N′-(5-Bromofuran-2-carbonyl)isonicotinohydrazide
Abstract
1. Introduction
2. Results and Discussion
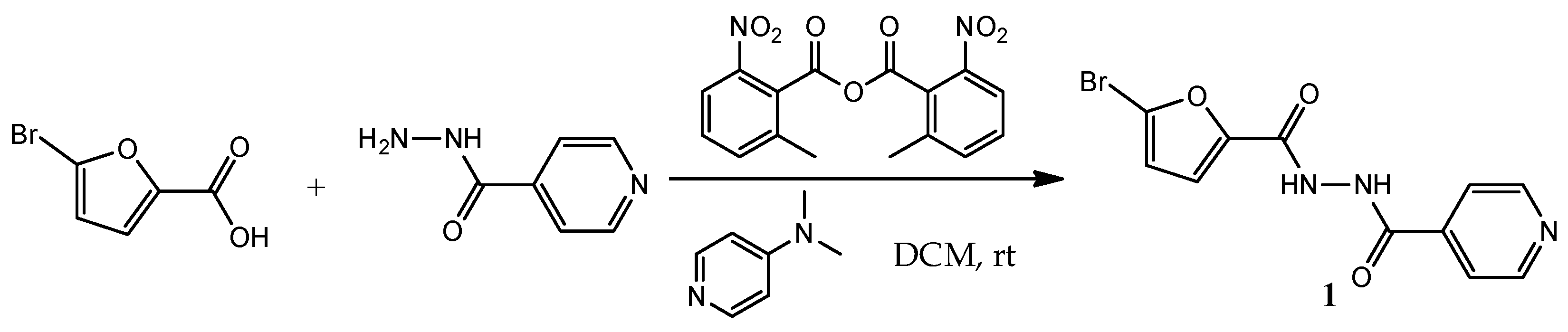
2.1. Chemistry
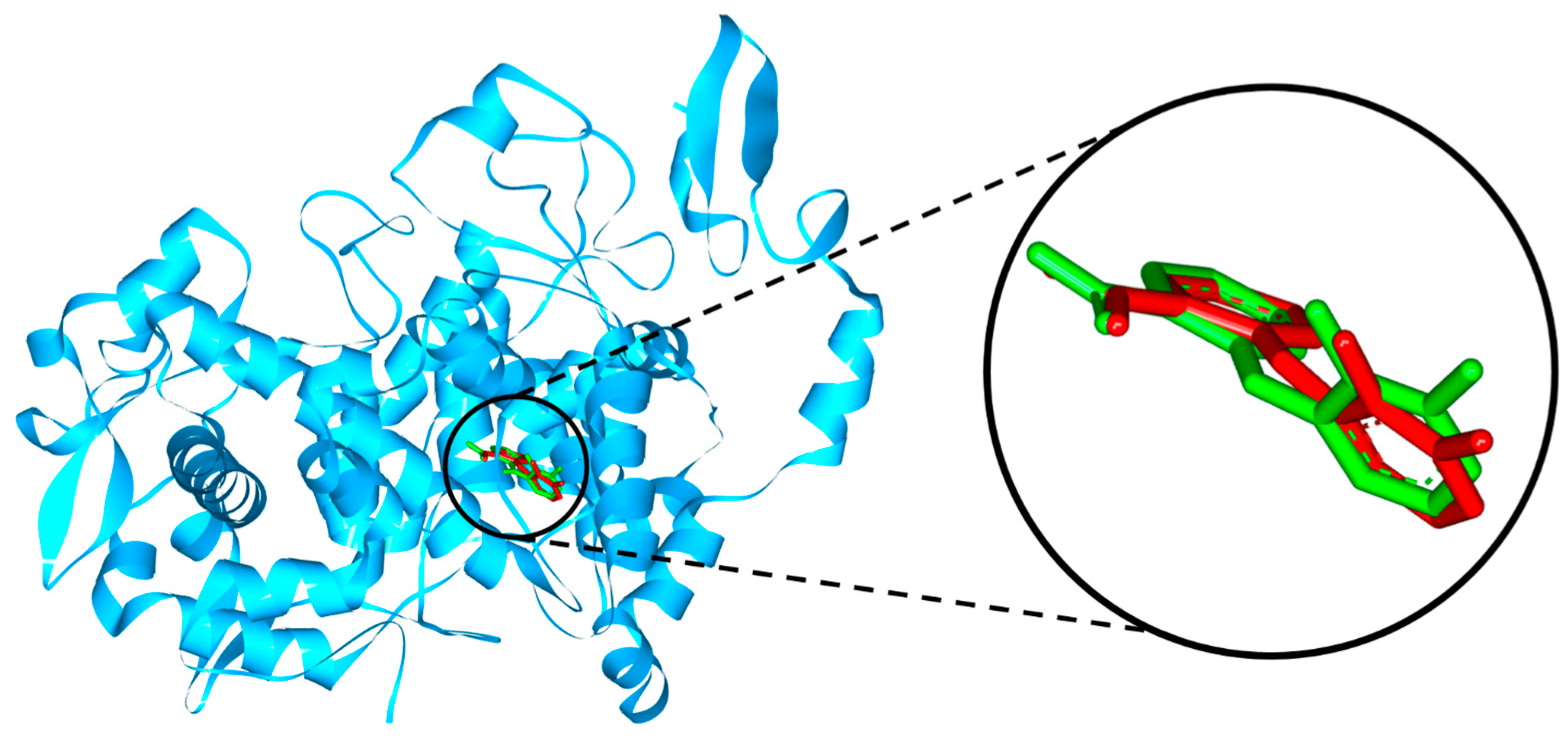
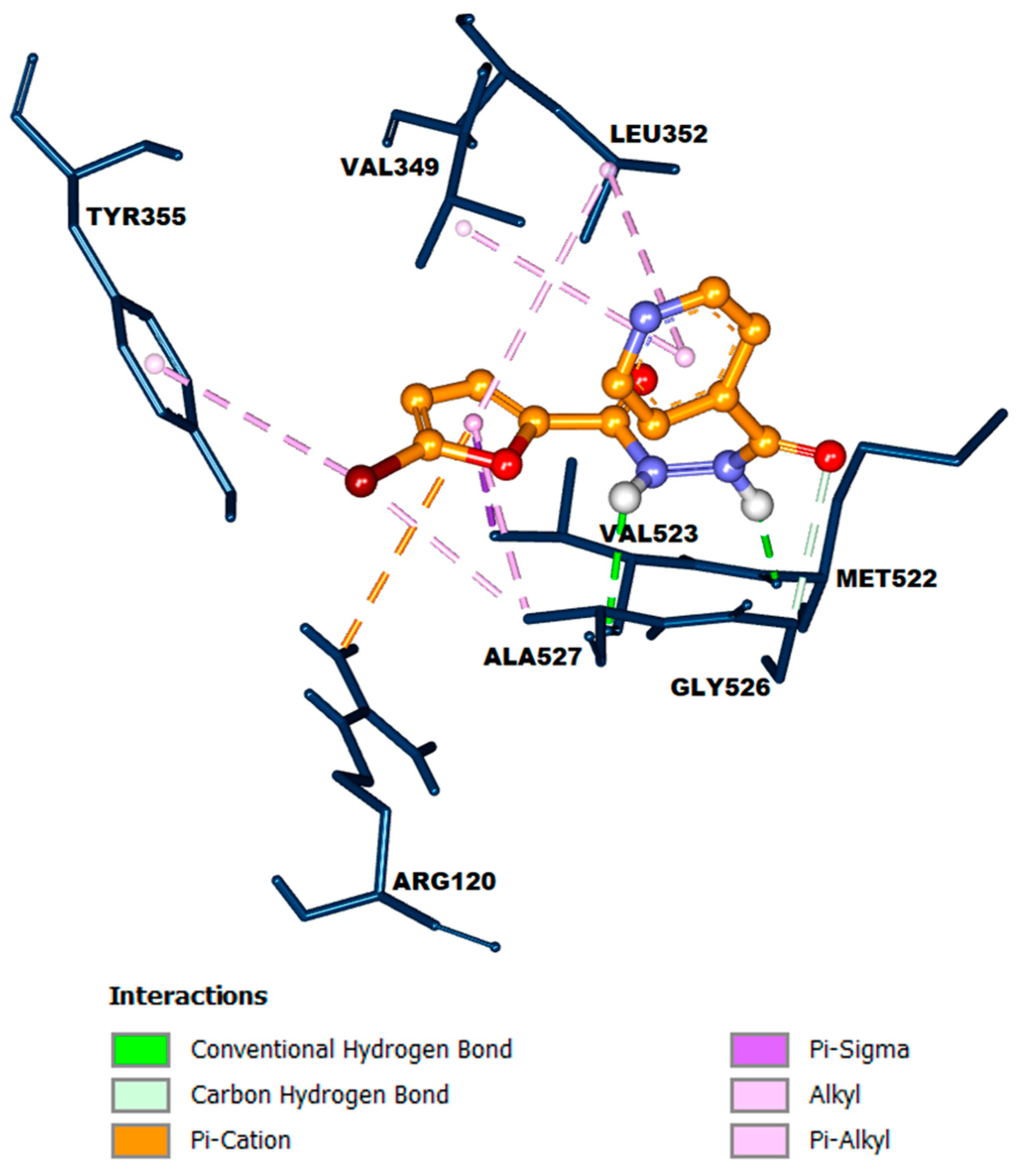
2.2. Molecular Docking Study
3. Materials and Methods
3.1. Synthesis of N′-(5-Bromofuran-2-carbonyl)isonicotinohydrazide (1)
3.2. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tikhomirov, A.S.; Lin, C.Y.; Volodina, Y.L.; Dezhenkova, L.G.; Tatarskiy, V.V.; Schols, D.; Shtil, A.A.; Kaur, P.; Chueh, P.J.; Shchekotikhin, A.E. New Antitumor Anthra[2,3-b]Furan-3-Carboxamides: Synthesis and Structure-Activity Relationship. Eur. J. Med. Chem. 2018, 148, 128–139. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Liu, Q.; Li, A.; Wang, W.; Gu, W. Design, Synthesis, and Antifungal Activity of Novel Thiophene/Furan-1,3,4-Oxadiazole Carboxamides as Potent Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem. 2021, 69, 13373–13385. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, N.; Alves, S.H.; Coelho, H.S.; Borchhardt, D.M.; Machado, P.; Flores, K.M.; da Silva, F.M.; Spader, T.B.; Santurio, J.M.; Bonacorso, H.G.; et al. Synthesis, Antimicrobial Activity, and QSAR Studies of Furan-3-Carboxamides. Bioorg. Med. Chem. 2007, 15, 1947–1958. [Google Scholar] [CrossRef]
- Zhan, W.; Xu, L.; Dong, X.; Dong, J.; Yi, X.; Ma, X.; Qiu, N.; Li, J.; Yang, B.; Zhou, Y.; et al. Design, Synthesis and Biological Evaluation of Pyrazol-Furan Carboxamide Analogues as Novel Akt Kinase Inhibitors. Eur. J. Med. Chem. 2016, 117, 47–58. [Google Scholar] [CrossRef]
- He, M.; Li, Y.-J.; Shao, J.; Li, Y.-S.; Cui, Z.-N. Synthesis and Biological Evaluation of 2,5-Disubstituted Furan Derivatives Containing 1,3-Thiazole Moiety as Potential α-Glucosidase Inhibitors. Bioorg. Med. Chem. Lett. 2023, 83, 129173. [Google Scholar] [CrossRef]
- Monier, M.; El-Mekabaty, A.; Elattar, K.M. Five-Membered Ring Systems with One Heteroatom: Synthetic Routes, Chemical Reactivity, and Biological Properties of Furan-Carboxamide Analogues. Synth. Commun. 2018, 48, 839–875. [Google Scholar] [CrossRef]
- Narasimhan, B.; Belsare, D.; Pharande, D.; Mourya, V.; Dhake, A. Esters, Amides and Substituted Derivatives of Cinnamic Acid: Synthesis, Antimicrobial Activity and QSAR Investigations. Eur. J. Med. Chem. 2004, 39, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, X.; Zhang, H.J.; Li, N.; Xiao, Y.; Zhu, H.L.; Ye, Y.H. Synthesis, Structure, and Biological Assay of Cinnamic Amides as Potential EGFR Kinase Inhibitors. Med. Chem. Res. 2013, 22, 986–994. [Google Scholar] [CrossRef]
- Dunetz, J.R.; Magano, J.; Weisenburger, G.A. Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals. Org. Process Res. Dev. 2016, 20, 140–177. [Google Scholar] [CrossRef]
- Shwetha, B.; Sudhanva, M.S.; Jagadeesha, G.S.; Thimmegowda, N.R.; Hamse, V.K.; Sridhar, B.T.; Thimmaiah, K.N.; Ananda Kumar, C.S.; Shobith, R.; Rangappa, K.S. Furan-2-Carboxamide Derivative, a Novel Microtubule Stabilizing Agent Induces Mitotic Arrest and Potentiates Apoptosis in Cancer Cells. Bioorg. Chem. 2021, 108, 104586. [Google Scholar] [CrossRef] [PubMed]
- Métro, T.X.; Martinez, J.; Lamaty, F. 1,1′-Carbonyldiimidazole and Mechanochemistry: A Shining Green Combination. ACS Sustain. Chem. Eng. 2017, 5, 9599–9602. [Google Scholar] [CrossRef]
- Shiina, I.; Kawakita, Y.I. The Effective Use of Substituted Benzoic Anhydrides for the Synthesis of Carboxamides. Tetrahedron 2004, 60, 4729–4733. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Aijijiyah, N.P.; Wati, F.A.; Rahayu, R.; Srilistiani, A.; Mahzumi, F.; Aulia, T.; Santoso, L.; Pamela, E.; Ramadhani, E.Y.; Ilfahmi, Y.A.; et al. Synthesis, α-Glucosidase Inhibitory Activity, and Molecular Docking of Cinnamamides. Med. Chem. Res. 2023, 32, 723–735. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Santoso, M.; Pamela, E.; Ramadhani, E.Y.; Ilfahmi, Y.A.; Aijijiyah, N.P.; Purnomo, A.S.; Putra, S.R. 4-Methoxyphenethyl (E)-3-(o-Tolyl)acrylate. Molbank 2022, 2022, M1519. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadhani, E.Y.; Aijijiyah, N.P.; Santoso, E.; Atmaja, L.; Santoso, M. N′-(5-Bromofuran-2-carbonyl)isonicotinohydrazide. Molbank 2023, 2023, M1706. https://doi.org/10.3390/M1706
Ramadhani EY, Aijijiyah NP, Santoso E, Atmaja L, Santoso M. N′-(5-Bromofuran-2-carbonyl)isonicotinohydrazide. Molbank. 2023; 2023(3):M1706. https://doi.org/10.3390/M1706
Chicago/Turabian StyleRamadhani, Ersya Yanu, Nur Pasca Aijijiyah, Eko Santoso, Lukman Atmaja, and Mardi Santoso. 2023. "N′-(5-Bromofuran-2-carbonyl)isonicotinohydrazide" Molbank 2023, no. 3: M1706. https://doi.org/10.3390/M1706
APA StyleRamadhani, E. Y., Aijijiyah, N. P., Santoso, E., Atmaja, L., & Santoso, M. (2023). N′-(5-Bromofuran-2-carbonyl)isonicotinohydrazide. Molbank, 2023(3), M1706. https://doi.org/10.3390/M1706




