Abstract
A new triazol-3-one resulted unexpectedly from the reduction reaction of a heterocyclic thioketone using sodium borohydride in pyridine containing a small amount of water. The structure of the new compound was characterised using FT-IR, 1D and 2D NMR, and HRMS spectroscopic methods.
1. Introduction
Triazoles are a class of five-membered heterocycles that contain three nitrogen and two carbon atoms. On the basis of the way nitrogen and carbon atoms connect between each other, there exist two triazole isomers: 1,2,3-triazoles and 1,2,4-triazoles. These compounds have been extensively studied for more than a century due to their remarkable properties, making them promising candidates for drug development and treatment of various diseases [1]. Some of the most known drugs that contain the 1,2,4-triazole moiety are alprazolam, fluconazole, ribavirin, and posaconazole.
1,2,4-triazoles that contain the sulphanyl functional group directly attached to the heterocyclic core have also been studied for their potential use as bioactive compounds. The first reported synthesis of 3-sulphanyl-1,2,4-triazole was reported by Freund in 1896 [2]. Some of the biological activities of 1,2,4-triazole thiols include anticancer [3,4], enzyme inhibition capacity [5], antioxidant [6], antimicrobial [7], anti-inflammatory [8], antituberculous [9], and many more.
Several methods are commonly used to synthesise 3-sulphanyl-1,2,4-triazoles, including reactions involving the reaction of isothiocyanates with hydrazides [10] and the reaction of 1,3,4-oxadiazoles with hydrazine [11], by thermal cyclization of acylated thiosemicarbazides [12] or by reaction between carboxylic acids with hydrazinecarbothiohydrazides [13].
S-alkylated compounds can be prepared by reacting the corresponding sulphanyl compound with a halogenated compound under various reaction conditions. Some of the reported S-alkylation protocols include the use of potassium carbonate [14], potassium hydroxide [15], caesium carbonate [16], sodium ethoxide [17], and ultrasound [18].
1,2,4-triazole-3-ones are heterocyclic compounds that have biological activities such as anticonvulsant [19], anti-inflammatory [20], and antimicrobial [21,22]. In addition, these compounds can be used as high-energy materials [23].
Taking into account the aforementioned information, our aim was to synthesise novel sulphanyl-alkylated 1,2,4-triazoles. During an intermediate synthesis step, an unexpected outcome occurred when the carbonyl intermediate was reduced using sodium borohydride, leading to the formation of a triazolone through a new, unreported method.
2. Results and Discussion
2-((4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)thio)-1-phenylethan-1-one (2) was synthesized using an S-alkylation reaction of the 4-(4-ethoxyphenyl)-5-(4-methoxyphenyl)-4H-1,2,4-triazole-3-thiol (1). Initially, the scope of the present work was to synthesise a secondary racemic alcohol from the aforementioned ketone by reduction of carbonylic functional group using sodium borohydride. Therefore, a modified procedure from the literature was applied for the reduction reaction. The synthesis followed Scheme 1:
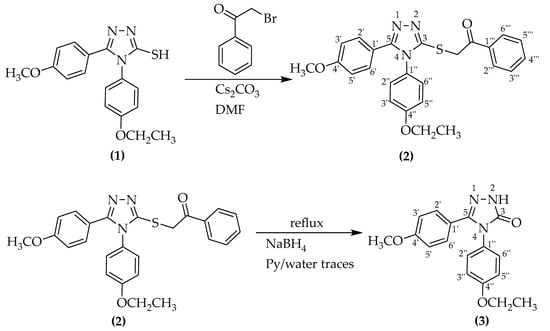
Scheme 1.
Synthesis scheme for the novel compound (3).
For the S-alkylation reaction, a modified reported protocol was followed [16]. The caesium carbonate was used in excess in relation to triazole thiol. As an alkylation agent, 2-bromo-1-phenylethan-1-one was used. During the reaction, in the basic medium provided by caesium carbonate, an intermediate thiolate salt is formed. Due to the conjugation of the triazole ring and the presence of the sulphur atom with its lone pair of electrons, the salt possesses a nucleophilic character, so it readily reacts with the α-haloketone. In addition to the aromatic and ether functional groups, the resulting compound carries a ketone and thioether functional group. Like other carbonylic compounds, it can be reduced to an alcohol by using different reduction agents.
For the reduction of the obtained ketone, sodium borohydride was chosen as the reduction agent, following modified literature procedures [24]. An excess of the borohydride was used to compensate for the eventual loss of reduction agent by decomposition or secondary reactions. Surprisingly, the reduction reaction did not produce racemic secondary alcohol, but rather a different compound (3). First, it possessed an unusually high melting point, which is not characteristic of similar secondary racemic alcohols. From NMR and later HRMS data, it was concluded that the obtained compound carries a ketone functional group but it is directly attached to the triazole ring. At first sight, it appears that the reaction of heterocyclic thioketones with sodium borohydride in the pyridine/water mixture at reflux temperature leads to the formation of triazolone (3) instead of the secondary alcohol. A first conclusion is that instead of the reduction of carbonyl group, a nucleophilic substitution of the alkylsulphanyl functional group by the hydroxyl functional group in the basic medium took place. More research in this direction is required to elucidate the real mechanism of the reaction. It is not clear what effect the alkyl substituents or reaction conditions (temperature, solvent, etc.) have on the triazolone formation rate. In Scheme 2, the expected and actual reaction paths are represented.
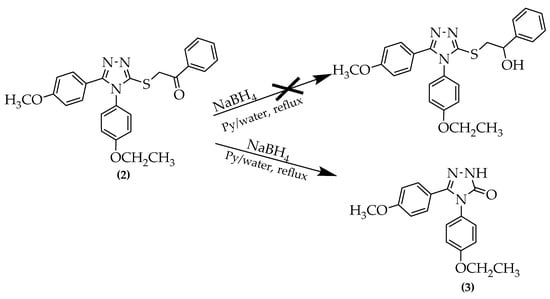
Scheme 2.
Unexpected formation of (3) by reduction of compound (2).
2.1. Explanation of Experimental NMR Data for Compound (2)
From the 1H-15N HMBC spectrum, the signals corresponding to aromatic protons 2″-H and 6″-H (7.16 ppm) were identified by the long-range coupling with 4-N nitrogen atom (174.9 ppm). In the 1H-13C HMBC spectrum, the following long-range couplings could be observed: coupling of the carbonylic carbon (C=O) (193.3 ppm) with aromatic protons 2′′′-H and 6′′′-H (8.04 ppm), coupling of carbon atom 3-C (152.2 ppm) with methylene protons (S-CH2) (4.95 ppm), coupling of carbon atom 5-C (155.1 ppm) with aromatic protons 2′-H and 6′-H (7.36 ppm), coupling of carbon atom 4′-C (160.6 ppm) with methyl protons (O-CH3) (3.77 ppm), and the coupling of carbon atom 4″-C (159.9 ppm) with methylene protons (O-CH2) (4.07 ppm). In Table 1, long-range correlations between protons and carbon atoms (1H-13C HMBC experiment) and long-range correlation between protons and nitrogen atom (1H-15N HMBC) are presented.

Table 1.
Experimental NMR data * for the compound (2).
2.2. Explanation of Experimental NMR Data for Compound (3)
Using 2D NMR data, we could assign signals to all three nitrogen atoms of 1,2,4-triazole heterocycle as follows: from the 1H-15N HSQC spectrum, the direct coupling between nitrogen atom 2-N (164.5 ppm) and the directly attached proton 2-H (11.98 ppm). In the 1H-15N HMBC spectrum, long-range couplings between nitrogen atoms 4-N (154.1 ppm) and 1-N (254.5 ppm) and the proton 2-H (11.98 ppm) could be observed. The chemical shift identification of the nitrogen atom 4-N was performed based on its coupling with two aromatic protons 2″-H and 6″-H (7.16 ppm). In the long-range coupling spectrum 1H-13C HMBC, the couplings of the two triazolic carbon atoms 5-C (145.8 ppm) and 3-C (155.3 ppm) with the triazolic proton 2-H (11.98 ppm) were observed. The signal at 145.8 ppm was assigned to the 5-C carbon atom due to the couplings with aromatic protons 2′-H and 6′-H (7.22 ppm). The signals corresponding to 4′-C carbon (160.6 ppm) and 4″-C carbon (158.7 ppm) atoms were assigned based on the long-range couplings with methyl protons (O-CH3) (3.72 ppm) and with methylene protons (O-CH2) (4.03 ppm). In Table 2, direct bonding of protons to nitrogen atoms (1H-15N HSQC experiment), long-range correlations between protons and carbon atoms (1H-13C HMBC experiment), and long-range correlation between protons and nitrogen atom (1H-15N HMBC) are presented.

Table 2.
Experimental NMR data * for the compound (3).
3. Materials and Methods
The reagents used were purchased from commercial sources and used as received. 4-(4-ethoxyphenyl)-5-(4-methoxyphenyl)-4H-1,2,4-triazole-3-thiol (1) was synthesized earlier in our laboratory following the modified procedures from the literature [25,26,27].
The 1H-NMR, 13C-NMR, and 15N NMR spectra were recorded on a Bruker Avance III 500 MHz spectrometer. Chemical shifts (δ) have been measured in ppm and coupling constants (J) in Hz. The samples were dissolved in DMSO-d6 or CDCl3. TMS was used as an internal standard.
IR spectra were recorded on a Jasco FT/IR-410 spectrophotometer (Jasco Corporation, Tokyo, Japan) in KBr pellets.
Melting points were measured on a Böetius PHMK apparatus (Veb Analytik, Dresden) and were uncorrected.
The high-resolution MS (HRMS) spectrum was recorded on a Bruker Maxis II QTOF spectrometer (Bruker Daltonics, Bremen, Germany) with electrospray ionisation (ESI) in positive mode. The compound was initially dissolved in DMSO and further diluted 1:100 with acetonitrile. MS spectrum processing and isotope pattern simulations have been performed with Compass Data Analysis V.4.4 (Bruker Daltonics).
4. Experimental
4.1. Synthesis of 2-((4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)thio)-1-phenylethan-1-one (2)
In a 100 mL round bottom flask equipped with a magnetic stirrer, 4-(4-ethoxyphenyl)-5-(4-methoxyphenyl)-4H-1,2,4-triazole-3-thiol (1 g, 3 mmol) was added together with 20 mL of DMF.
After complete dissolution of triazole-3-thiol (1), caesium carbonate (0.992 g, 3 mmol) was added to the solution. A white precipitate formation was observed. The solution was stirred for 1 h to ensure that the whole triazole-3-thiol quantity was transformed into caesium 4-(4-ethoxyphenyl)-5-(4-methoxyphenyl)-4H-1,2,4-triazole-3-thiolate salt.
A solution of 2-bromo-1-phenylethan-1-one (0.642 g, 3.2 mmol) was prepared in 10 mL of 96% ethanol. Then, the obtained solution was added dropwise to the caesium thiolate salt under stirring. A slightly pink colouration of the solution was observed. After adding 2-bromo-1-phenylethan-1-one, the reaction mass was left to stir for 24 h. Reaction completion was monitored using TLC, using a 1:1 (v/v) hexane:ethyl acetate mixture as an eluent.
After 24 h, a white precipitate was observed in reaction mass, whereas the colour of the solution changed to a bright yellow one. The reaction mass was added dropwise to 150 mL of distilled water under vigorous stirring. A white precipitate was separated using vacuum filtration. After separation, the precipitate was dried at room temperature.
The precipitate was recrystallised from 35 mL of 96% ethanol.
920 mg of compound was obtained, and the isolation yield was 68%, melting point: 163–165 °C; 1H NMR (CDCl3, 500 MHz) δ (ppm): 8.04 (d, 2H, J = 7.2 Hz, 2‴-H, 6‴-H), 7.62–7.58 (m, 1H, 4‴-H), 7.50–7.47 (m, 2H, 3‴-H, 5‴-H), 7.36 (d, 2H, J = 8.9 Hz, 2′-H, 6′-H), 7.16 (d, 2H, J = 8.7 Hz, 2″-H, 6″-H), 6.95 (d, 2H, J = 8.7 Hz, 3″-H, 5″-H), 6.79 (d, 2H, J = 8.9Hz, 3′-H, 5′-H), 4.95 (s, 2H, -S-CH2-), 4.07 (q, 2H, -CH2-CH3), 3.77 (s, 3H, -CH3), 1.45 (t, 3H, -CH2-CH3); 13C NMR (CDCl3, 125 MHz) δ (ppm): 193.3 (-C=O), 160.6 (4′-C), 159.9 (4″-C), 155.1 (5-C), 152.2 (3-C), 135.3 (1‴-C), 133.8 (4‴-C), 129.5 (2′-C, 6′-C), 128.8 (3‴, 5‴-C), 128.6 (2‴-C, 6‴-C), 128.5 (2″-C, 6″-C), 126.4 (4″-C), 119.1 (1′-C), 115.6 (2″-C, 6″-C), 113.9 (3′-C, 5′-C), 63.9 (-CH2-CH3), 59.2 (-CH3), 41.1 (-S-CH2), 14.7 (-CH2-CH3); 15N NMR (CDCl3, 50 MHz) δ (ppm): 174.9 (4-N); FT-IR (cm−1):, 3046 (νCar-H), 3008 (νCar-H), 2951 (νasCH3), 2933 (νasCH3), 1679 (νC=O), 1609 (νC=N triazole), 1578 (νsk.ar.), 1510 (νsk.ar.), 1479 (νsk.ar.), 1254 (νasCOC).
4.2. Synthesis of 4-(4-Ethoxyphenyl)-5-(4-methoxyphenyl)-2,4-dihydro-3H-1,2,4-triazol-3-one (3)
In a 100 mL round bottom flask equipped with a magnetic stirrer, 2-((4-(4-ethoxyphenyl)-5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)thio)-1-phenylethan-1-one (0.72 g, 2 mmol) was added to 10 mL of pyridine and 1 mL of distilled water, then the heating of the reaction mixture with stirring was started.
During 30 min, small portions of sodium borohydride (0.302 g, 8 mmol) were added to the reaction mass. After this, the reaction mass was refluxed for 1 h. Reaction completion was monitored using TLC, using a 1:1 (v/v) hexane:ethyl acetate mixture as an eluent.
After completion of the reaction, the solution was cooled to room temperature, then using a 10 % HCl solution, the pH was adjusted to 6.5–7. The solution was added dropwise to 50 mL of water under vigorous stirring. A white precipitate was formed. The precipitate was recrystallised from 10 mL of 96% ethanol.
280 mg of crystalline white compound was obtained, and the isolation yield was 45%, melting point: 229–231 °C; 1H NMR (DMSO-d6, 500 MHz) δ (ppm): 11.98 (s, 1H, -NH), 7.22 (d, 2H, J = 8.8 Hz, 2′-H, 6′-H), 7.16 (d, 2H, J = 8.8 Hz, 2″-H, 6″-H), 6.97 (d, 2H, J = 8.8 Hz, 3″-H, 5″-H), 6.88 (d, 2H, J = 8.8 Hz, 3′-H, 5′-H), 4.03 (q, 2H, J = 6.9 Hz, -O-CH2), 3.72 (s, 3H, -O-CH3), 1.33 (t, 3H, -CH2CH3); 13C NMR (DMSO-d6, 125 MHz) δ (ppm): 160.6 (4′-C), 158.7 (4″-C), 155.3 (3-C), 145.8 (5-C), 129.6 (2″-C, 6″-C), 129.5 (2′-C, 6′-C), 126.7 (1″-C), 119.9 (1′-C), 115.3 (3″-C, 5″-C), 114.4 (3′-C, 5′-C), 63.8 (-O-CH2), 55.6 (-O-CH3), 15.0 (-CH2-CH3); 15N NMR (DMSO-d6, 50 MHz) δ (ppm): 254.5 (1-N), 164.5 (2-N), 154.1 (4-N), FT-IR (cm−1): 2974 (νasCH3-OR), 1698 (νC=O), 1612 (νsk.ar.), 1513 (νsk.ar.), 1298 (νasCOC), 838 (γ1,4-disubst. phenyl); HRMS-ESI (m/z): [M + Na+] for C17H17N3O3 Na, calcd. 334.11621, found 334.11619.
All spectra are reported in supplementary materials.
5. Conclusions
A new triazolone (3) was synthesized by a new, unreported method, which probably followed a nucleophilic substitution of the alkylsulphanyl functional group with hydroxide anion. The reported method can potentially be used for the synthesis of similar triazolones.
Supplementary Materials
The following supporting information can be downloaded online. Figure S1. 1H NMR spectrum of the compound (2), Figure S2. 13C NMR spectrum of the compound (2), Figure S3. 13C DEPT135 spectrum of the compound (2), Figure S4. COSY 1H-1H spectrum of the compound (2), Figure S5. HSQC 1H-13C spectrum of the compound (2), Figure S6. HMBC 1H-15N spectrum of the compound (2), Figure S7. HMBC 1H-13C spectrum of the compound (2), Figure S8. 1H NMR spectrum of the compound (3), Figure S9. 13C NMR spectrum of the compound (3), Figure S10. 13C DEPT135 spectrum of the compound (3), Figure S11. COSY 1H-1H spectrum of the compound (3), Figure S12. HSQC 1H-13C spectrum of the compound (3), Figure S13. HSQC 1H-15N spectrum of the compound (3), Figure S14. HMBC 1H-13C spectrum of the compound (3), Figure S15. HMBC 1H-15N spectrum of the compound (3), Figure S16. FT-IR spectrum of the compound (2), Figure S17. FT-IR spectrum of the compound (3), and Figure S18. HRMS spectrum of the compound (3).
Author Contributions
Designed the experiments, V.B. and V.-N.B.; performed the experiments, I.B.; analysed the spectral data, V.B. and C.D.; wrote the manuscript, I.B.; supervision and funding acquisitions, V.B. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P2-2.1-PED-2019-3414, within PNCDI III.
Data Availability Statement
The data presented in this study are available within the article or supplementary material.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ferreira, V.F.; da Rocha, D.R.; da Silva, F.C.; Ferreira, P.G.; Boechat, N.A.; Magalhães, J.L. Novel 1H-1,2,3-, 2H-1,2,3-, 1H-1,2,4- and 4H-1,2,4-Triazole Derivatives: A Patent Review (2008–2011). Expert Opin. Ther. Pat. 2013, 23, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, Ş.G.; Çıkla-Süzgün, P. Recent Advances Bioactive 1,2,4-Triazole-3-Thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef] [PubMed]
- Šermukšnytė, A.; Kantminienė, K.; Jonuškienė, I.; Tumosienė, I.; Petrikaitė, V. The Effect of 1,2,4-Triazole-3-Thiol Derivatives Bearing Hydrazone Moiety on Cancer Cell Migration and Growth of Melanoma, Breast, and Pancreatic Cancer Spheroids. Pharmaceuticals 2022, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brahmbhatt, J.G.; Pandya, P.A.; Daraji, D.G.; Patel, H.D.; Rawal, R.M.; Baran, S.K. Design, Synthesis and Biological Evaluation of Novel 5-(4-Chlorophenyl)-4-Phenyl-4H-1,2,4-Triazole-3-Thiols as an Anticancer Agent. J. Mol. Struct. 2021, 1231, 130000. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Farahat, M.M.K.; Awad, L.F.; Balbaa, M.; Yusef, H.; Badawy, M.E.I.; Abd Al Moaty, M.N. New 4-(Arylidene)Amino-1,2,4-Traizole-5-Thiol Derivatives and Their Acyclo Thioglycosides as α-Glucosidase and α-Amylase Inhibitors: Design, Synthesis, and Molecular Modelling Studies. J. Mol. Struct. 2022, 1259, 132733. [Google Scholar] [CrossRef]
- Ihnatova, T.; Kaplaushenko, A.; Frolova, Y.; Pryhlo, E. Synthesis and Antioxidant Properties of Some New 5-Phenethyl-3-Thio-1,2,4-Triazoles. Pharmacia 2021, 68, 129–133. [Google Scholar] [CrossRef]
- Domyati, D.; Zabin, S.A.; Elhenawy, A.A.; Abdelbaset, M. Preparation, Antimicrobial Activity and Docking Study of Vanadium Mixed Ligand Complexes Containing 4-Amino-5-Hydrazinyl-4H-1,2,4-Triazole-3-Thiol and Aminophenol Derivatives. Processes 2021, 9, 1008. [Google Scholar] [CrossRef]
- Arustamyan, Z.S.; Margaryan, R.E.; Aghekyan, A.A.; Panosyan, G.A.; Muradyan, R.E.; Tumajyan, A.E. Synthesis and Anti-Inflammatory Properties of Substituted 5-(Tetrahydro-4-Phenyl-2H-Pyran-4-Yl)-4H-1,2,4-Triazole-3-Thiols. Russ. J. Org. Chem. 2021, 57, 195–202. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Kandeel, M.; Pillay, M.; Deb, P.K.; Abdallah, H.H.; Mahomoodally, M.F.; Chopra, D. Anti-Tubercular Properties of 4-Amino-5-(4-Fluoro-3- Phenoxyphenyl)-4H-1,2,4-Triazole-3-Thiol and Its Schiff Bases: Computational Input and Molecular Dynamics. Antibiotics 2020, 9, 559. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Ashraf, M.; Rehman, J.; Saleem, R.S.Z. Synthesis, in Vitro and in Silico Studies of S-Alkylated 5-(4-Methoxyphenyl)-4-Phenyl-4H-1,2,4-Triazole-3-Thiols as Cholinesterase Inhibitors. Pak. J. Pharm. Sci. 2018, 31, 2697–2708. [Google Scholar]
- Ünver, Y.; Deniz, S.; Çelik, F.; Akar, Z.; Küçük, M.; Sancak, K. Synthesis of New 1,2,4-Triazole Compounds Containing Schiff and Mannich Bases (Morpholine) with Antioxidant and Antimicrobial Activities. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. S3), 89–95. [Google Scholar] [CrossRef]
- Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Šiugždaitė, J.; Mickevičius, V.; Beresnevičius, Z.J. Synthesis and Biological Activity of 1,3,4-Oxa(Thia)Diazole, 1,2,4-Triazole-5-(Thio)One and S-Substituted Derivatives of 3-((2-Carboxyethyl)Phenylamino)Propanoic Acid. Res. Chem. Intermed. 2016, 42, 4459–4477. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Sayadi, M. Syntheses of Some Novel and Symmetrical Bis(4-Amino-4H-1,2,4-Triazole-3-Thiols). J. Sulphur. Chem. 2012, 33, 647–652. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Haukka, M.; Soliman, S.M.; Barakat, A.; Boraei, A.T.A.; Aboelmagd, A. Stereoselective Synthesis of New 4-Aryl-5-Indolyl-1,2,4-Triazole S- and N-β-Galactosides: Characterizations, X-Ray Crystal Structure and Hirshfeld Surface Analysis. Crystals 2023, 13, 797. [Google Scholar] [CrossRef]
- Il’inykh, E.S.; Kim, D.G.; Valova, M.S.; Fedorova, O.V. Synthesis and Optical Properties of New S-Derivatives of 5,5′-(1,4-Phenylene)Bis(4H-1,2,4-Triazole-3-Thiol) and 5,5′,5″-(Benzene-1,3,5-Triyl)Tris(4H-1,2,4-Triazole-3-Thiol). Russ. J. Gen. Chem. 2019, 89, 2571–2576. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Smith, R.A.; Nischwitz, A.K.; Gavin, T. A Mild and Highly Convenient Chemoselective Alkylation of Thiols Using Cs2CO3–TBAI. Tetrahedron Lett. 2005, 46, 8931–8935. [Google Scholar] [CrossRef]
- Socea, L.; Barbuceanu, S.; Socea, B.; Draghici, C.; Apostol, T.-V.; Pahontu, E.; Olaru, O. New Heterocyclic Compounds from 1,2,4-Triazoles Class with Potential Cytotoxic Activity. Rev. Chim. 2017, 68, 2503–2508. [Google Scholar] [CrossRef]
- Deligeorgiev, T.; Kaloyanova, S.; Lesev, N.; Vaquero, J.J. An Easy and Fast Ultrasonic Selective S-Alkylation of Hetaryl Thiols at Room Temperature. Ultrason. Sonochem. 2010, 17, 783–788. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.-B.; Cao, X.; Liu, D.-C.; Shu, B.; Quan, Z.-S. Design, Synthesis and Anticonvulsant Activity Evaluation of Novel 4-(4-Substitutedphenyl)-3-Methyl-1H-1,2,4-Triazol-5(4H)-Ones. Drug Res. 2013, 64, 40–46. [Google Scholar] [CrossRef]
- An, F.; Xuan, X.; Liu, Z.; Bian, M.; Shen, Q.; Quan, Z.; Zhang, G.; Wei, C. Anti-Inflammatory Activity of 4-(4-(Heptyloxy)Phenyl)-2,4-Dihydro-3H-1,2,4-Triazol-3-One via Repression of MAPK/NF-ΚB Signaling Pathways in β-Amyloid-Induced Alzheimer’s Disease Models. Molecules 2022, 27, 5035. [Google Scholar] [CrossRef]
- Ustabaş, R.; Süleymanoğlu, N.; Ünver, Y.; Direkel, Ş. 5-(4-Bromobenzyl)-4-(4-(5-Phenyl-1,3,4-Oxadiazole-2-Yl)Phenyl)-2,4-Dihydro-3H-1,2,4-Triazole-3-One: Synthesis, Characterization, DFT Study and Antimicrobial Activity. J. Mol. Struct. 2020, 1214, 128217. [Google Scholar] [CrossRef]
- Malbec, F.; Milcent, R.; Vicart, P.; Bure, A.M. Synthesis of New Derivatives of 4-Amino-2,4-Dihydro-1,2,4-Triazol-3-One as Potential Antibacterial Agents. J. Heterocycl. Chem. 1984, 21, 1769–1774. [Google Scholar] [CrossRef]
- Sirach, R.R.; Dave, P.N. 3-Nitro-1,2,4-Triazol-5-One (NTO): High Explosive Insensitive Energetic Material. Chem. Heterocycl. Comp. 2021, 57, 720–730. [Google Scholar] [CrossRef]
- Kikugawa, Y.; Yamada, S.; Nagashima, H.; Kaji, K. The Reaction of Substituted Ureas with Sodium Borohydride in Pyridine. Tetrahedron. Lett. 1969, 10, 699–702. [Google Scholar] [CrossRef]
- Molla, E.; Abser, N.; Islam, M. Synthesis and Characterization of Some 4-Aryl Substituted Thiosemicarbazides, N-Alkyloxybenzaldehydes Containing Long Alkyl Chains and Their Corresponding Thiosemicarbazones. Jahangirnagar. Univ. J. Sci. 2018, 41, 31–42. [Google Scholar]
- Siwek, A.; Wujec, M.; Dobosz, M.; Wawrzycka-Gorczyca, I. Study of Direction of Cyclization of 1-Azolil-4-Aryl/Alkyl-Thiosemicarbazides. Heteroat. Chem. 2010, 21, 521–532. [Google Scholar] [CrossRef]
- Pareek, A.K.; Joseph, P.E.; Seth, D.S. Convenient Synthesis, Characterization of Some Novel Substituted 3-Methyl-2-Pyrazoline-5-Ones and Substituted 3,5-Dimethyl Pyrazoles. Orient. J. Chem. 2010, 26, 1467–1471. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).