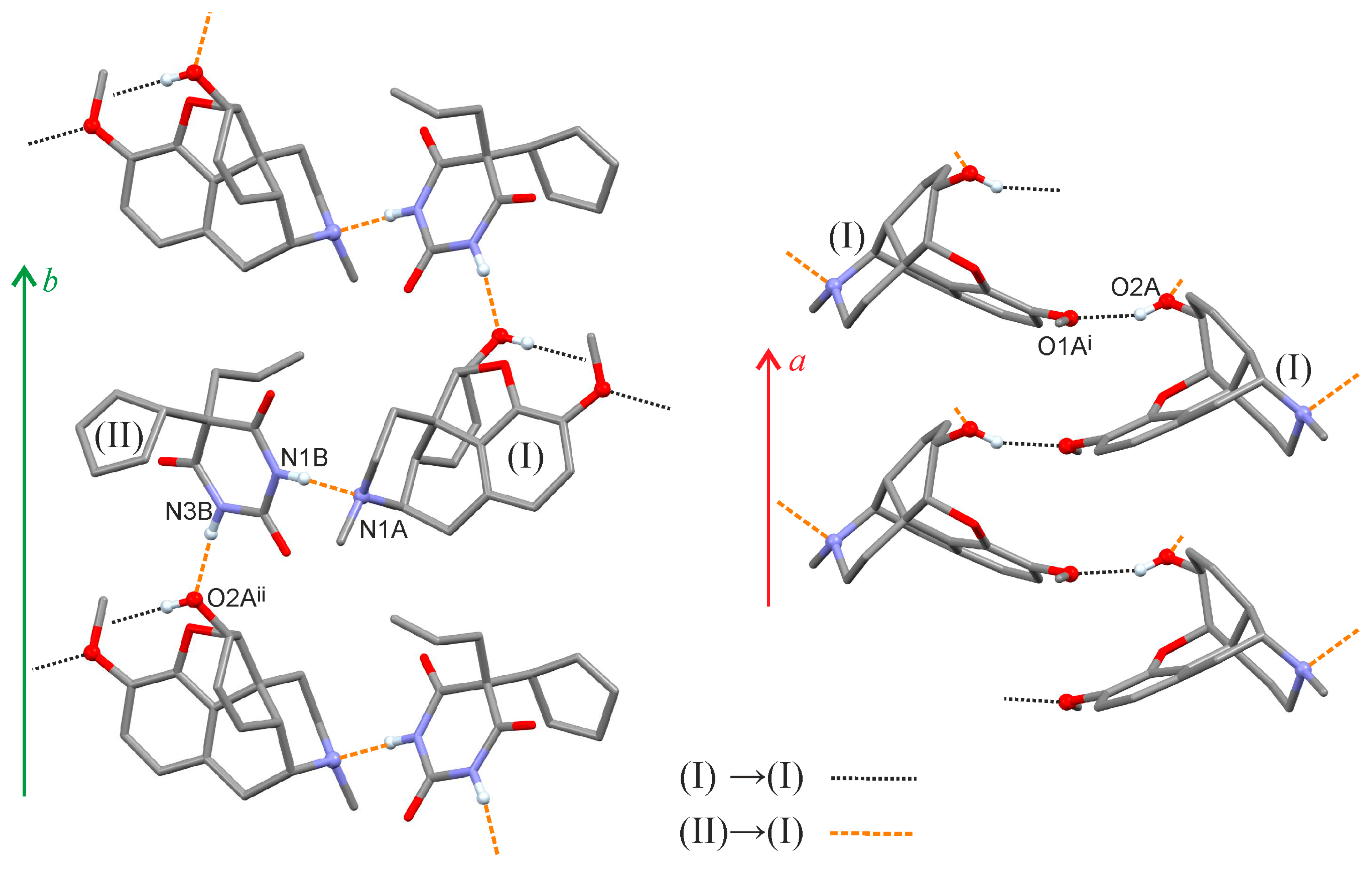
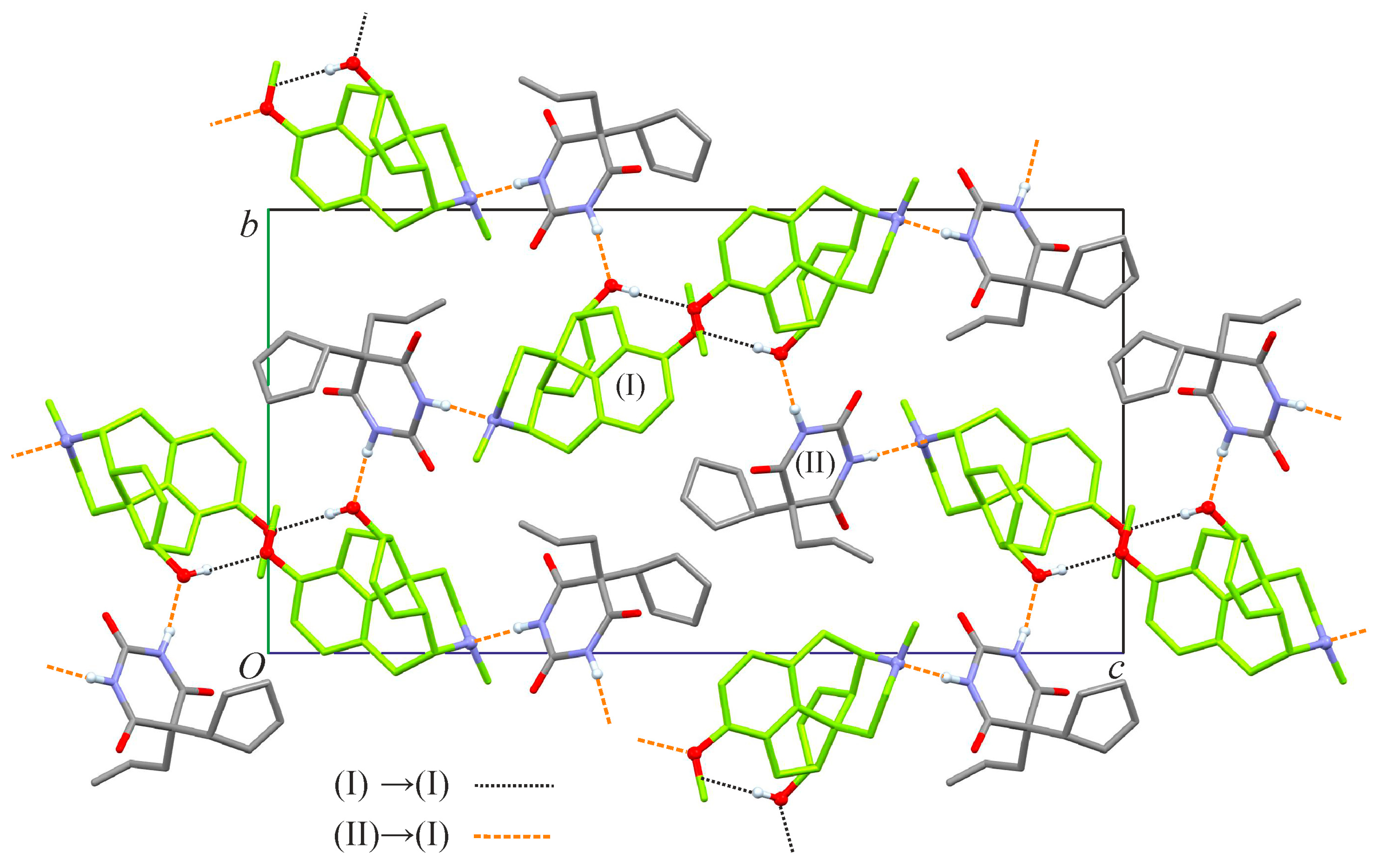
2.1. Crystal Structure
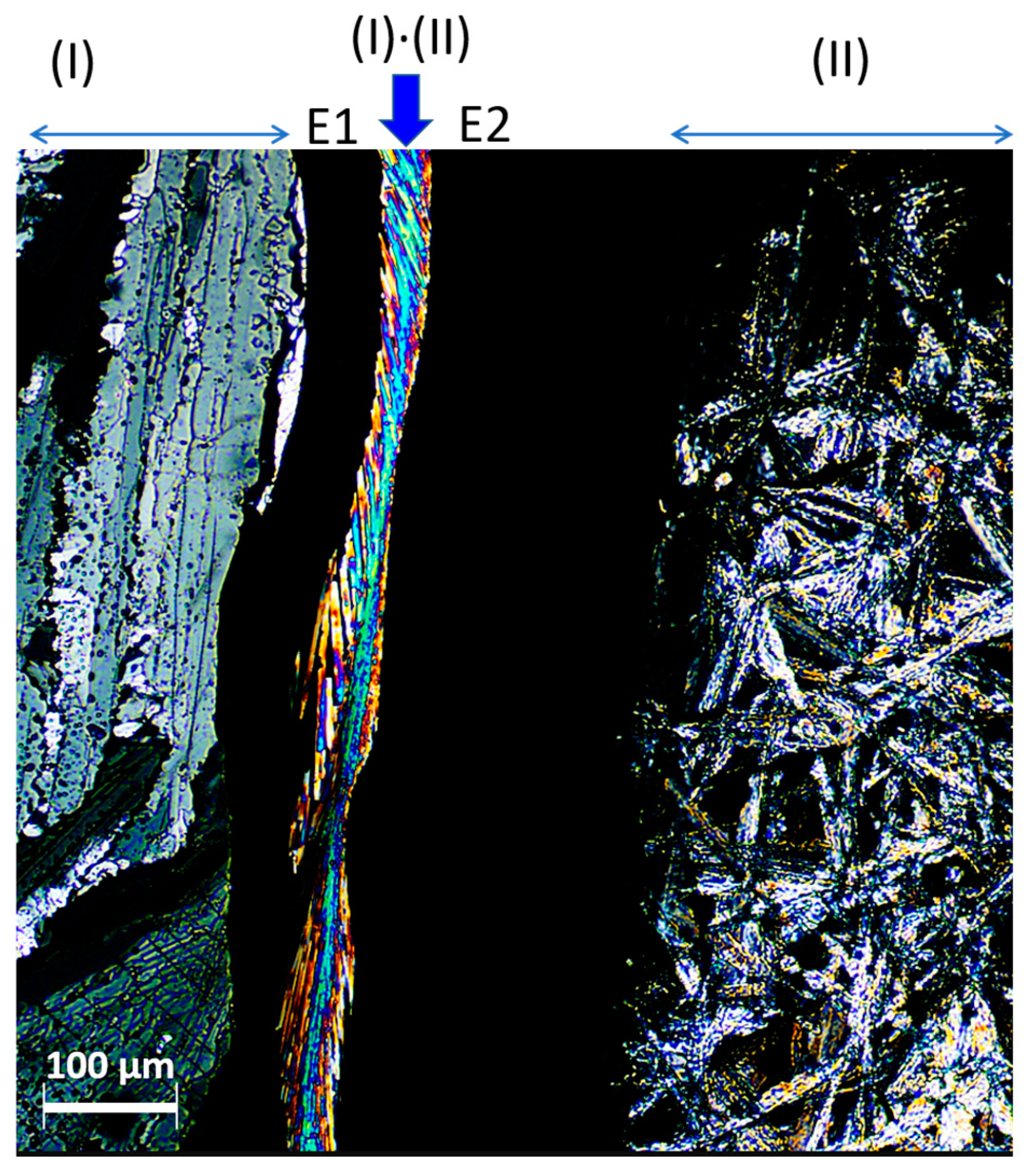
2.2. Hot-Stage Microscopy
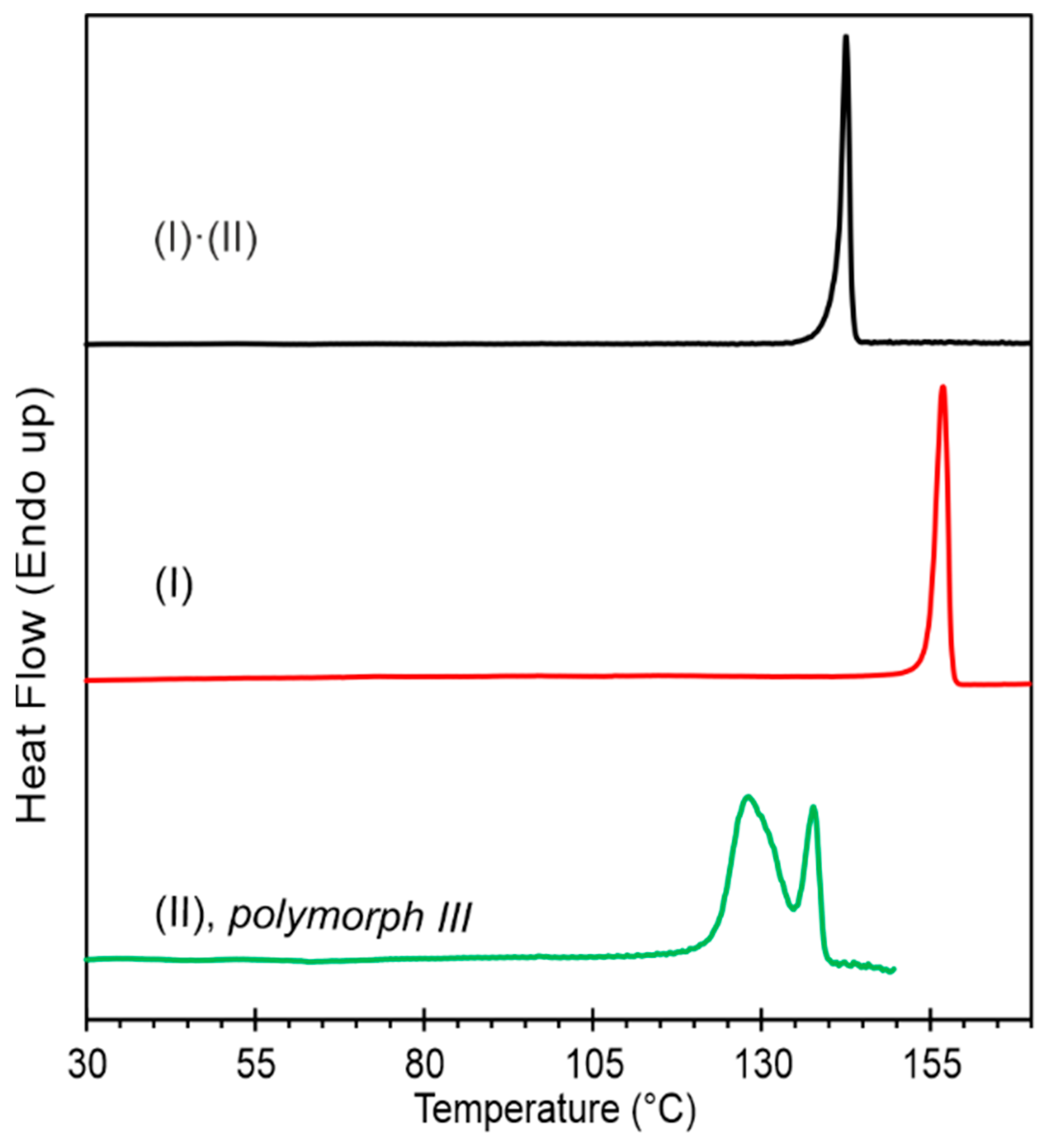
2.3. Differential Scanning Calorimetry (DSC)
3. Materials and Methods
3.1. Preparation of the Cocrystal (I)·(II)
3.2. Single-Crystal Structure Determination
3.3. Hot Stage Microscopy and Contact Preparation Method
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wisniak, J. Pierre-Jean Robiquet. Educ. Quim. 2013, 24, 139–149. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines: 22nd List (2021); World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Eddy, N.B.; Friebel, H.; Hahn, K.-J.; Halbach, H. Codeine and its alternates for pain and cough relief: 1. Codeine, exclusive of its antitussive action. Bull. World Health Organ. 1968, 38, 673–741. [Google Scholar] [CrossRef] [PubMed]
- Eddy, N.B.; Friebel, H.; Hahn, K.-J.; Halbach, H. Codeine and its alternates for pain and cough relief: 3. The antitussive action of codeine—Mechanism, methodology and evaluation. Bull. World Health Organ. 1969, 40, 425–454. [Google Scholar] [PubMed]
- Atkinson, A.J.; Adler, H.F.; Ivy, A.C. Motility of the human colon: The normal pattern, dyskinesia and the effekt of drugs. J. Am. Med. Soc. 1943, 121, 646–652. [Google Scholar] [CrossRef]
- International Narcotics Control Board. List of Narcotic Drugs under International Control: Yellow List; International Narcotics Control Board: Vienna, Austria, 2020. [Google Scholar]
- United States Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry: Regulatory Classification of Pharmaceutical Co-Crystals. 2018 Revision 1. Available online: https://www.fda.gov/files/drugs/published/Regulatory-Classification-of-Pharmaceutical-Co-Crystals.pdf (accessed on 20 July 2023).
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Zhang, C.; Xiong, Y.; Jiao, F.; Wang, M.; Li, H. Redefining the Term of “Cocrystal” and Broadening Its Intention. Cryst. Growth Des. 2019, 19, 1471–1478. [Google Scholar] [CrossRef]
- Centolella, A.P.; Nelson, J.W.; Kolloff, H.G. Barbiturates Containing the Δ2-Cyclopentenyl Group. J. Am. Chem. Soc. 1943, 65, 2091–2092. [Google Scholar] [CrossRef]
- Vander Brook, M.J.; Cartland, G.F. A Pharmacologic Study of 5-Allyl-5-Δ2-cyclopentenyl Barbituric Acid (cyclopal). J. Pharmacol. Exp. Ther. 1944, 80, 119–125. [Google Scholar]
- Langes, C.; Gelbrich, T.; Griesser, U.J.; Kahlenberg, V. Codeine dihydrogen phosphate hemihydrate. Acta Crystallogr. C 2009, 65, o419–o422. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Arzeno, H.B.; Barton, D.H.R.; Davies, S.G.; Lusinchi, X.; Meunier, B.; Pascard, C. Synthesis of 10(S)-methylcodeine and 10(S)-methylmorphine. Nouv. J. Chim. 1980, 4, 369–375. [Google Scholar]
- Kartha, G.; Ahmed, F.R.; Barnes, W.H. Refinement of the crystal structure of codeine hydrobromide dihydrate, and establishment of the absolute configuration of the codeine molecule. Acta Crystallogr. 1962, 15, 326–333. [Google Scholar] [CrossRef]
- Rosenberger, L.; von Essen, C.; Khutia, A.; Kühn, C.; Georgi, K.; Hirsch, A.K.H.; Hartmann, R.W.; Badolo, L. Crystalline sponge affinity screening: A fast tool for soaking condition optimization without the need of X-ray diffraction analysis. Eur. J. Pharm. Sci. 2021, 164, 105884. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.E.; Gelbrich, T.; Kahlenberg, V.; Griesser, U.J. Insights into Hydrate Formation and Stability of Morphinanes from a Combination of Experimental and Computational Approaches. Mol. Pharm. 2014, 11, 3145–3163. [Google Scholar] [CrossRef] [PubMed]
- Runčevski, T.; Petruševski, G.; Makreski, P.; Ugarkovic, S.; Dinnebier, R.E. On the hydrates of codeine phosphate: The remarkable influence of hydrogen bonding on the crystal size. Chem. Commun. 2014, 50, 6970–6972. [Google Scholar] [CrossRef]
- Scheins, S.; Messerschmidt, M.; Morgenroth, W.; Paulmann, C.; Luger, P. Electron Density Analyses of Opioids: A Comparative Study. J. Phys. Chem. A 2007, 111, 5499–5508. [Google Scholar] [CrossRef] [PubMed]
- Brandstätter-Kuhnert, M.; Aepkers, M. Molekülverbindungen, Mischkristallbildung und neue Polymorphiefälle bei Barbituraten. Microchim. Acta 1962, 50, 1041–1054. [Google Scholar] [CrossRef]
- Zencirci, N.; Gelbrich, T.; Kahlenberg, V.; Griesser, U.J. Crystallization of metastable polymorphs of phenobarbital by isomorphic seeding. Cryst. Growth Des. 2009, 9, 3444–3456. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Kofler, A. Mikro-Thermoanalyse organischer Zweistoffsysteme. Naturwissenschaften 1943, 31, 553–557. [Google Scholar] [CrossRef]
- Kofler, L.; Kofler, A. Thermo-Mikro-Methoden zur Kennzeichnung organischer Stoffe und Stoffgemische; Wagner: Innsbruck, Austria, 1954. [Google Scholar]
| Compound | (I)·(II) |
| Moiety formula | C18H21NO3 · C12H14N2O3 |
| Empirical formula | C30H35N3O6 |
| Formula weight | 533.61 |
| Temperature (K) | 173(2) |
| Wavelength (Å) | 1.5418 |
| Crystal system | Orthorhombic |
| Space group | P212121 |
| a (Å) | 6.9914(3) |
| b (Å) | 14.1455(7) |
| c (Å) | 27.1767(11) |
| Unit cell volume (Å3) | 2687.7(2) |
| Z/Z’ | 4/1 |
| Reflections collected/Rint | 9191/0.0447 |
| Data/restraints/parameters | 4466/133/404 |
| Goodness-of-fit on F2 | 1.027 |
| R1 [I > 2 σ(I)] | 0.0500 |
| wR2 (all data) | 0.1289 |
| Largest diff. peak and hole (e · Å−3) | 0.197 and −0.189 |
| CCDC no. | 2278182 |
| D—H⋯A | dD—H | dH⋯A | dD⋯A | <(DHA) |
|---|---|---|---|---|
| O2A—H2A⋯O1Ai | 0.838(14) | 1.99(3) | 2.784(4) | 157(6) |
| N1B—H1B⋯N1A | 0.886(13) | 1.974(15) | 2.859(5) | 176(4) |
| N3B—H3B⋯O2Aii | 0.881(13) | 1.945(18) | 2.811(5) | 167(5) |
| Symmetry codes: (i) x − 1/2, −y + 1/2, −z; (ii) −x, y − 1/2, −z + 1/2. | ||||
| Parameter | Value a |
|---|---|
| Tfus (°C) b | |
| DSC (onset) | 141.1 ± 0.2 |
| HSM (melting equilibrium) | 142 |
| ΔfusH (kJ mol−1) c | 47.6 ± 0.1 |
| ΔfusS (J mol−1 K−1) d | 115.0 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelbrich, T.; Schinke, J.; Griesser, U.J. Cocrystal of Codeine and Cyclopentobarbital. Molbank 2023, 2023, M1722. https://doi.org/10.3390/M1722
Gelbrich T, Schinke J, Griesser UJ. Cocrystal of Codeine and Cyclopentobarbital. Molbank. 2023; 2023(3):M1722. https://doi.org/10.3390/M1722
Chicago/Turabian StyleGelbrich, Thomas, Jascha Schinke, and Ulrich J. Griesser. 2023. "Cocrystal of Codeine and Cyclopentobarbital" Molbank 2023, no. 3: M1722. https://doi.org/10.3390/M1722
APA StyleGelbrich, T., Schinke, J., & Griesser, U. J. (2023). Cocrystal of Codeine and Cyclopentobarbital. Molbank, 2023(3), M1722. https://doi.org/10.3390/M1722







