Diethyl 2-((aryl(alkyl)amino)methylene)malonates: Unreported Mycelial Growth Inhibitors against Fusarium oxysporum
Abstract
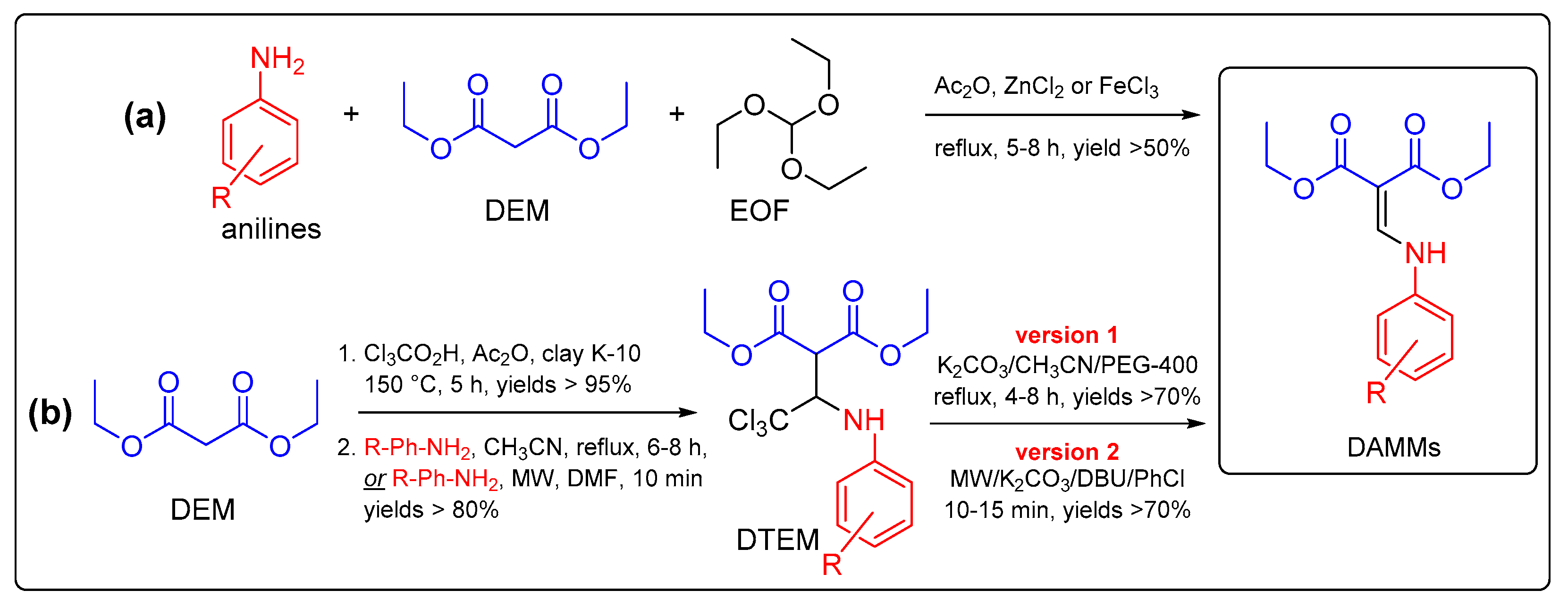
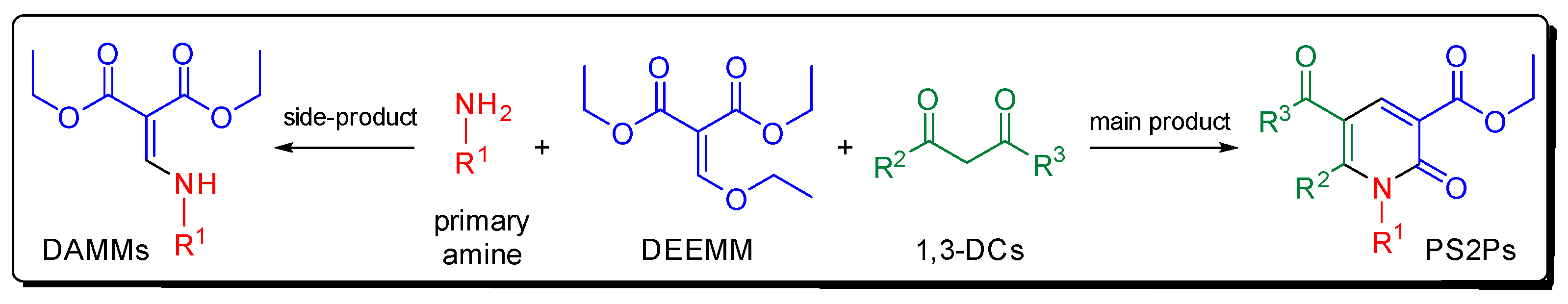
1. Introduction
2. Results and Discussion
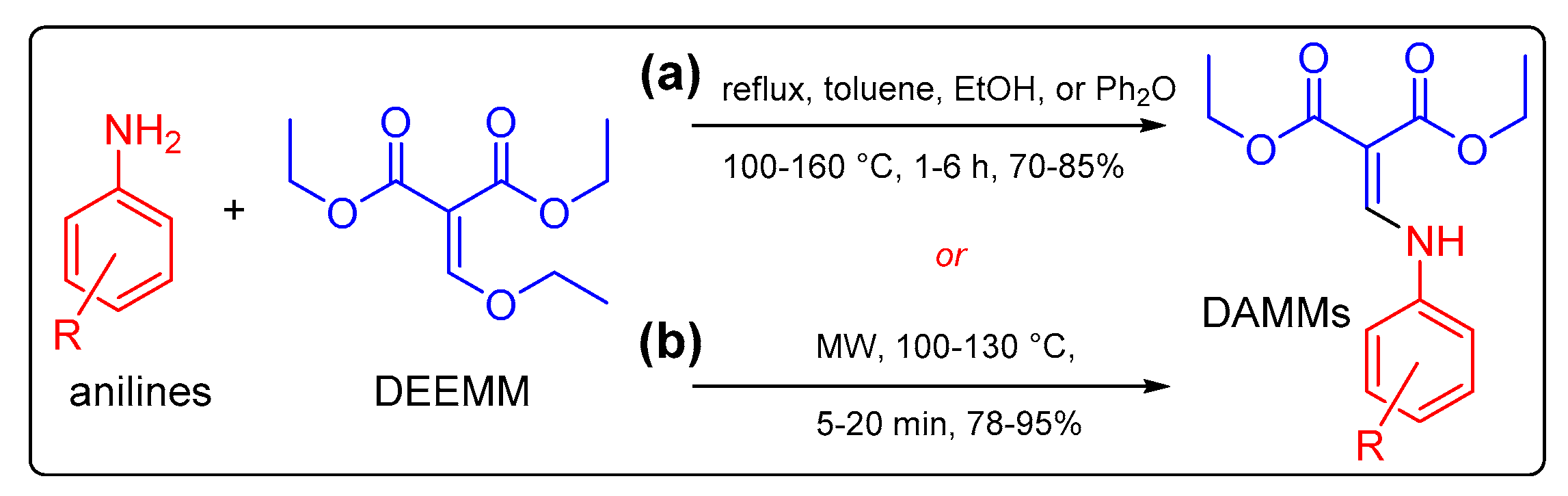
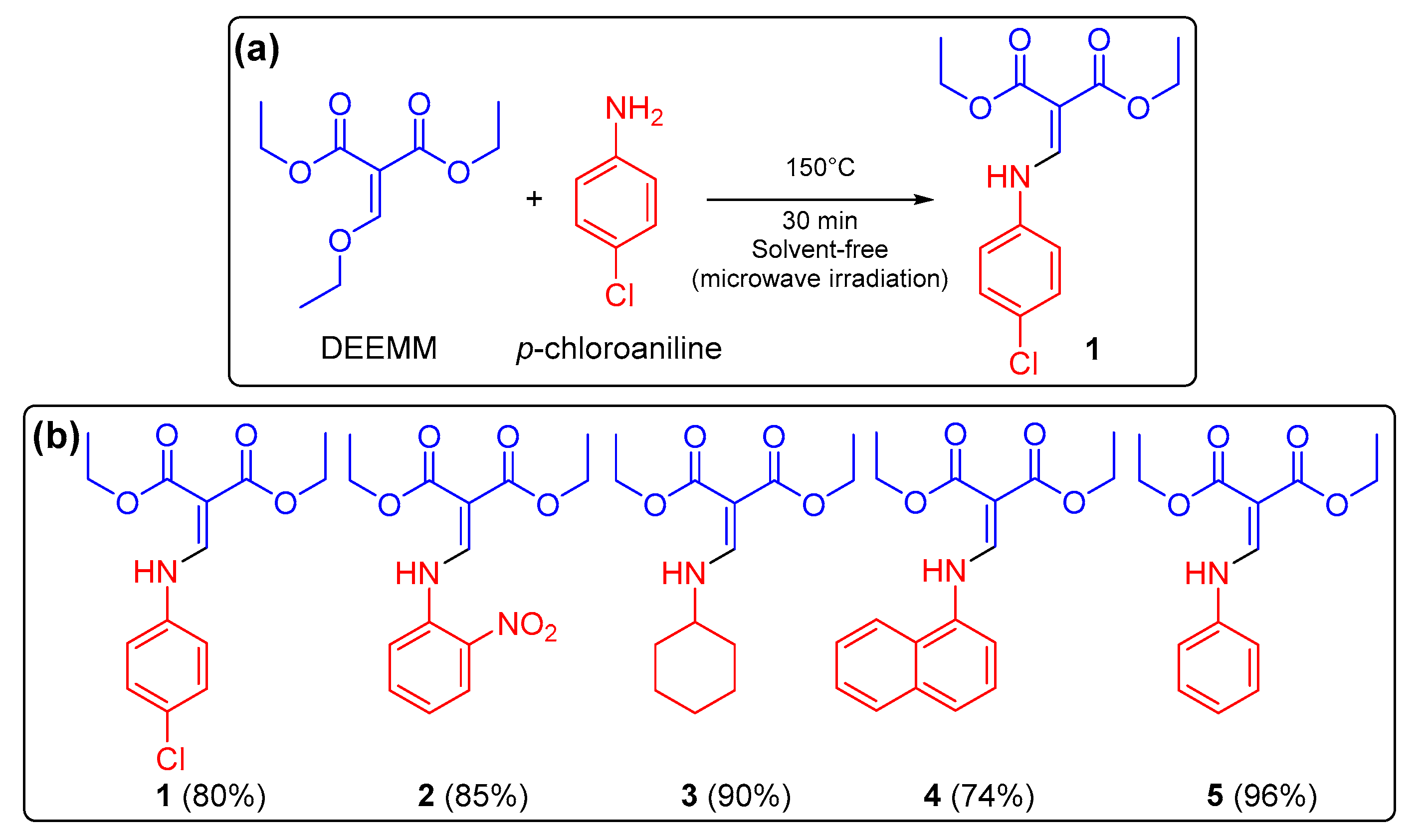
2.1. Synthesis of the DAMMs 1–5
2.2. Antifungal Activity of DAMMs 1–5
3. Materials and Methods
3.1. General
3.2. General Procedure for the Microwave-Assisted Synthesis of Diethyl 2-((aryl(alkyl)amino) methylene)malonates 1–5
3.3. Antifungal Activity
3.3.1. Fungicidal and Fungistatic Effect
3.3.2. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hu, M.; Chen, S. Non-Target Site Mechanisms of Fungicide Resistance in Crop Pathogens: A Review. Microorganisms 2021, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.; de Hoog, G.S.; Meis, J.F. Multiresistant Fusarium Pathogens on Plants and Humans: Solutions in (from) the Antifungal Pipeline? Infect. Drug Resist. 2019, 12, 3727–3737. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Sun, R.; Chen, X.; Yang, L.; Huang, C. Microwave-Assisted, Solvent-Free, Three-Component Domino Protocol: Efficient Synthesis of Polysubstituted-2-Pyridone Derivatives. ChemistrySelect 2018, 3, 4635–4638. [Google Scholar] [CrossRef]
- Larson, P.G.; Ferguson, D.M. 4-Amino-2-Butyl-7-Methoxycarbonylthiazolo[4,5-c]Quinoline. Molbank 2021, 2021, 1305. [Google Scholar] [CrossRef]
- Banerji, B.; Conejo-Garcia, A.; McNeill, L.A.; McDonough, M.A.; Buck, M.R.G.; Hewitson, K.S.; Oldham, N.J.; Schofield, C.J. The Inhibition of Factor Inhibiting Hypoxia-Inducible Factor (FIH) by β-Oxocarboxylic Acids. Chem. Commun. 2005, 43, 5438–5440. [Google Scholar] [CrossRef]
- Ilangovan, A.; Kumar, R.G. 2,2-Bis(Ethoxycarbonyl)Vinyl (BECV) as a Versatile Amine Protecting Group for Selective Functional-Group Transformations. Chem.–Eur. J. 2010, 16, 2938–2943. [Google Scholar] [CrossRef]
- Forezi, L.D.S.M.; Tolentino, N.M.C.; De Souza, A.M.T.; Castro, H.C.; Montenegro, R.C.; Dantas, R.F.; Oliveira, M.E.I.M.; Silva, F.P.; Barreto, L.H.; Burbano, R.M.R.; et al. Synthesis, Cytotoxicity and Mechanistic Evaluation of 4-Oxoquinoline-3- Carboxamide Derivatives: Finding New Potential Anticancer Drugs. Molecules 2014, 19, 6651–6670. [Google Scholar] [CrossRef]
- Venkatesan, P.; Thamotharan, S.; Percino, M.J.; Ilangovan, A. Intramolecular Resonance Assisted N–H⋅⋅⋅O=C Hydrogen Bond and Weak Noncovalent Interactions in Two Asymmetrically Substituted Geminal Amido-Esters: Crystal Structures and Quantum Chemical Exploration. J. Mol. Struct. 2021, 1246, 131210. [Google Scholar] [CrossRef]
- Deshmukh, A.R.A.S.; Panse, D.G.; Bhawal, B.M. A Clay Catalyzed Method for Diethyl 2,2,2- Trichloroethylidenepropanedioate, an Efficient Intermediate for the Synthesis of Enamino Esters. Synth. Commun. 1999, 29, 1801–1809. [Google Scholar] [CrossRef]
- Ilangovan, A.; Kumar, R.; Kaushik, M. Lewis Acid Mediated Selective Monohydrolysis of Geminal Diesters: Synthesis of Functionalized Malonic Acid Half Esters. Synlett 2012, 23, 2093–2097. [Google Scholar] [CrossRef]
- Arya, F.; Bouquant, J.; Chuche, J. A Convenient Synthesis of 3-Formyl-4(1 h)-Pyridones. Synthesis (Stuttgart) 1983, 1983, 946–948. [Google Scholar] [CrossRef]
- Tsoung, J.; Bogdan, A.R.; Kantor, S.; Wang, Y.; Charaschanya, M.; Djuric, S.W. Synthesis of Fused Pyrimidinone and Quinolone Derivatives in an Automated Hight-Temperature and High-Pressure Flow Reactor. J. Org. Chem. 2017, 82, 1073–1084. [Google Scholar] [CrossRef]
- Muñoz, H.; Tamariz, J.; Zamora, H.S.; Lázaro, M.; Labarrios, F. Preparation of Propanedioic Acid, (Anilinometwlene) Alkyl Esters by Direct Condensation. Synth. Commun. 1987, 17, 549–554. [Google Scholar] [CrossRef]
- Bhujanga Rao, A.K.S.; Radhakrishna, A.S.; Rao, C.G.; Singh, B.B.; Bhatnagar, S.P. An Improved Procedure for the Preparation of Ethyl α-Carbethoxy-β-(Arylamino)Acrylates. Org. Prep. Proced. Int. 1988, 20, 93–95. [Google Scholar] [CrossRef]
- Wang, Z. Gould-Jacobs Reaction. In Comprehensive Organic Name Reactions and Reagents; Wang, Z., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1252–1255. ISBN 9780470638859. [Google Scholar]
- Li, J.J. Gould–Jacobs Reaction. In Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications; Li, J.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 289–290. ISBN 978-3-319-03979-4. [Google Scholar]
- Loupy, A.; Song, S.J.; Cho, S.J.; Park, D.K.; Kwon, T.W. Solvent-free Microwave Michael Addition between EMME and Various Nucleophiles. Synth. Commun. 2005, 35, 79–87. [Google Scholar] [CrossRef]
- Lager, E.; Andersson, P.; Nilsson, J.; Pettersson, I.; Nielsen, E.Ø.; Nielsen, M.; Sterner, O.; Liljefors, T. 4-Quinolone Derivatives: High-Affinity Ligands at the Benzodiazepine Site of Brain GABAA Receptors. Synthesis, Pharmacology, and Pharmacophore Modeling. J. Med. Chem. 2006, 49, 2526–2533. [Google Scholar] [CrossRef]
- Shaik, A.; Angira, D.; Thiruvenkatam, V. Insights into Supramolecular Assembly Formation of Diethyl Aryl Amino Methylene Malonate (DAM)Derivatives Assisted via Non-Covalent Interactions. J. Mol. Struct. 2019, 1192, 178–185. [Google Scholar] [CrossRef]
- Santos, C.; Pimentel, L.; Canzian, H.; Oliveira, A.; Junior, F.; Dantas, R.; Hoelz, L.; Marinho, D.; Cunha, A.; Bastos, M.; et al. Hybrids of Imatinib with Quinoline: Synthesis, Antimyeloproliferative Activity Evaluation, and Molecular Docking. Pharmaceuticals 2022, 15, 309. [Google Scholar] [CrossRef]
- Jang, T.-S.; Ku, I.W.; Jang, M.S.; Keum, G.; Kang, S.B.; Chung, B.Y.; Kim, Y. Indium-Mediated One-Pot Three-Component Reaction of Aromatic Amines, Enol Ethers, and Allylic Bromides. Org. Lett. 2006, 8, 195–198. [Google Scholar] [CrossRef]
- Malvacio, I.; Vera, D.; Moyano, E. Microwave Assisted Synthesis of Ethyl-Quinolon-4-One-3-Carboxylates and Hydrolysis to Quinolon-4-One-3-Carboxylic Acids. Curr. Microw. Chem. 2014, 1, 52–58. [Google Scholar] [CrossRef]
- Cernuchová, P.; Vo-Thanh, G.; Milata, V.; Loupy, A. Solvent-Free Synthesis of Quinolone Derivatives. Heterocycles 2004, 64, 177–191. [Google Scholar] [CrossRef]
- Li, X.; Xu, J. Determination on Temperature Gradient of Different Polar Reactants in Reaction Mixture under Microwave Irradiation with Molecular Probe. Tetrahedron 2016, 72, 5515–5520. [Google Scholar] [CrossRef]
- Borrego-Muñoz, P.; Cardenas, D.; Ospina, F.; Coy-Barrera, E.; Quiroga, D. Second-Generation Enamine-Type Schiff Bases as 2-Amino Acid-Derived Antifungals against Fusarium Oxysporum: Microwave-Assisted Synthesis, in Vitro Activity, 3D-QSAR, and in Vivo Effect. J. Fungi 2023, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Muñoz, P.; Becerra, L.D.; Ospina, F.; Coy-Barrera, E.; Quiroga, D. Synthesis (Z) vs (E) Selectivity, Antifungal Activity against Fusarium Oxysporum, and Structure-Based Virtual Screening of Novel Schiff Bases Derived from L-Tryptophan. ACS Omega 2022, 7, 24714–24726. [Google Scholar] [CrossRef]
- Ul Haq, I.; Sarwar, M.K.; Faraz, A.; Latif, M.Z. Synthetic Chemicals: Major Component of Plant Disease Management. In Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches; Ul Haq, I., Ijaz, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 53–81. ISBN 978-3-030-35955-3. [Google Scholar]
- Marentes-Culma, R.; Orduz-Díaz, L.L.; Coy-Barrera, E. Targeted Metabolite Profiling-Based Identification of Antifungal 5-n-Alkylresorcinols Occurring in Different Cereals against Fusarium oxysporum. Molecules 2019, 24, 770. [Google Scholar] [CrossRef]
- Cole, M.D. Key Antifungal, Antibacterial and Anti-Insect Assays-a Critical Review. Biochem. Syst. Ecol. 1994, 22, 837–856. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat; versión 24; Universidad Nacional de Córdoba: Córdoba, Argentina, 2011; Available online: http://www.infostat.com.ar/ (accessed on 12 January 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cely-Veloza, W.-F.; Quiroga, D.; Coy-Barrera, E. Diethyl 2-((aryl(alkyl)amino)methylene)malonates: Unreported Mycelial Growth Inhibitors against Fusarium oxysporum. Molbank 2023, 2023, M1630. https://doi.org/10.3390/M1630
Cely-Veloza W-F, Quiroga D, Coy-Barrera E. Diethyl 2-((aryl(alkyl)amino)methylene)malonates: Unreported Mycelial Growth Inhibitors against Fusarium oxysporum. Molbank. 2023; 2023(2):M1630. https://doi.org/10.3390/M1630
Chicago/Turabian StyleCely-Veloza, Willy-Fernando, Diego Quiroga, and Ericsson Coy-Barrera. 2023. "Diethyl 2-((aryl(alkyl)amino)methylene)malonates: Unreported Mycelial Growth Inhibitors against Fusarium oxysporum" Molbank 2023, no. 2: M1630. https://doi.org/10.3390/M1630
APA StyleCely-Veloza, W.-F., Quiroga, D., & Coy-Barrera, E. (2023). Diethyl 2-((aryl(alkyl)amino)methylene)malonates: Unreported Mycelial Growth Inhibitors against Fusarium oxysporum. Molbank, 2023(2), M1630. https://doi.org/10.3390/M1630







