Synthesis of a New α-Azidomethyl Styrene from Safrole via a Dearomative Rearrangement
Abstract
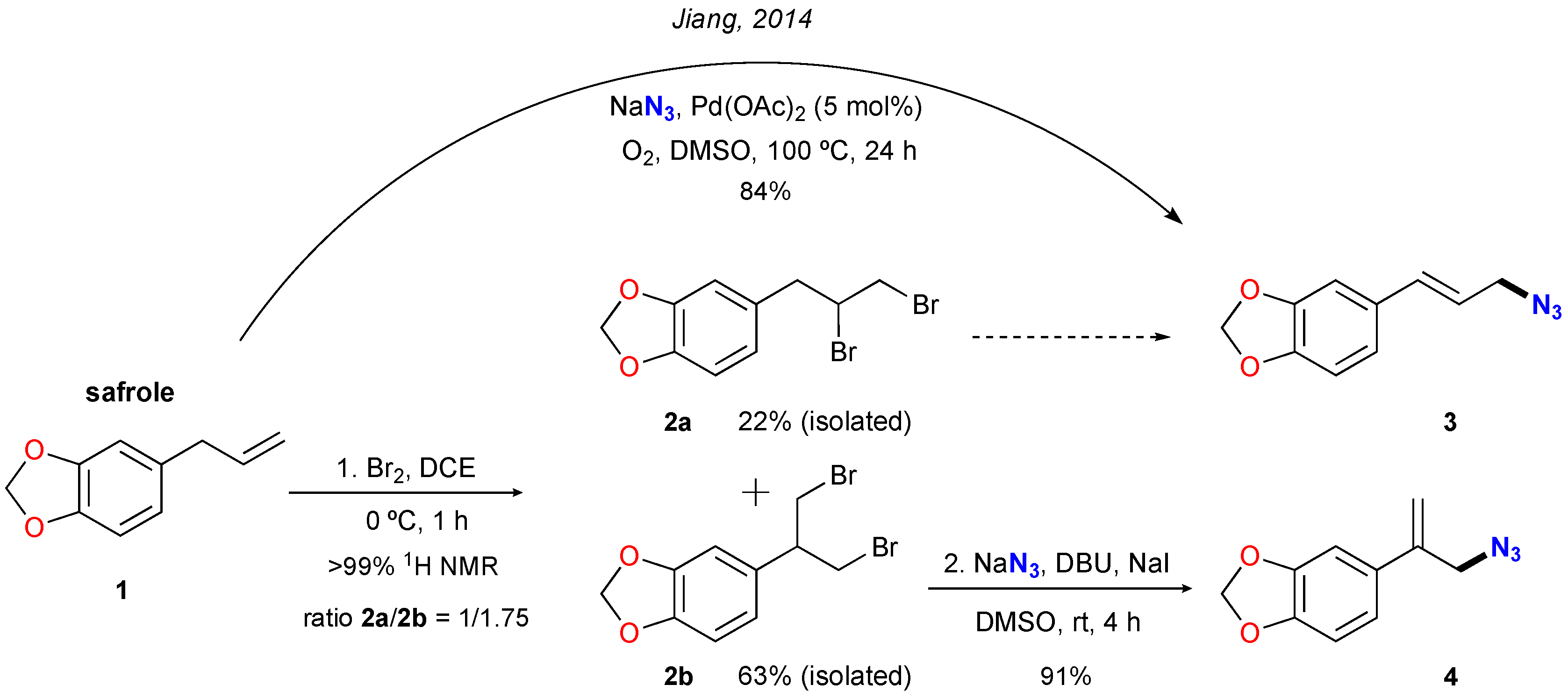
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Dibromide Derivatives 2a and 2b
3.3. Synthesis of Organic Azide 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nag, S.; Batra, S. Applications of Allylamines for the Syntheses of Aza-Heterocycles. Tetrahedron 2011, 67, 8959–9061. [Google Scholar] [CrossRef]
- Scriven, E.F.V.; Turnbull, K. Azides: Their Preparation and Synthetic Uses. Chem. Rev. 1988, 88, 297–368. [Google Scholar] [CrossRef]
- Askin, D.; Angst, C.; Danishefsky, S. A Total Synthesis of N-Acetylactinobolamine. J. Org. Chem. 1985, 50, 5005–5007. [Google Scholar] [CrossRef]
- Gagneux, A.; Winstein, S.; Young, W.G. Rearrangement of Allylic Azides. J. Am. Chem. Soc. 1960, 82, 5956–5957. [Google Scholar] [CrossRef]
- Tanimoto, H.; Kakiuchi, K. Recent Applications and Developments of Organic Azides in Total Synthesis of Natural Products. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef]
- Liu, Z.-K.; Zhao, Q.-Q.; Gao, Y.; Hou, Y.-X.; Hu, X.-Q. Organic Azides: Versatile Synthons in Transition Metal-Catalyzed C(Sp2)−H Amination/Annulation for N-Heterocycle Synthesis. Adv. Synth. Catal. 2021, 363, 411–424. [Google Scholar] [CrossRef]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic Azides: An Exploding Diversity of a Unique Class of Compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef]
- Aube, J.; Milligan, G.L. Intramolecular Schmidt Reaction of Alkyl Azides. J. Am. Chem. Soc. 1991, 113, 8965–8966. [Google Scholar] [CrossRef]
- Liu, R.; Gutierrez, O.; Tantillo, D.J.; Aubé, J. Stereocontrol in a Combined Allylic Azide Rearrangement and Intramolecular Schmidt Reaction. J. Am. Chem. Soc. 2012, 134, 6528–6531. [Google Scholar] [CrossRef]
- Sivaguru, P.; Ning, Y.; Bi, X. New Strategies for the Synthesis of Aliphatic Azides. Chem. Rev. 2021, 121, 4253–4307. [Google Scholar] [CrossRef]
- Carlson, A.S.; Topczewski, J.J. Allylic Azides: Synthesis, Reactivity, and the Winstein Rearrangement. Org. Biomol. Chem. 2019, 17, 4406–4429. [Google Scholar] [CrossRef]
- Chiba, S. Application of Organic Azides for the Synthesis of Nitrogen-Containing Molecules. Synlett 2011, 2012, 21–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.; Zhang, C.; Li, D.; Sun, P.; Li, J.; Wang, H.; Liu, J.; Qu, J. Base-Controlled Regiodivergent Azidation of Trifluoromethyl Alkenyl Triflates: Transition-Metal-Free Access to CF3-Containing Allyl Azides and Alkenyl Azides. J. Org. Chem. 2018, 83, 2858–2868. [Google Scholar] [CrossRef] [PubMed]
- Nishina, Y.; Morita, J.; Ohtani, B. Direct Bromination of Hydrocarbons Catalyzed by Li2MnO3 under Oxygen and Photo-Irradiation Conditions. RSC Adv. 2013, 3, 2158–2162. [Google Scholar] [CrossRef]
- Bräse, S.; Banert, K. Organic Azides: Syntheses and Applications; Wiley: Chichester, UK, 2010; ISBN 978-0-470-51998-1. [Google Scholar]
- Rokade, B.V.; Gadde, K.; Prabhu, K.R. Copper-Catalyzed Direct Transformation of Secondary Allylic and Benzylic Alcohols into Azides and Amides: An Efficient Utility of Azide as a Nitrogen Source. Eur. J. Org. Chem. 2015, 2015, 2706–2717. [Google Scholar] [CrossRef]
- Rueping, M.; Vila, C.; Uria, U. Direct Catalytic Azidation of Allylic Alcohols. Org. Lett. 2012, 14, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Ravi, V.; Sreedhar, B. Efficient Synthesis of Allylic Azides and One-Pot Regioselective Synthesis of 1,4-Disubstituted 1,2,3-Triazoles from Homoallyl Alcohols. Tetrahedron Lett. 2010, 51, 4037–4041. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. On the PdCl2-Catalyzed Synthesis of Allylic Azides and Allylic Sulfonamides from Homoallylic Alcohols. Tetrahedron Lett. 2011, 52, 5217–5219. [Google Scholar] [CrossRef]
- Srinu, G.; Srihari, P. A Catalytic Approach for the Synthesis of Allylic Azides from Aryl Vinyl Carbinols. Tetrahedron Lett. 2013, 54, 2382–2385. [Google Scholar] [CrossRef]
- Rokhum, L.; Bez, G. A Practical One-Pot Synthesis of Azides Directly from Alcohols. J. Chem. Sci. 2012, 124, 687–691. [Google Scholar] [CrossRef]
- Khrakovsky, D.A.; Tao, C.; Johnson, M.W.; Thornbury, R.T.; Shevick, S.L.; Toste, F.D. Enantioselective, Stereodivergent Hydroazidation and Hydroamination of Allenes Catalyzed by Acyclic Diaminocarbene (ADC) Gold(I) Complexes. Angew. Chem. Int. Ed. 2016, 55, 6079–6083. [Google Scholar] [CrossRef]
- Goswami, P.P.; Suding, V.P.; Carlson, A.S.; Topczewski, J.J. Direct Conversion of Aldehydes and Ketones into Azides by Sequential Nucleophilic Addition and Substitution: Direct Conversion of Aldehydes and Ketones into Azides by Sequential Nucleophilic Addition and Substitution. Eur. J. Org. Chem. 2016, 2016, 4805–4809. [Google Scholar] [CrossRef]
- Chen, H.; Yang, W.; Wu, W.; Jiang, H. Palladium-Catalyzed Regioselective Azidation of Allylic C–H Bonds under Atmospheric Pressure of Dioxygen. Org. Biomol. Chem. 2014, 12, 3340–3343. [Google Scholar] [CrossRef]
- Huang, X.; Fulton, B.; White, K.; Bugarin, A. Metal-Free, Regio- and Stereoselective Synthesis of Linear (E)-Allylic Compounds Using C, N, O, and S Nucleophiles. Org. Lett. 2015, 17, 2594–2597. [Google Scholar] [CrossRef]
- Ojo, O.S.; Miranda, O.; Baumgardner, K.C.; Bugarin, A. Practical Regio- and Stereoselective Azidation and Amination of Terminal Alkenes. Org. Biomol. Chem. 2018, 16, 9354–9358. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.S.; Bugarin, A. One-Pot Synthesis of α-Alkyl Styrene Derivatives. ACS Omega 2021, 6, 20619–20628. [Google Scholar] [CrossRef]
- Costa, P.R.R. Safrol e Eugenol: Estudo Da Reatividade Química e Uso Em Síntese de Produtos Naturais Biologicamente Ativos e Seus Derivados. Quim. Nova 2000, 23, 357–369. [Google Scholar] [CrossRef]
- Saraiva, M.C.; Santos, D.F.; Costa, P.R.R.; Rabi, J.A. Safrole as Starting Material for the Synthesis of 7-Methoxy-3′,4′-Methylenedioxy Isoflavone. J. Braz. Chem. Soc. 1990, 1, 91–93. [Google Scholar] [CrossRef]
- Perković, I.; Raić-Malić, S.; Fontinha, D.; Prudêncio, M.; de Carvalho, L.P.; Held, J.; Tandarić, T.; Vianello, R.; Zorc, B.; Rajić, Z. Harmicines—Harmine and Cinnamic Acid Hybrids as Novel Antiplasmodial Hits. Eur. J. Med. Chem. 2020, 187, 111927. [Google Scholar] [CrossRef]
- Gombos, L.G.; Werner, L.; Schollmeyer, D.; Martínez-Huitle, C.A.; Waldvogel, S.R. Selective Electrochemical Dibromination of Terpenes and Naturally Derived Olefins. Eur. J. Org. Chem. 2022, 2022, e202200857. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isbel, S.R.; Bugarin, A. Synthesis of a New α-Azidomethyl Styrene from Safrole via a Dearomative Rearrangement. Molbank 2023, 2023, M1713. https://doi.org/10.3390/M1713
Isbel SR, Bugarin A. Synthesis of a New α-Azidomethyl Styrene from Safrole via a Dearomative Rearrangement. Molbank. 2023; 2023(3):M1713. https://doi.org/10.3390/M1713
Chicago/Turabian StyleIsbel, Stephen R., and Alejandro Bugarin. 2023. "Synthesis of a New α-Azidomethyl Styrene from Safrole via a Dearomative Rearrangement" Molbank 2023, no. 3: M1713. https://doi.org/10.3390/M1713
APA StyleIsbel, S. R., & Bugarin, A. (2023). Synthesis of a New α-Azidomethyl Styrene from Safrole via a Dearomative Rearrangement. Molbank, 2023(3), M1713. https://doi.org/10.3390/M1713





