4,7-Dimethoxy-6-propyl-2H-1,3-benzodioxole-5-carbaldehyde
1. Introduction
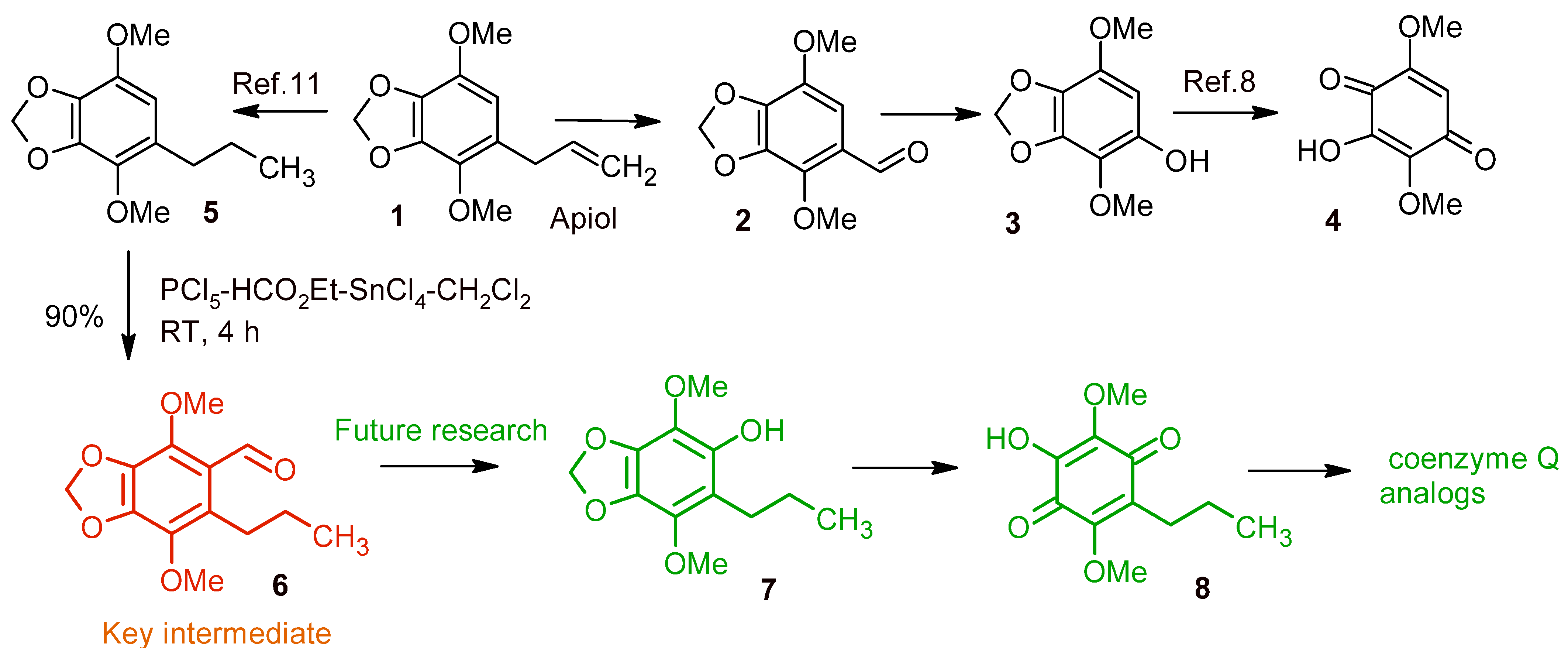
2. Results and Discussion
3. Materials and Methods
Synthesis of 4,7-Dimethoxy-6-propyl-2H-1,3-benzodioxole-5-carbaldehyde 6
- Preparation of the formylation mixture: Ethyl formate as a single portion (11.0 g, 148 mmol) was added to a suspension of PCl5 (26.7 g, 128 mmol) in dry CH2Cl2 (40 mL) and refluxed for 4 h (CaCl2 tube). A solution of dichloromethyl methyl ether (128 mmol) was obtained.
- A solution of 4,7-dimethoxy-5-propyl-1,3-benzodioxole 5 [9] (2.1 g, 9.3 mmol) in formylation mixture (12.8 mmol) and dry CH2Cl2 (15 mL) was added dropwise to a solution of SnCl4 (7.0 g, 27 mmol) in dry CH2Cl2 (15 mL) at −10 °C. The reaction mixture was kept for 1 h at 0 °C and poured into water (100 mL). The organic layer was separated, washed with water (3 × 50 mL), and dried over MgSO4. After removal of the solvent, the product was recrystallized from EtOH. White powder; 2.1 g (90%); mp 69–71 °C (EtOH); 1H NMR (DMSO-d6) δ: 0.90 (t, 3H, Me, J 7.3 Hz), 1.34–1.42 (m, 2H, CH2), 2.815 (t, 2H, ArCH2, J 7.7 Hz), 3.83 (s, 3H, OMe), 3.95 (s, 3H, OMe), 6.16 (s, 2H, OCH2 O), 10.25 (s, 1H, CHO). 13C NMR (DMSO-d6) δ: 14.12, 23.85, 27.01, 60.08, 60.68, 102.60, 120.04, 132.35, 136.33, 136.90, 142.59, 143.93, 189.79. EIMS m/z: 252 ([M]+, 90%), 251 (38), 237 (30), 235 (18), 223 (100), 209 (57), 208 (31), 207 (17), 193 (18), 179 (18), 151 (12), 121 (11), 92 (19), 91 (28), 79 (34), 77 (50), 69 (22), 67 (25), 66 (29), 65 (35), 64 (22), 63 (29), 53 (43), 51 (39), 41 (26). IR (KBr) νmax: 1609, 1674 (CO), calculated for C13H16O5 C, 61.90; H, 6.39; found C, 61.96; H, 6.42.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, H.-L.; Thiyagarajan, V.; Shen, P.-C.; Mathew, D.C.; Lin, K.-Y.; Liao, Y.-W.; Hseu, Y.-C. Anti-EMT Properties of CoQ0 Attributed to PI3K/AKT/NFKB/MMP-9 Signaling Pathway Through ROS-mediated Apoptosis. J. Exp. Clin. Cancer Res. 2019, 38, 186. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Thiyagarajan, V.; Tsou, H.-T.; Lin, K.-Y.; Chen, H.-J.; Lin, C.-M.; Liao, J.-W.; Yang, H.-L. In Vitro and in Vivo Anti-tumor Activity of CoQ0 Against Melanoma Cells: Inhibition of Metastasis and Induction of Cell-cycle Arrest and Apoptosis Through Modulation of Wnt/β-catenin Signaling Pathways. Oncotarget 2016, 7, 22409–22426. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Tsai, T.-J.; Korivi, M.; Liu, J.-Y.; Chen, H.-J.; Lin, C.-M.; Yang, H.-L. Antitumor Properties of Coenzyme Q0 Against Human Ovarian Carcinoma Cells via Induction of ROS-mediated Apoptosis and Cytoprotective Autophagy. Sci. Rep. 2017, 7, 8062. [Google Scholar] [CrossRef] [PubMed]
- Yafei, J.; Wanmei, X.; Wenhu, J.; Yue, W. Practical Synthesis of 2,3,4,5-Tetramethoxytoluene. Synth. Commun. 2006, 36, 1961–1965. [Google Scholar] [CrossRef]
- Semenov, V.V.; Rusak, V.A.; Chartov, E.M.; Zaretsky, M.I.; Konyushkin, L.D.; Firgang, S.I.; Chizhov, A.O.; Elkin, V.V.; Latin, N.N.; Bonashek, V.M.; et al. Polyalkoxybenzenes from Plant Raw Materials 1. Isolation of Polyalkoxybenzenes from CO2 Extracts of Umbelliferae Plant Seeds. Russ. Chem. Bull. Int. Ed. 2007, 56, 2448–2455. [Google Scholar] [CrossRef]
- Tsyganov, D.V.; Chernysheva, N.B.; Salamandra, L.K.; Konyushkin, L.D.; Atamanenko, O.P.; Semenova, M.N.; Semenov, V.V. Synthesis of Polyalkoxy-3-(4-Methoxyphenyl)Coumarins with Antimitotic Activity from Plant Allylpolyalkoxybenzenes. Mendeleev Commun. 2013, 23, 147–149. [Google Scholar] [CrossRef]
- Lien, H.; Kuo, P.; Huang, C.; Kao, J.; Lin, H.; Yang, D.; Lai, Y. Study of the Anti-Proliferative Activity of 5-Substituted 4,7-Dimethoxy-1,3-Benzodioxole Derivatives of SY-1 from Antrodia camphorata on Human COLO 205 Colon Cancer Cells. Evid.-Based Complement. Altern. 2009, 2011, 450529. [Google Scholar] [CrossRef]
- Shi, L.-S.; Chao, C.-H.; Shen, D.-Y.; Chan, H.-H.; Chen, C.-H.; Liao, Y.-R.; Wu, S.-J.; Leu, Y.-L.; Shen, Y.-C.; Kuo, Y.-H.; et al. Biologically Active Constituents from the Fruiting Body of Taiwanofungus camphorates. Bioorg. Med. Chem. 2011, 19, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Varakutin, A.E.; Muravsky, E.A.; Shinkarev, I.Y.; Khrustalev, V.N.; Semenov, V.V. Hydrogenation of Plant Polyalkoxybenzene Derivatives: Convenient Access to Coenzyme Q0 Analogues. Mendeleev Commun. 2020, 30, 599–601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganov, D.V.; Semenov, V.V. 4,7-Dimethoxy-6-propyl-2H-1,3-benzodioxole-5-carbaldehyde. Molbank 2023, 2023, M1676. https://doi.org/10.3390/M1676
Tsyganov DV, Semenov VV. 4,7-Dimethoxy-6-propyl-2H-1,3-benzodioxole-5-carbaldehyde. Molbank. 2023; 2023(3):M1676. https://doi.org/10.3390/M1676
Chicago/Turabian StyleTsyganov, Dmitry V., and Victor V. Semenov. 2023. "4,7-Dimethoxy-6-propyl-2H-1,3-benzodioxole-5-carbaldehyde" Molbank 2023, no. 3: M1676. https://doi.org/10.3390/M1676
APA StyleTsyganov, D. V., & Semenov, V. V. (2023). 4,7-Dimethoxy-6-propyl-2H-1,3-benzodioxole-5-carbaldehyde. Molbank, 2023(3), M1676. https://doi.org/10.3390/M1676






