1-(((6-(Methoxycarbonyl)-5-oxononan-4-yl)oxy)carbonyl)cyclopropane-1-carboxylic Acid
Abstract
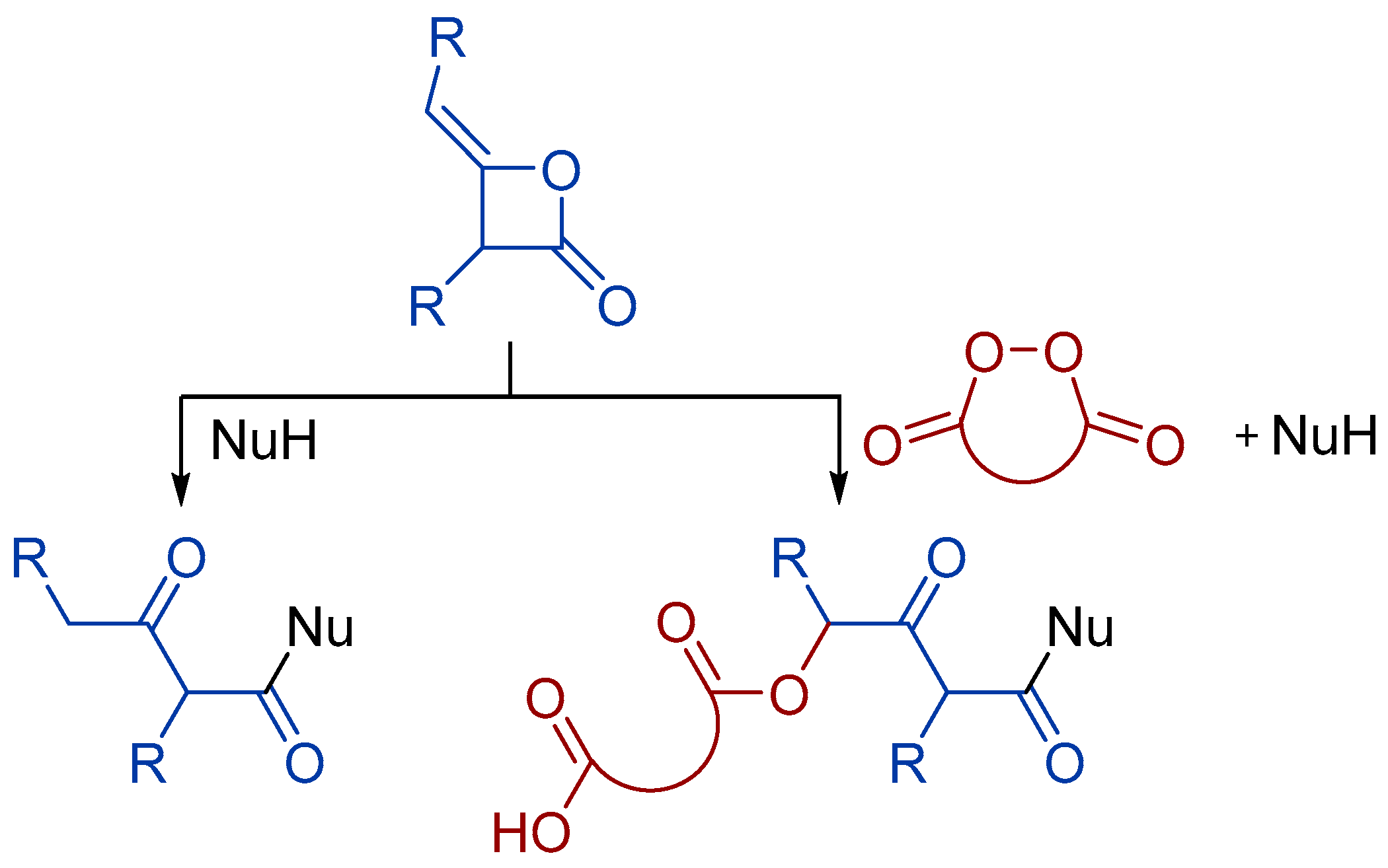
1. Introduction
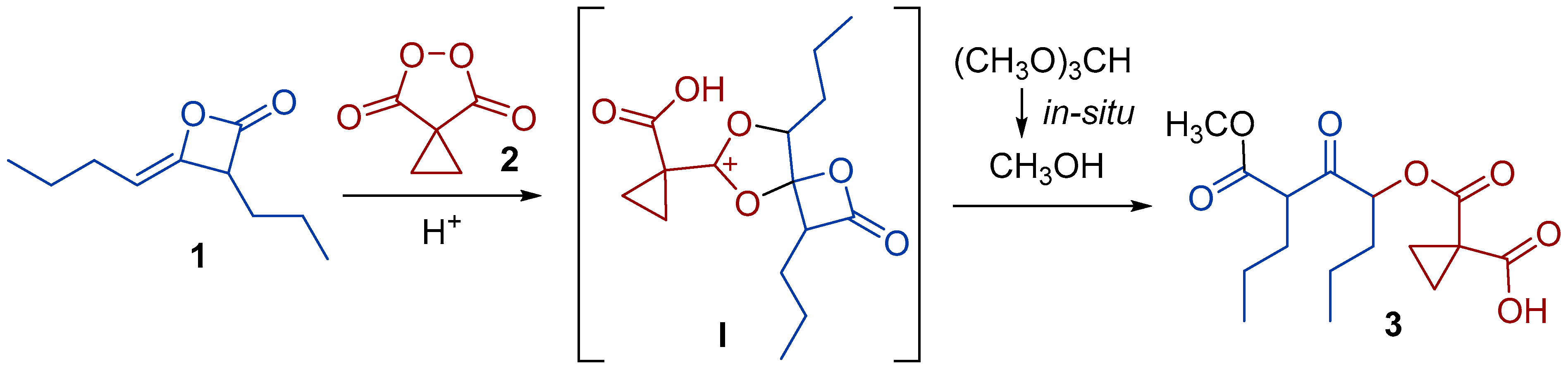
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 4-Butylidene-3-propyloxetan-2-one (1)
3.3. Synthesis of Cyclopropyl Malonoyl Peroxide (2)
3.4. Synthesis of 1-(((6-(Methoxycarbonyl)-5-oxononan-4-yl)oxy)carbonyl)cyclopropane-1-carboxylic acid (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wedekind, E. Ueber die Gewinnung von Säureanhydriden mit Hülfe von tertiären Aminen. Ber. Dtsch. Chem. Ges. 1901, 34, 2070–2077. [Google Scholar] [CrossRef]
- Staudinger, H. Über ketene. 4. Mitteilung: Reaktionen des diphenylketens. Ber. Dtsch. Chem. Ges. 1907, 40, 1145–1148. [Google Scholar] [CrossRef]
- Staudinger, H.; Klever, H. Über Ketene. 5. Mitteilung. Reaktionen des Dimethylketens. Ber. Dtsch. Chem. Ges. 1907, 40, 1149–1153. [Google Scholar] [CrossRef]
- Clemens, R.J. Diketene. Chem. Rev. 1986, 86, 241–318. [Google Scholar] [CrossRef]
- Clemens, R.J.; Witzeman, J.S. Acetic Acid and Its Derivatives; Marcel Dekker, Inc.: New York, NY, USA, 1992; Volume 16, pp. 173–175. [Google Scholar]
- Taeschler, C. Ketenes, ketene dimers, and related substances. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000; pp. 1–54. [Google Scholar] [CrossRef]
- Hirao, T.; Fujii, T.; Ohshiro, Y. VO (OR) Cl2-induced cyclization of diketene via ring opening. J. Organomet. Chem. 1991, 407, C1–C4. [Google Scholar] [CrossRef]
- Nishino, H.; Nguyen, V.-H.; Yoshinaga, S.; Kurosawa, K. Formation of Tetrahydrofuran Derivatives and Acetonylation of Alkenes Using Carbon Radicals Derived from Manganese (III) Oxidation of Diketene. J. Org. Chem. 1996, 61, 8264–8271. [Google Scholar] [CrossRef]
- Van Ha, N.; Nishino, H. Formation of endoperoxides from Mn (III)-induced reaction of 1,1-diarylethene, diketene and ethanol. Vietnam. J. Chem. 2015, 53, 210–214. [Google Scholar]
- Perrin, C.L.; Arrhenius, T. Malonic anhydride. J. Am. Chem. Soc. 1978, 100, 5249–5251. [Google Scholar] [CrossRef]
- O’Murchu, C.D. Process for Preparing Malonic Anhydride. EP0496362A2, 29 July 1992. [Google Scholar]
- Greene, F.D. Cyclic Diacyl Peroxides. I. Monomeric Phthaloyl Peroxide. I. J. Am. Chem. Soc. 1956, 78, 2246–2250. [Google Scholar] [CrossRef]
- Adam, W.; Rucktaeschel, R. Cyclic peroxides. V.. alpha.-Lactone intermediate via photodecarboxylation of a monomeric malonyl peroxide. J. Am. Chem. Soc. 1971, 93, 557–559. [Google Scholar] [CrossRef]
- Zhao, R.; Chang, D.; Shi, L. Recent Advances in Cyclic Diacyl Peroxides: Reactivity and Selectivity Enhancement Brought by the Cyclic Structure. Synthesis 2017, 49, 3357–3365. [Google Scholar] [CrossRef]
- Kawamura, S.; Mukherjee, S.; Sodeoka, M. Recent advances in reactions using diacyl peroxides as sources of O- and C-functional groups. Org. Biomol. Chem. 2021, 19, 2096–2109. [Google Scholar] [CrossRef] [PubMed]
- Vil’, V.A.; Gorlov, E.S.; Terent’ev, A.O. 4,4′-(Butane-1,4-diyl) bis (4-methyl-1,2-dioxolane-3,5-dione). Molbank 2022, 2022, M1497. [Google Scholar] [CrossRef]
- Greene, F.D. Cyclic diacyl peroxides. II. Reaction of phthaloyl peroxide with cis-and trans-stilbene. J. Am. Chem. Soc. 1956, 78, 2250–2254. [Google Scholar] [CrossRef]
- Griffith, J.C.; Jones, K.M.; Picon, S.; Rawling, M.J.; Kariuki, B.M.; Campbell, M.; Tomkinson, N.C. Alkene syn dihydroxylation with malonoyl peroxides. J. Am. Chem. Soc. 2010, 132, 14409–14411. [Google Scholar] [CrossRef]
- Yuan, C.; Axelrod, A.; Varela, M.; Danysh, L.; Siegel, D. Synthesis and reaction of phthaloyl peroxide derivatives, potential organocatalysts for the stereospecific dihydroxylation of alkenes. Tetrahedron Lett. 2011, 52, 2540–2542. [Google Scholar] [CrossRef]
- Jones, K.M.; Tomkinson, N.C. Metal-free dihydroxylation of alkenes using cyclobutane malonoyl peroxide. J. Org. Chem. 2012, 77, 921–928. [Google Scholar] [CrossRef]
- Rawling, M.J.; Tomkinson, N.C. Metal-free syn-dioxygenation of alkenes. Org. Biomol. Chem. 2013, 11, 1434–1440. [Google Scholar] [CrossRef]
- Alamillo-Ferrer, C.; Davidson, S.C.; Rawling, M.J.; Theodoulou, N.H.; Campbell, M.; Humphreys, P.G.; Kennedy, A.R.; Tomkinson, N.C. Alkene anti-dihydroxylation with malonoyl peroxides. Org. Lett. 2015, 17, 5132–5135. [Google Scholar] [CrossRef]
- Alamillo-Ferrer, C.; Karabourniotis-Sotti, M.; Kennedy, A.R.; Campbell, M.; Tomkinson, N.C. Alkene dioxygenation with malonoyl peroxides: Synthesis of γ-lactones, isobenzofuranones, and tetrahydrofurans. Org. Lett. 2016, 18, 3102–3105. [Google Scholar] [CrossRef]
- Alamillo-Ferrer, C.; Curle, J.M.; Davidson, S.C.; Lucas, S.C.; Atkinson, S.J.; Campbell, M.; Kennedy, A.R.; Tomkinson, N.C. Alkene oxyamination using malonoyl peroxides: Preparation of pyrrolidines and isoxazolidines. J. Org. Chem. 2018, 83, 6728–6740. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Liang, Y.; Hernandez, T.; Berriochoa, A.; Houk, K.N.; Siegel, D. Metal-free oxidation of aromatic carbon–hydrogen bonds through a reverse-rebound mechanism. Nature 2013, 499, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Camelio, A.M.; Liang, Y.; Eliasen, A.M.; Johnson, T.C.; Yuan, C.; Schuppe, A.W.; Houk, K.; Siegel, D. Computational and Experimental Studies of Phthaloyl Peroxide-Mediated Hydroxylation of Arenes Yield a More Reactive Derivative, 4, 5-Dichlorophthaloyl Peroxide. J. Org. Chem. 2015, 80, 8084–8095. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.; Kubczyk, T.M.; Rowley, J.H.; Sproules, S.; Tomkinson, N.C. Arene oxidation with malonoyl peroxides. Org. Lett. 2015, 17, 2618–2621. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Z.; Li, S.; Zhang, P.-P.; Huang, Z.-H.; Zhang, W.-B.; Gong, J.; Yang, Z. A chiral pool approach for asymmetric syntheses of (−)-antrocin,(+)-asperolide C, and (−)-trans-ozic acid. Chem. Commun. 2016, 52, 12426–12429. [Google Scholar] [CrossRef] [PubMed]
- Pilevar, A.; Hosseini, A.; Šekutor, M.; Hausmann, H.; Becker, J.; Turke, K.; Schreiner, P.R. Tuning the Reactivity of Peroxo Anhydrides for Aromatic C–H Bond Oxidation. J. Org. Chem. 2018, 83, 10070–10079. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-K.; Liu, L.; Wang, J.-J.; Qian, F.; Yang, M.-Q.; Zhang, L.-Q.; Fu, J.-G.; Li, Y.-M.; Feng, C.-G. An asymmetric synthesis of (+)-Scrodentoid A from dehydroabietic acid. Tetrahedron 2021, 85, 132031. [Google Scholar] [CrossRef]
- Tavakoli, A.; Dudley, G.B. Synthesis of coprinol and several alcyopterosin sesquiterpenes by regioselective [2+ 2+ 2] alkyne cyclotrimerization. J. Org. Chem. 2022, 87, 14909–14914. [Google Scholar] [CrossRef]
- Eliasen, A.M.; Christy, M.; Claussen, K.R.; Besandre, R.; Thedford, R.P.; Siegel, D. Dearomatization Reactions Using Phthaloyl Peroxide. Org. Lett. 2015, 17, 4420–4423. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Vil’, V.A.; Gorlov, E.S.; Nikishin, G.I.; Pivnitsky, K.K.; Adam, W. Lanthanide-catalyzed oxyfunctionalization of 1, 3-diketones, acetoacetic esters, and malonates by oxidative C–O coupling with malonyl peroxides. J. Org. Chem. 2016, 81, 810–823. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Vil’, V.A.; Gorlov, E.S.; Rusina, O.N.; Korlyukov, A.A.; Nikishin, G.I.; Adam, W. Selective Oxidative Coupling of 3H-Pyrazol-3-ones, Isoxazol-5(2H)-ones, Pyrazolidine-3,5-diones, and Barbituric Acids with Malonyl Peroxides: An Effective C-O Functionalization. ChemistrySelect 2017, 2, 3334–3341. [Google Scholar] [CrossRef]
- Vil’, V.A.; Gorlov, E.S.; Bityukov, O.V.; Barsegyan, Y.A.; Romanova, Y.E.; Merkulova, V.M.; Terent’ev, A.O. C− O coupling of Malonyl Peroxides with Enol Ethers via [5+2] Cycloaddition: Non-Rubottom Oxidation. Adv. Synth. Catal. 2019, 361, 3173–3181. [Google Scholar] [CrossRef]
- Vil’, V.A.; Gorlov, E.S.; Yu, B.; Terent’ev, A.O. Oxidative α-acyloxylation of acetals with cyclic diacyl peroxides. Org. Chem. Front. 2021, 8, 3091–3101. [Google Scholar] [CrossRef]
- Vil’, V.A.; Gorlov, E.S.; Shuingalieva, D.V.; Kunitsyn, A.Y.; Krivoshchapov, N.V.; Medvedev, M.G.; Alabugin, I.V.; Terent’ev, A.O. Activation of O-electrophiles via structural and solvent effects: SN2@O reaction of cyclic diacyl peroxides with enol acetates. J. Org. Chem. 2022, 87, 13980–13989. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Vil’, V.A.; Barsegyan, Y.A.; Terent’ev, A.O.; Alabugin, I.V. Carboxylate as a Non-innocent L-Ligand: Computational and Experimental Search for Metal-Bound Carboxylate Radicals. Org. Lett. 2022, 24, 3817–3822. [Google Scholar] [CrossRef]
- Vil’, V.A.; Barsegyan, Y.A.; Kuhn, L.; Terent’ev, A.O.; Alabugin, I.V. Creating, Preserving, and Directing Carboxylate Radicals in Ni-Catalyzed C(sp3)–H Acyloxylation of Ethers, Ketones, and Alkanes with Diacyl Peroxides. Organometallics 2023. [Google Scholar] [CrossRef]
- Tsedilin, A.M.; Fakhrutdinov, A.N.; Eremin, D.B.; Zalesskiy, S.S.; Chizhov, A.O.; Kolotyrkina, N.G.; Ananikov, V.P. How sensitive and accurate are routine NMR and MS measurements? Mendeleev Commun. 2015, 25, 454–456. [Google Scholar] [CrossRef]
- Sung, K.; Wu, S.-Y. Convenient and efficient syntheses of β-keto esters and β-keto amides directly from α-alkylacetyl chlorides. Synth. Commun. 2001, 31, 3069–3074. [Google Scholar] [CrossRef]
- Beutler, U.; Boehm, M.; Fuenfschilling, P.C.; Heinz, T.; Mutz, J.-P.; Onken, U.; Mueller, M.; Zaugg, W. A High-Throughput Process for Valsartan. Org. Process. Res. Dev. 2007, 11, 892–898. [Google Scholar] [CrossRef]
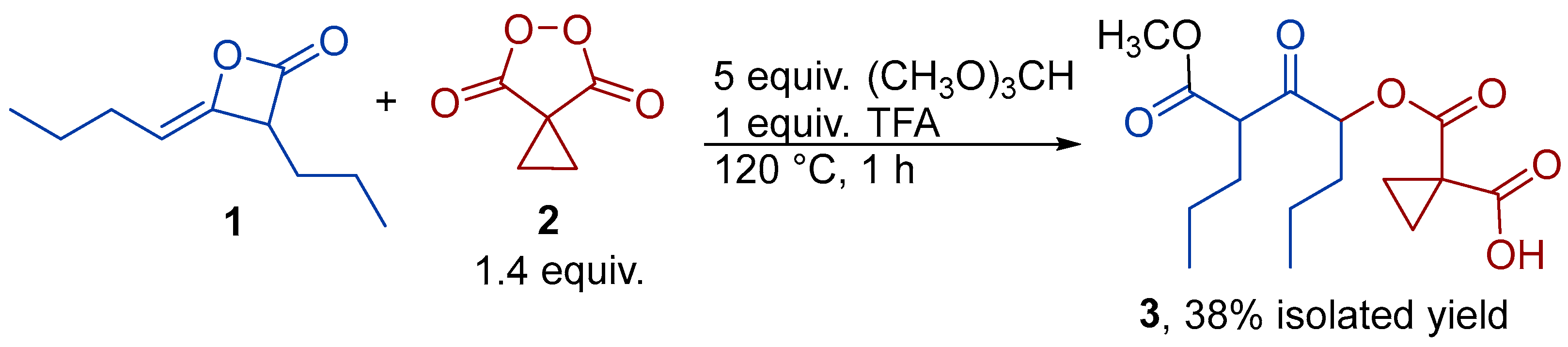
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorlov, E.S.; Shuingalieva, D.V.; Ilovaisky, A.I.; Vil’, V.A.; Terent’ev, A.O. 1-(((6-(Methoxycarbonyl)-5-oxononan-4-yl)oxy)carbonyl)cyclopropane-1-carboxylic Acid. Molbank 2023, 2023, M1651. https://doi.org/10.3390/M1651
Gorlov ES, Shuingalieva DV, Ilovaisky AI, Vil’ VA, Terent’ev AO. 1-(((6-(Methoxycarbonyl)-5-oxononan-4-yl)oxy)carbonyl)cyclopropane-1-carboxylic Acid. Molbank. 2023; 2023(2):M1651. https://doi.org/10.3390/M1651
Chicago/Turabian StyleGorlov, Evgenii S., Diana V. Shuingalieva, Alexey I. Ilovaisky, Vera A. Vil’, and Alexander O. Terent’ev. 2023. "1-(((6-(Methoxycarbonyl)-5-oxononan-4-yl)oxy)carbonyl)cyclopropane-1-carboxylic Acid" Molbank 2023, no. 2: M1651. https://doi.org/10.3390/M1651
APA StyleGorlov, E. S., Shuingalieva, D. V., Ilovaisky, A. I., Vil’, V. A., & Terent’ev, A. O. (2023). 1-(((6-(Methoxycarbonyl)-5-oxononan-4-yl)oxy)carbonyl)cyclopropane-1-carboxylic Acid. Molbank, 2023(2), M1651. https://doi.org/10.3390/M1651







